Corticosteroides para el accidente cerebrovascular isquémico agudo
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Double‐blind, placebo controlled | |
| Participants | USA 28 dexamethasone | |
| Interventions | Dexamethasone versus placebo Intervention: dexamethasone 12 mg i.v. stat, 4 mg i.m. 6 hourly for 3 days, 4 mg i.m. 8 hourly for 3 days, 4 mg i.m. 12 hourly for 2 days, 4 mg i.m. 24 hourly for 2 days: total dose: 120 mg dexamethasone Placebo: unspecified All patients: prophylactic ulcer diet and antacid (30 cc Maalox) 4 times a day where oral intake possible Duration: 10 days | |
| Outcomes | Death at 14 days | |
| Notes | Exclusions: more than 300 red blood cells per cc in the cerebrospinal fluid; history of gastro‐intestinal bleeding or symptomatic duodenal ulcer Follow‐up: 14 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, but assumed computer‐generated by Merck, Sharp & Dohme |
| Allocation concealment (selection bias) | Unclear risk | "randomisation was performed on a consecutive admission basis". Blind until last patient assessed |
| Blinding (performance bias and detection bias) | Low risk | "coded vials which contained either Decadron [dexamethasone] or a similarly appearing placebo" |
| Incomplete outcome data (attrition bias) | Low risk | 5 patients withdrawn (3 placebo, 2 dexamethasone). These were not included in analyses |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Double‐blind, placebo controlled | |
| Participants | India 13 Betamethasone | |
| Interventions | Betamethasone versus placebo Intervention: betamethasone 10 mg stat, 10 mg/day in divided doses for 2 days, 8 mg/day in divided doses for 3 days, 6 mg/day in divided doses for 3 days, 4 mg/day for 3 days, 2 mg/day for 10 days: total dose: 94 mg betamethasone Placebo: unspecified Duration: 21 days | |
| Outcomes | Death at 21 days | |
| Notes | Exclusions: not stated Follow‐up: 21 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "ampoules of the drug and placebo were similar in appearance" |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors do not indicate if there were any patients excluded or lost to follow‐up prior to analysis |
| Methods | Double‐blind, placebo controlled | |
| Participants | New Zealand 24 betamethasone | |
| Interventions | Betamethasone versus placebo Interventions: betamethasone 12 mg i.m. stat, 4 mg i.m. 8 hourly for 1 day, 4 mg i.m. 8 hourly for 9 days, 4 mg i.m. 2 hourly for 2 days, 2 mg i.m. 2 hourly for 2 days: total dose: 345 mg betamethasone Placebo: vitamin C Duration: 14 days | |
| Outcomes | Death at 12 weeks | |
| Notes | Exclusions: subarachnoid haemorrhage; severe diabetes; pre‐existing steroid treatment; known peptic ulceration Follow‐up: 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated, but probably prepared by outsider to study |
| Allocation concealment (selection bias) | Unclear risk | "each centre received packages of betamethasone or identical placebo in randomised order within blocks of six. As patients were entered into the trial they were given the medication designated by the next number in the treatment block" |
| Blinding (performance bias and detection bias) | Low risk | "packages of betamethasone or identical placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors do not indicate if there were any patients excluded or lost to follow‐up prior to analysis |
| Methods | Double‐blind, placebo controlled | |
| Participants | UK 61 dexamethasone | |
| Interventions | Dexamethasone versus placebo Interventions: dexamethasone 4.2 mg i.m. 6 hourly for 10 days, 4.2 mg i.m. 8 hourly for 1 day, 4.2 mg i.m. 12 hourly for 2 days, 4.2 mg i.m. once for 1 day: total dose: 201.6 mg dexamethasone Placebo: water Duration: 14 days | |
| Outcomes | Death at day 10 and at 3 and 12 months | |
| Notes | Exclusions: previous stroke; considered to have intracranial tumour, injury or subarachnoid haemorrhage; diabetes mellitus; peptic ulcer; already receiving steroids. Follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | "each of the four wards contained a series of sealed envelopes containing randomised treatment instructions ... After entering into the trial the next envelope was opened" |
| Blinding (performance bias and detection bias) | Low risk | "treatment ampoules identified only by a code number", "the treatment code was available to [one of the trial organisers], who did not participate in assessment" |
| Incomplete outcome data (attrition bias) | Low risk | 16 patients excluded after randomisation and not included in analysis. 4 patients lost to follow‐up (2 placebo, 2 treatment) not included in analysis |
| Methods | Double‐blind, placebo controlled | |
| Participants | Canada 26 dexamethasone | |
| Interventions | Dexamethasone versus placebo. Intervention: dexamethasone 8 mg stat bolus, 4 mg 6 hourly gradually decreased over 12 days: total dose: 140 mg dexamethasone Placebo: unspecified Duration: 12 days | |
| Outcomes | Death at day 29 | |
| Notes | Exclusions: recent peptic ulcer; concurrent infection, psychiatric disturbances Follow‐up: 29 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with author suggests random number tables |
| Allocation concealment (selection bias) | Unclear risk | "patients ... were randomly allocated on admission to a placebo or steroid group in a double‐blind manner" |
| Blinding (performance bias and detection bias) | Low risk | "each patient received either dexamethasone or matching placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 patient lost to follow‐up but included in analysis, not stated if any patients were excluded after randomisation: "53 patients completed the study" |
| Methods | Double‐blind, placebo controlled | |
| Participants | Canada 54 dexamethasone | |
| Interventions | Dexamethasone versus placebo Intervention: dexamethasone 24 mg p.o./i.v. 6 hourly with progressive reduction of dose until day 12: total dose: 480 mg dexamethasone Placebo: unspecified Duration: 12 days | |
| Outcomes | Death at day 21 | |
| Notes | Exclusions: cerebral haemorrhage by CT, mild stroke, massive previous stroke, terminal stroke, dementia, diabetes mellitus, concurrent sepsis, gastrointestinal haemorrhage, or cardiac embolic source Follow‐up: 21 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | "consecutive patients admitted to the stroke unit were entered into the study", "patients were assigned to receive either dexamethasone or placebo according to random number" |
| Blinding (performance bias and detection bias) | Low risk | "material was supplied in identical‐appearing vials bearing the patients number in the study" |
| Incomplete outcome data (attrition bias) | Unclear risk | 13 patients excluded after randomisation and not included in analysis. ? patients lost to follow‐up (likely 0) |
| Methods | Double‐blind, placebo controlled | |
| Participants | Nigeria 5 dexamethasone | |
| Interventions | Dexamethasone versus placebo Interventions: dexamethasone 100 mg stat, 16 mg 6 hourly for 2 days: total dose: 228 mg dexamethasone Placebo: water Duration: 2 days | |
| Outcomes | Death at 1 and 6 months | |
| Notes | Exclusions: presentation after 24 hours; primary subarachnoid haemorrhage; symptoms suggestive of head injury, subdural, tumour; brainstem stroke; diabetes mellitus; peptic ulcer; sepsis; previous stroke in same hemisphere; impairment of consciousness Follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "by simple ballot" |
| Allocation concealment (selection bias) | Unclear risk | "patients were sequentially paired and randomised", "the code was broken after the first 20 patients ... by [one author] who was not involved in assessment of patients" |
| Blