نسخه سفالیک خارجی برای نمایش بریچ پیش از دوران ترم
چکیده
پیشینه
نشان داده شده که نسخه سفالیک خارجی (external cephalic version; ECV) از جنین بریچ در دوران ترم (پس از 37 هفته) در کاهش تعداد نمایشهای بریچ و زایمان سزارین موثر است، اما نرخ موفقیت نسبتا پائینی دارد. این مرور مطالعاتی را بررسی میکند که ECV را پیش از دوران ترم (پیش از هفته 37 بارداری) آغاز میکنند.
اهداف
ارزیابی اثربخشی سیاست آغاز ECV پیش از دوران ترم (پیش از هفته 37 بارداری) برای نمایش بریچ بر نمایش جنین هنگام تولد، روش زایمان، و نرخ زایمان زودرس، موربیدیتی پریناتال، مردهزایی یا مورتالیتی نوزادان.
روشهای جستوجو
پایگاه ثبت کارآزماییهای گروه بارداری و زایمان در کاکرین (Cochrane Pregnancy and Childbirth Group’s Trials Register) (31 مارچ 2015) و فهرست منابع مطالعات بازیابیشده را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) از انجام ECV پیش از دوران ترم (هفته 37 بارداری) یا آغاز آن پیش از دوران ترم، در مقایسه با گروه کنترل شامل زنانی (در نمایش بریچ) که برای آنها ECV انجام نشده یا ECV در دوران ترم انجام شد. کارآزماییهای خوشهای‐تصادفیسازی شده برای ورود واجد شرایط بودند اما هیچ موردی از این نوع شناسایی نشد. شبه‐RCTها یا مطالعاتی با طراحی متقاطع، واجد شرایط برای ورود نبودند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم کارآزماییها را برای ورود و خطر سوگیری (bias) ارزیابی کردند، دادهها را استخراج و دقت آنها را بررسی کردند. مطالعات از نظر خطر سوگیری و برای پیامدهای مهم ارزیابی شدند، کیفیت کلی شواهد نیز با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) ارزیابی شد.
نتایج اصلی
پنج مطالعه (با 2187 زن) گنجانده شدهاند. کورسازی مداخله امکانپذیر نبود، و مشخص نیست که عدم کورسازی چه تاثیری بر پیامدهای گزارششده خواهد داشت. برای دیگر حوزههای «خطر سوگیری»، مطالعات در معرض خطر پائین یا نامشخص سوگیری قرار داشتند.
یک مطالعه، گزارشی را از ECV ارائه داد که پیش از هفته 37 بارداری انجام و تکمیل شده، و با عدم استفاده از ECV مقایسه شد. تفاوتی در نرخ نمایش غیر‐سفالیک در بدو تولد یافت نشد (خطر نسبی (RR): 1.04؛ 95% فاصله اطمینان (CI): 0.64 تا 1.69؛ 102 شرکتکننده). یک مطالعه گزارشی را از سیاست ECV ارائه داد که پیش از دوران ترم (هفته 33) و تا هفته 40 بارداری شروع شده و در مقایسه با عدم‐استفاده از ECV میتوانست تا زمان زایمان تکرار شود. این مطالعه کاهش نرخ نمایش غیر‐سفالیک را در بدو تولد نشان داد (RR: 0.59؛ 95% CI؛ 0.45 تا 0.77؛ 179 شرکتکننده).
سه مطالعه گزارشی را از ECV ارائه کردند که بین هفتههای 34 و 35 بارداری آغاز شده و با شروع در هفتههای 37 تا 38 بارداری مقایسه شد. نتایج تجمعی نشان داد که ECV اولیه خطر نمایش غیر‐سفالیک هنگام تولد (RR: 0.81؛ 95% CI؛ 0.74 تا 0.90؛ 1906 شرکتکننده؛ سه مطالعه؛ I² = 0%، شواهد با کیفیت بالا)، عدم‐دستیابی به زایمان واژینال سفالیک (RR: 0.90؛ 95% CI؛ 0.83 تا 0.97؛ 1888 شرکتکننده؛ سه مطالعه؛ I² = 0%؛ شواهد با کیفیت بالا)، و زایمان بریچ واژینال (RR: 0.44؛ 95% CI؛ 0.25 تا 0.78؛ 1888 شرکتکننده؛ سه مطالعه؛ I² = 0%؛ شواهد با کیفیت بالا) را کاهش داد. تفاوت بین گروهها از نظر خطر سزارین دارای اهمیت آماری نبود (RR: 0.92؛ 95% CI؛ 0.85 تا 1.00؛ 1888 شرکتکننده؛ سه مطالعه؛ I² = 0%، شواهد با کیفیت بالا). شواهدی وجود داشت مبنی بر اینکه خطر زایمان زودرس با ECV زودهنگام در مقایسه با ECV پس از 37 هفته افزایش یافت (6.6% در گروه ECV و 4.3% در گروه کنترل) (RR: 1.51؛ 95% CI؛ 1.03 تا 2.21؛ 1888 شرکتکننده، سه مطالعه؛ I² = 0%، شواهد با کیفیت بالا). تفاوت بارزی بین گروهها برای نمره آپگار پائین نوزاد در پنج دقیقه یا مرگومیر پریناتال (مردهزایی به علاوه مورتالیتی نوزادی تا هفت روز) وجود نداشت (شواهد برای هر دو پیامد با کیفیت پائین).
نتیجهگیریهای نویسندگان
در مقایسه با عدم آغاز ECV، شروع ECV پیش از دوران ترم، نمایش غیر‐سفالیک را در بدو تولد کاهش میدهد. در مقایسه با ECV در دوران ترم، شروع ECV در هفتههای 34 تا 35 ممکن است از نظر کاهش نرخ نمایش غیر‐سفالیک و خطر زایمان بریچ واژینال مزیتی داشته باشد. با این حال، ECV زودهنگام ممکن است خطر زایمان زودرس دیرهنگام را افزایش دهد، و مهم است که هر یک از پژوهشهای آینده پیامدهای مربوط به موربیدیتی نوزاد را گزارش کنند. نتایج مرور نشان میدهد که زمانبندی پروسیجر ECV نیاز به بحث دقیقی با زنان دارد تا آنها بتوانند تصمیمات آگاهانه بگیرند.
PICO
خلاصه به زبان ساده
نسخه سفالیک خارجی برای نمایش بریچ پیش از دوران ترم
نوزادانی که از طرف باسن به دنیا میآیند (در وضعیت بریچ) ممکن است هنگام تولد مشکلات بیشتری نسبت به نوزادانی داشته باشند که از طرف سر به دنیا میآیند (در وضعیت سفالیک)، زیرا در حالت اول ممکن است سر نوزاد با کمی تاخیر و با وارد کردن فشار روی بند ناف هنگام عبور از کانال زایمان خارج شود. در طول نسخه سفالیک خارجی (external cephalic version; ECV)، با فشار دادن آرام روی شکم مادر، نوزاد بریچ به وضعیت سر رو به پائین برگردانده میشود. پژوهشها نشان میدهند که ECV پس از 37 هفته، تعداد نوزادان وضعیت بریچ را در دوران ترم کامل و تعداد زایمانهای سزارین را کاهش میدهد.
این مرور شامل پنج مطالعه تصادفیسازی و کنترل شده با مجموع 2187 زن بود، مطالعات در معرض خطر پائین یا نامشخص سوگیری (bias) قرار داشتند، اگرچه «کورسازی» زنان و کارکنان نسبت به این مداخله امکانپذیر نبود. نتایج نشان داد که اگر ECV حدود اواسط سه ماهه سوم (هفتههای 32 تا 34) انجام شود، احتمال اینکه نوزاد در دوران ترم کاملا در وضعیت سر رو به پائین قرار بگیرد، افزایش مییابد. سه کارآزمایی شامل 1888 زن نشان دادند که شروع ECV بین 34 تا 36 هفته در مقایسه با شروع ECV پس از 37 هفته (در دوران ترم) باعث 19% کاهش در نرخ نمایش غیر‐سفالیک در بدو تولد، 10% کاهش در خطر عدم‐موفقیت در زایمان واژینال سفالیک و کاهش قابلتوجه در احتمال زایمان واژینال بریچ شد، با این حال، ECV زودهنگام ممکن است بهطور قابلتوجهی شانس زایمان زودرس دیرهنگام را افزایش دهد. کیفیت شواهد برای این پیامدها در سطح بالا درجهبندی شدند. شواهد در مورد مزایا و معایب احتمالی نسخه سفالیک خارجی (ECV) زودهنگام (پیش از 37 هفته) نیاز به بحث دقیق با زنان در مورد زمانبندی پروسیجر ECV دارد تا آنها بتوانند در این زمینه آگاهانه تصمیم بگیرند.
Authors' conclusions
Summary of findings
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
Background
Description of the condition
By late pregnancy most babies in singleton pregnancies are positioned with their heads down ready for the birth; babies presenting in the breech position (bottom first) may be at increased risk during vaginal birth as there may be delay in birth of the head and compression of the umbilical cord as the head passes through the bony pelvis. About 3% to 4% of all pregnant women who reach full term will have a fetus presenting by the breech, and breech birth is associated as having a higher risk for the neonate regardless of mode of birth (Schutte 1985).
Breech presentation may be caused by an underlying fetal or maternal abnormality, or may be an apparently chance occurrence, or related to an otherwise benign variant such as cornual placental position. In the latter instances, breech presentation places a healthy fetus and mother at increased risk of a complicated vaginal delivery or caesarean section. It is not surprising that, over the years, the possibility of manipulating the baby from the breech to the cephalic presentation has intrigued obstetric caregivers.
Description of the intervention
During an external cephalic version (ECV) a breech baby is turned to the head down position by gently pushing on the mother's abdomen. ECV before term came into routine obstetric practice on the basis of the self‐evident immediate effectiveness of the procedure as well as reassuring results from several non‐randomised studies, and in spite of the negative results of the only controlled trial reported prior to 1980 (Brosset 1956). The popularity of ECV before term waned after the mid‐1970s, partly because of reports of a substantial perinatal mortality associated with the procedure (Bradley‐Watson 1975), and the increasing perception of caesarean section as a safer option than ECV or breech delivery.
How the intervention might work
For the singleton fetus in breech presentation, caesarean section has been shown to be safer for the fetus than vaginal birth (Hofmeyr 2003). Even though many women would prefer a vaginal birth (Gamble 2000; Geary 1997; Hildingsson 2002; Turnbull 1999), most would choose caesarean section if there is a medical indication, resulting in the majority of fetuses in breech presentation now being born by caesarean. The risks associated with caesarean section are low, but caesarean section is not without maternal risk and, in developed countries, remains the largest contributing factor to the incidence of maternal mortality and morbidity following childbirth (Liu 2007; Minkoff 2003). Estimates of the incidence of mortality associated with elective caesarean section were nearly tripled compared to vaginal birth (Cooper 2002; Hall 1999), and severe maternal morbidity has been shown to be increased five fold (Liu 2007). Among breech presenting fetuses, a Cochrane review of planned caesarean section versus planned vaginal delivery for breech pregnancy at term, reported that even though 45% of women in the planned vaginal delivery group were delivered by caesarean section, planned caesarean section was associated with an increase in maternal morbidity (risk ratio 1.29, 95% confidence interval 1.03 to 1.61) (Hofmeyr 2003). In addition to the increase in immediate morbidity following caesarean section, intra‐abdominal adhesions may occur after caesarean section resulting in subsequent infertility (LaSala 1987). The presence of the uterine scar puts future pregnancies at increased risk of complications such as ectopic pregnancy, placenta previa, accreta and abruptio, and uterine rupture (Dashe 2002; Gilliam 2002; Lydon‐Rochelle 2001; Minkoff 2003). A further deterrent to caesarean section is that the procedure requires the expertise of an obstetrician or other physician with surgical training, and limits the role for low‐risk obstetrical care providers such as midwives and family practitioners
A review of strategies to reduce caesarean section rates identified external cephalic version (ECV) as the only clinical intervention with demonstrated Level 1 evidence for reducing primary caesarean section rates overall (Walker 2002). ECV undertaken at term has been shown to be effective in moderately decreasing the rate of non‐cephalic presentation at birth and in avoiding caesarean section (Hofmeyr 2015).
It has been hypothesised that compared to waiting until term, beginning the ECV procedure somewhat earlier in pregnancy before the breech is engaged in the pelvis and while there are maximal levels of amniotic fluid present may further decrease the rate of non‐cephalic presentation at birth and promote cephalic vaginal birth (Hutton 2011b).
Why it is important to do this review
Prior to the mid‐1970s, ECV was usually attempted before term because of the belief that the procedure would seldom be successful at term. Subsequent studies showed that with the use of tocolysis, ECV could be achieved in a substantial proportion of women with breech presentation at term (Cluver 2015). ECV at term differs in many fundamental ways from that performed before term. These include the fact that the fetus is mature and may be delivered more readily in the event of complications, and that spontaneous version without ECV attempt, or reversion after successful ECV, may be less common at term. A Cochrane review of ECV at term (beginning at 37 weeks) reported an increased likelihood that the fetus will be cephalic at delivery, and reduced caesarean sections (Hofmeyr 2015). Thus ECV has been recommended for all women with a breech fetus at term, where there is no contraindication. However, the procedure is often unsuccessful, particularly in North American and European settings, (Hofmeyr 2015; Hutton 1999) and in a study comparing outcomes when ECV was begun earlier (34 to 35 weeks' gestation) compared to at term (after 37 weeks' gestation) reported a clinically important decrease in the proportion of women with non‐cephalic presentation at birth (Hutton 2011b).
Readers are referred to other reviews of the topic (Hofmeyr 1989; Hofmeyr 1991; Hofmeyr 1992; Hofmeyr 1993). See also related Cochrane reviews: 'External cephalic version for breech presentation at term' (Hofmeyr 2015); 'Interventions for helping to turn breech babies to head first presentation when using external cephalic version' (Cluver 2015); and, 'Cephalic version by postural management for breech presentation' (Hofmeyr 2012).
Objectives
To assess the effectiveness of a policy of beginning external cephalic version (ECV) before term for breech presentation on fetal presentation at birth, method of delivery, and the rate of preterm birth, perinatal morbidity, stillbirth and neonatal mortality, using the best available evidence.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, comparing the effects of external cephalic version (ECV) before term or commenced before term with a control group (no ECV attempt or ECV at term). Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐RCTs or studies using a cross‐over design were not eligible for inclusion. We planned to include studies reported in abstract form provided sufficient information was provided to allow us to assess risk of bias.
Types of participants
Women with a live singleton fetus in breech presentation before term.
Types of interventions
External cephalic version attempt before term (37 weeks' gestation) or commenced before term, compared with a no ECV attempt or ECV at term. The comparisons fall into the following three categories.
-
ECV before term compared with no ECV.
-
A policy of initiating ECV before term but continuing if necessary up to term compared with no ECV.
-
A policy of beginning ECV before term compared with a policy of beginning ECV after 37 weeks.
Studies recruiting women both before and at term would be eligible for inclusion in comparison one provided results were reported separately for women in the preterm group.
Types of outcome measures
Outcomes were included if they were determined to be clinically meaningful, data were available for analysis according to original allocation, irrespective of protocol violations and data were available in format suitable for analysis. As part of the assessment of risk of bias we assessed whether reasonable measures were taken to minimise observer bias, and confirmed that missing data were insufficient to materially influence conclusions.
Primary outcomes
-
Rate of non‐cephalic presentation at birth
-
Vaginal cephalic birth not achieved (caesarean section plus vaginal breech delivery)
-
Method of delivery (caesarean section, breech vaginal birth, vaginal cephalic birth)
Secondary outcomes
-
Preterm birth
-
Perinatal outcomes including serious morbidity (trialist defined), stillbirth, neonatal mortality and perinatal mortality
-
Infant Apgar score < seven at five minutes
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 March 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional searching carried out in the previous version of this review (Hutton 2006), see: Appendix 1.]
Searching other resources
We manually searched the reference lists of all retrieved articles and contacted expert in this research field.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeHutton 2006.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search (this section of the review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses.
For this update the quality of the evidence has been assessed using the GRADE approach (Schunemann 2009) for the comparison ECV commenced before term versus ECV at term. This comparison was considered to be the most clinically relevant, as external cephalic version at term has been demonstrated to be effective in reducing the chance of non‐cephalic presentation at birth and caesarean section and should be regarded as the standard of care (Hofmeyr 2015). Comparisons of early ECV with no ECV are now mainly of historical interest. The quality of the evidence was assessed for the following outcomes.
-
Rate of non‐cephalic presentation at birth.
-
Vaginal cephalic birth not achieved (caesarean section plus vaginal breech delivery).
-
Caesarean birth.
-
Breech vaginal birth.
-
Preterm birth.
-
Perinatal mortality (stillbirth plus neonatal death up to seven days).
-
Apgar score less than seven at five minutes.
GRADE profiler (GRADEpro 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials if they were otherwise eligible. In this version of the review no such trials were identified. If cluster trials are eligible for future updates we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials have not been included.
Studies with multiple treatment arms
In this version of the review we have not included any trials with more than two treatment arms; if such trials are included in updates we will use the methods described in the Handbook to analyse findings.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. If we use random‐effects analyses in updates, the random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses. We planned subgroup analysis for:
-
nulliparous versus multiparous women (as nulliparity is a well established to be associated with decreased likelihood of success of ECV);
-
type of breech (frank breech, where the fetus has hips flexed and legs extended making the ECV more difficult versus non‐frank);
-
use of tocolytics versus no tocolytics, (as tocolytics have been shown to increase the likelihood of success in ECV at term, and variation in use may explain heterogeneity between trials);
-
gestational age at randomisation ( 33 weeks 0 days to 34 weeks 6 days; and 35 weeks 0 days to 36 weeks 6 days).
We planned subgroup analysis for primary outcomes only. In this version of the review the study samples however were insufficient to make this analysis meaningful. We will carry out planned subgroup analysis if more data become available in future updates. We will assess subgroup differences by interaction tests available in RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this would make any difference to the overall result. In this version of the review too few studies were included to allow these additional analyses.
Results
Description of studies
Results of the search
In the previous published version of this review, three studies were included (Hutton 2003; Mensink 1980; Van Veelen 1989). In this update, two further studies were identified and were assessed as eligible for inclusion (Akhtar 2013; Hutton 2011). SeeCharacteristics of included studies.
In this version of the review, altogether, five studies were excluded (Brosset 1956; Dafallah 2004; El‐Muzaini 2008; Kasule 1985; Rust 2005) and one is still ongoing (Belizan 1989).Two controlled trials which had been included in an earlier version of this review were subsequently excluded for concerns relating to methodological soundness (Brosset 1956; Kasule 1985). Neither of these trials used random assignment to treatment groups. The Brosset 1956 study states that "cases were divided into two groups" while in the Kasule 1985 trial women were "allocated to a version or non‐version group depending on the day they attended antenatal clinic".
Included studies
Comparison one: ECV before term (from 32 weeks with one repeat attempt) compared with no ECV
Mensink 1980 included women in early third trimester (as early as 32 weeks' gestation) in a randomised controlled trial undertaken in Gronigen, The Netherlands. Allocation was undertaken using randomised sealed envelopes, stratified by parity. Breech was verified by ultrasound. Women with a singleton breech presentation before term (from 32 weeks) were included. Women with any contraindication to external version were excluded. External cephalic version (ECV) was attempted without tocolysis by an assistant in training (n = 50) compared with no ECV attempt (n = 52). If the ECV failed, a further attempt was made by an obstetrician one week later. Outcomes included: non‐cephalic births; caesarean section; one minute Apgar score less than seven; umbilical vein pH less than 7.2; neurological deficit in newborn; and perinatal mortality; neonatal morbidity at the time of delivery was reported, but this was not defined.
Comparison two: ECV commencing before term (33 to 40 weeks with repeated attempts) compared with no ECV
Van Veelen 1989 enrolled 180 healthy white Dutch women with uncomplicated pregnancy at 33 to 40 weeks' gestation and a live singleton breech fetus attending antenatal clinic of Ikazia Hospital, Rotterdam, The Netherlands. Random allocation of women used sealed envelopes, and was stratified by parity. Repeated ECV was performed between 33 and 40 weeks' gestation up to four times with no tocolysis, analgesia or anaesthesia compared with no ECV. The outcomes included: presentation at delivery; mode of delivery; neonatal outcome including perinatal death.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation)
In this update three studies are now included in this comparison (Akhtar 2013; Hutton 2003; Hutton 2011).
Hutton 2003 is an international multicentre randomised controlled trial (n = 233). All nulliparous women with any breech presentation and multiparous women with a frank breech presentation were eligible for the trial if they had a live singleton fetus and a gestational age of between 34 weeks, 0 days and 36 weeks 0 days. Women were excluded if they had a parity greater than four, if they planned to move to a non‐trial centre, or if there was any contraindication to labour or vaginal birth (such as placenta previa, or previous classical caesarean section), to ECV (such as fetal heart rate abnormalities, abruptio placenta, fetal anomalies, uterine anomalies, oligohydramnios, rupture of membranes, over distended uterus) or to early ECV (such as fetus engaged in the pelvis, an increased risk of preterm labour, increased risk of abruptio placenta). ECV was begun between 34 weeks 0 days and 36 weeks 0 days in the early group (n = 117); and between 37 weeks 0 days and 38 weeks 0 days in the delayed group (n = 116). Tocolysis was recommended to be used either routinely or selectively in both groups; analgesia was permitted. The primary outcome was presentation at delivery; other outcomes included: caesarean section; serious fetal complication; preterm birth less than 37 weeks; women's views about ECV. The study was funded by Canadian Institutes of Health Research.
The Hutton 2011 study included 68 centres in 21 countries with ECV carried out by clinicians who were experienced in the procedure and with birth facilities that were deemed to meet Canadian standards. One‐thousand, five‐hundred and forty‐three women were randomised. The study recruited women with a singleton fetus in a breech presentation, between gestation ages of 33 weeks 0 days and 35 weeks six days. Women with contraindications to ECV (e.g. fetal heart rate abnormalities, placental abruption, major life‐threatening fetal anomalies, uterine anomalies, hyper‐extended fetal head, rupture of fetal membranes, severe oligohydramnios or hydramnios); contraindications to early ECV (e.g. increased risk of preterm labour or placental abruption); or contraindications to labour or vaginal birth (e.g. placenta praevia, previous classical caesarean section); or if they had been prior participants in the trial; were at increased risk of unstable lie (such as grand multiparity); or if they planned to give birth by caesarean section even if the fetus turned to a cephalic position, or if they planned a vaginal birth if the fetus remained breech were excluded. In the early ECV group (n = 767), ECV carried out between 34 weeks 0 days and 35 weeks six days gestation, and within seven days of randomisation. In the delayed ECV group (n = 774) ECV carried out at or after 37 weeks' gestation. In both groups fetal presentation was confirmed by ultrasound, fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman.
The study by Akhtar 2013 is a single‐centre, parallel‐group randomised controlled trial carried out in a hospital in Pakistan. The study included women with a singleton fetus with breech presentation between 33 and 35 weeks' gestation (n = 123 women). Women with contraindications to ECV, contraindications to early ECV or contraindications to labour or vaginal birth (e.g. fetal heart rate abnormalities, vaginal bleeding, rupture of membranes, placental abruption, fetal growth restriction, previous CS, low amniotic fluid index, fetal weight greater than 4 kg) or women unwilling to undergo ECV were excluded. In the early ECV group, ECV was carried out between 34 (238 days) and 35 weeks of gestation (n = 63). In the delayed ECV group ECV was carried out at or after 37 weeks. No tocolytics were used in either group and women were monitored for three hours before and one hour after the procedure. Up to two‐three attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities (n = 60).
Excluded studies
Five studies were excluded. Two studies were excluded for methodological reasons; it was not clear in the study by Brosset 1956 that allocation to groups was random and in the Kasule 1985 study allocation was by day of the week. Both of these studies are at high risk of selection bias. The remaining studies (Dafallah 2004; El‐Muzaini 2008; Rust 2005) were excluded because the intervention group mainly included women recruited at term and separate results were not available for women with preterm pregnancies. See Characteristics of excluded studies.
Ongoing studies
We have limited information on the study by Belizan 1989; it is not clear whether this study was completed, more information is set out in Characteristics of ongoing studies.
Risk of bias in included studies
See table Characteristics of included studies.
Allocation
Hutton 2003 and Hutton 2011 used a centralised telephone randomisation service and these studies were assessed as low risk of bias for sequence generation and allocation concealment. The remaining studies did not fully describe the methods used for generating the randomisation sequence. Mensink 1980 and Van Veelen 1989 used randomised, sealed envelopes to conceal allocation (it was not clear whether or not envelopes were opaque and sequentially numbered). Akhtar 2013 reported using the same methods as those used in the Hutton 2003 and Hutton 2011 trials but no further information was provided. All studies were stratified for parity at randomisation.
Blinding
Blinding women and care providers is not feasible for the intervention under study. It was not clear whether there was any attempt to achieve observer blinding in the collection of the outcome data in any of the studies. Although lack of blinding would not be likely to effect outcomes such as presentation at delivery, it is not clear whether lack of blinding had an impact on some of the other outcomes reported such as caesarean section.
Incomplete outcome data
All included studies were assessed to be at low risk of bias for this domain. There were no losses to follow‐up in Akhtar 2013, Mensink 1980 or Van Veelen 1989. Hutton 2003 reported one loss to follow‐up in the early ECV group following randomisation but prior to any ECV procedure being done. Hutton 2011 included more than 99% of women randomised in the analysis. All used an intention‐to‐treat approach to analyses.
Selective reporting
In the two multicentre trials (Hutton 2003; Hutton 2011) study protocols were available and there did not appear to have been any outcome reporting bias. In the remaining studies assessment of bias was from published reports and it was not clear whether all outcome data were reported.
Other potential sources of bias
In the Akhtar 2013 trial it was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject" (Hutton 2003; Hutton 2011). There was no further description of methods used. We contacted the author for more information but have not yet had a response (September 2014).
See Figure 1 and Figure 2 for a summary of findings for risk of bias.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison one: ECV attempt before term (with one repeat attempt) compared with no ECV: one trial involving 102 women (Mensink 1980)
Primary outcomes
The rate of non‐cephalic presentation at birth in the ECV group was 40% and in the no ECV group was 39% (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.64 to 1.69) Analysis 1.1. (The trial authors ascribe the low success rate to the gentleness with which ECV was attempted.) There was no clear difference between groups for failure to achieve vaginal cephalic birth (caesarean section plus breech vaginal birth) (RR 1.04, 95% CI 0.67 to 1.62) Analysis 1.2. (The rate of caesarean section was 14% in the ECV group and 8% in the no ECV group (RR 1.82, 95% CI 0.57 to 5.84) Analysis 1.3. The number of women undergoing vaginal breech delivery was comparable in the two groups (RR 0.87, 95% CI 0.49 to 1.52) Analysis 1.4.
Secondary outcomes
There was no clear evidence of differences between groups for other outcomes.
-
The rate of one minute Apgar scores less than seven (RR 0.62, 95% CI 0.25 to 1.59) Analysis 1.5.
-
The rate of stillbirth or neonatal mortality less than seven days (RR 0.35, 95% CI 0.04 to 3.22) Analysis 1.6.
Rates of premature delivery were similar in the two groups (two premature deliveries in the intervention group and three in the control group). "Perinatal morbidity" at the time of delivery was also reported although this was not defined (with one event in the intervention group and three in the control group).
Comparison two: ECV commencing before term compared with no ECV (repeated attempts): one trial involving 179 women (Van Veelen 1989)
Primary outcomes
The ECV group had 44% non‐cephalic presentation at birth compared to 74% in the no ECV group (RR 0.59, 95% CI 0.45 to 0.77); this difference between groups was statistically significant Analysis 2.1 . Women in the ECV group were at reduced risk of failing to achieve cephalic vaginal birth (RR 0.62, 95% CI 0.49 to 0.80) Analysis 2.2. The rate of caesarean section delivery was 11% in the ECV group compared to 14% in the no ECV group (RR 0.62, 95% CI 0.27 to 1.43) Analysis 2.3. The frequency of vaginal breech delivery was reduced in the ECV group (RR 0.63, 95% CI 0.46 to 0.85) Analysis 2.4.
Secondary outcomes
There was insufficient information on other outcomes.
-
The rate of five minute Apgar scores less than seven (one event in the intervention group) Analysis 2.5.
-
The rate of stillbirth or neonatal mortality less than seven days (one event in the control group) Analysis 2.6.
The authors reported no "major complications" in either group.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation): three trials involving 1906 women (Akhtar 2013; Hutton 2003; Hutton 2011)
Primary outcomes
The rate of non‐cephalic presentation at birth was lower when ECV was started before term (RR 0.81, 95% CI 0.74 to 0.90; participants = 1906; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.1. Women who had early ECV were at slightly less risk of failing to achieve a cephalic vaginal birth (RR 0.90, 95% CI 0.83 to 0.97; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.2. The rate of caesarean section was reduced when ECV was started before 37 weeks' gestation although the difference between groups did not reach statistic significance (RR 0.92, 95% CI 0.85 to 1.00; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.3. Women who were randomised to early ECV were at a considerably reduced risk of having a vaginal breech birth; the difference between groups for this outcome was statistically significant (RR 0.44, 95% CI 0.25 to 0.78; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.4.
Secondary outcomes
The rate of preterm birth less than 37 weeks was increased in the early ECV group (RR 1.51, 95% CI 1.03 to 2.21; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.7.
There was no strong evidence of differences between groups identified for:
-
the rate of five minute Apgar scores less than seven (RR 1.16, 95% CI 0.39 to 3.44; participants = 1759; studies = two; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.5;
-
the rate of stillbirth or neonatal mortality less than seven days (RR 0.23, 95% CI 0.04 to 1.34; participants = 1887; studies = three; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.6;
-
The studies by Hutton 2003 and Hutton 2011 reported several outcomes relating to neonatal outcome but these were not mutually exclusive and so a single composite outcome was reported: one or more serious fetal complication (RR 0.87, 95% CI 0.42 to 1.79; participants = 1761; studies = two; I2 = 0%) Analysis 3.8.
-
There no clear difference between groups for NICU stay for four days or longer (RR 2.50, 95% CI 0.49 to 12.63; participants = 232; studies = 1) Analysis 3.9.
Non‐prespecified outcome
One study reported maternal pain scores following the ECV attempt; pain scores were lower when ECV was commenced before term (mean difference (MD) ‐4.60, 95% CI ‐7.74 to ‐1.46; participants = 1533).
Discussion
Summary of main results
We have good evidence to support external cephalic version (ECV) beginning at term, that is after 37 weeks' gestation. A Cochrane review of ECV concluded that ECV is a useful manoeuvre to decrease both the rate of non‐cephalic presentation and caesarean section when it is begun after 37 weeks' gestation (Hofmeyr 2015), and the major obstetrical societies recommend that ECV be offered to low‐risk women with singleton breech pregnancies. Of the studies of ECV at term, those undertaken in European or American centres report a relatively low rate of success with ECV and a remarkably higher rate of non‐cephalic presentation at birth compared to the African trials. It is possible that there is a difference in the population characteristics. In a cohort study Hofmeyr 1986 reported higher rates of success with the ECV procedure in a group of African women compared to Caucasian women.
The studies of ECV before term are less straightforward.The Mensink 1980 trial which compared ECV prior to term with no ECV, undertook the procedure at an early stage in pregnancy (32 weeks' gestation), when the rates of spontaneous version remain high. Despite the findings from this early study of ECV before term which clearly showed no difference between the ECV and no ECV group, the more recent trials suggest that there may be benefit to beginning ECV prior to, but near term, particularly amongst those populations where success rates at term are low. The Van Veelen 1989 study beginning ECV as early as 33 weeks (but up to 40 weeks, with a mean of gestational age at ECV of 35 weeks) compared with no ECV showed a 30% decrease in the rate on non‐cephalic presentation. This trial showed that women in the ECV group were at reduced risk of failing to achieve cephalic vaginal birth, even though no difference was found in the rate of caesarean section. This is likely due to the higher proportion of women planning a vaginal breech birth when the fetus remained breech at term, as the study was undertaken prior to publication of findings from the Term Breech Trial (Hannah 2000), and a policy of vaginal breech delivery is evident. The study was too small to meaningfully rule out differences in Apgar scores less than seven at five minutes or in stillbirth or neonatal mortality less than seven days. In the Van Veelen 1989 study, the mean time of beginning ECV was 35 weeks' gestation, and it is unclear if the benefit that was found could be attributed to beginning the procedure earlier in pregnancy, or because some of the procedures were not initiated until after term.
Three trials compared beginning ECV early at between 34 and 36 weeks' gestation with ECV beginning at between 37 and 38 weeks' gestation (Akhtar 2013; Hutton 2003; Hutton 2011). Compared with women undergoing ECV at term, women who were randomised to ECV before term had a 19% decrease in the rate of non‐cephalic presentation at birth, a 10% reduction in the risk of failing to achieve a cephalic vaginal birth, a 8% decrease in the caesarean section rate, and a considerably reduced risk of undergoing a vaginal breech birth. The quality of the evidence for all of these outcomes was graded high quality. These findings are clinically important, and except for the finding relating to caesarean section these differences (favouring early ECV) were statistically significant. However, women randomised to early ECV appeared to be at increased risk of late preterm birth (risk increased by 51%), and although, overall, the number of women delivering their babies before term was relatively small (6.6% in the ECV group and 4.3% for controls) the possible increase in late preterm birth needs to be set against the positive outcomes associated with ECV.
Quality of the evidence
The trials included in the review were of mixed methodological quality. Three of the studies did not provide good descriptions of the methods used (Akhtar 2013; Mensink 1980; Van Veelen 1989). Blinding was not possible in these studies and it is difficult to know what impact lack of blinding had on outcomes. While the outcomes measured were objective and may not have been subject to detection bias it is possible that lack of blinding may have affected women's and care providers' behaviour and this may have had an effect on clinical decision‐making which could have influenced outcomes such as the decision whether or not to carry out a caesarean section. In two studies trial protocols were available; without this information it is difficult to assess possible outcome reporting bias.
Potential biases in the review process
The review process is subject to bias. We attempted to minimise bias by having two review authors independently involved in assessing risk of bias and carrying out data extraction. One of the review authors (E Hutton) was involved in two of the included trials (Hutton 2003; Hutton 2011); this author was not involved in data extraction or in assessing bias for these trials.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute.

Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality.
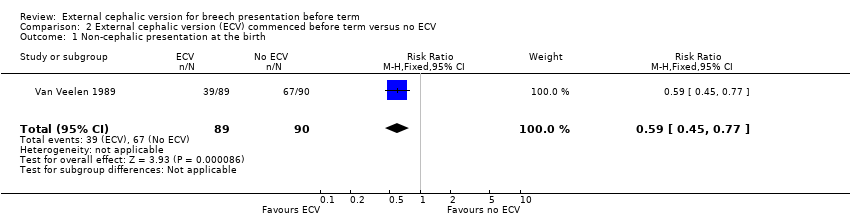
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
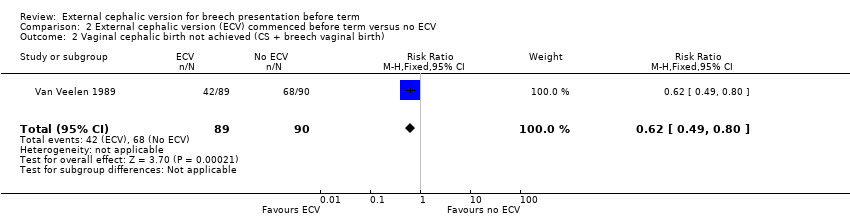
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth.
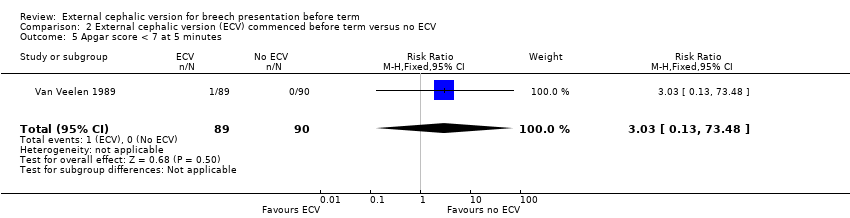
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days.
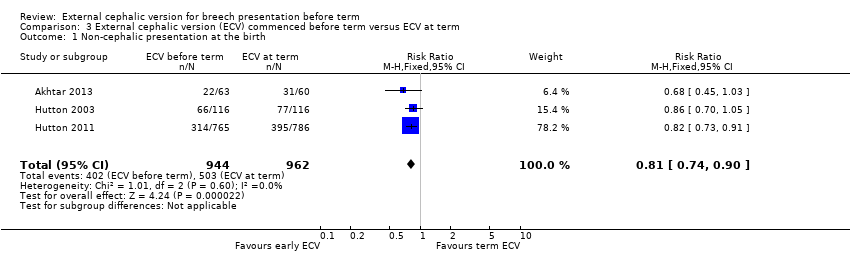
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth).

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth.
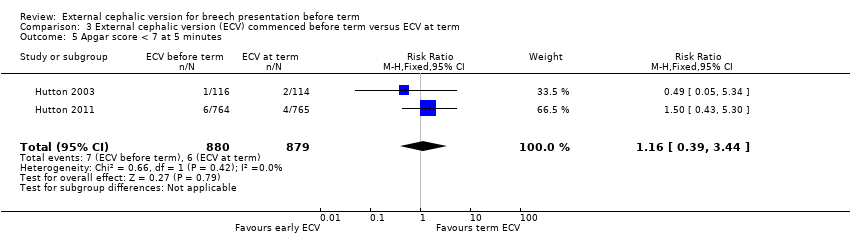
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation.

Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer.
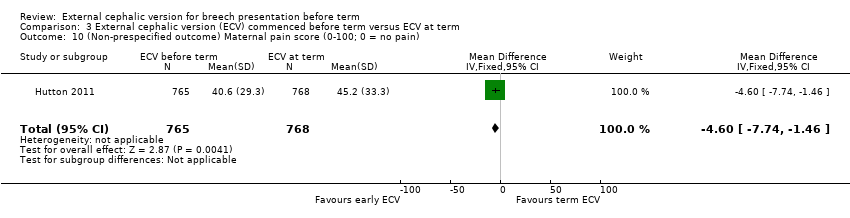
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain).
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 | 1906 | ⊕⊕⊕⊕ | ||
| 523 per 1000 | 424 per 1000 | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 | 1888 | ⊕⊕⊕⊕ | ||
| 600 per 1000 | 540 per 1000 | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 | |||||
| Caesarean section | Study population | RR 0.92 | 1888 | ⊕⊕⊕⊕ | ||
| 565 per 1000 | 519 per 1000 | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 | |||||
| Vaginal breech birth | Study population | RR 0.44 | 1888 | ⊕⊕⊕⊕ | ||
| 35 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 | 1759 | ⊕⊕⊝⊝ | ||
| 7 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 | 1887 | ⊕⊕⊝⊝ | ||
| 5 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 | 1888 | ⊕⊕⊕⊕ | ||
| 43 per 1000 | 66 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide 95% CI crossing the line of no effect and low event rate. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 3 Caesarean section Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| 4 Vaginal breech birth Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| 5 Apgar score < 7 at 1 minute Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| 6 Perinatal mortality Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 3 Caesarean section Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| 4 Vaginal breech birth Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| 6 Stillbirth and neonatal mortality < 7 days Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cephalic presentation at the birth Show forest plot | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3 Caesarean section Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 4 Vaginal breech birth Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| 5 Apgar score < 7 at 5 minutes Show forest plot | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| 6 Stillbirth or neonatal mortality < 7 days Show forest plot | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| 7 Preterm birth < 37 weeks Show forest plot | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| 8 One or more serious fetal complications following randomisation Show forest plot | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 9 NICU stay 4 days or longer Show forest plot | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) Show forest plot | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |

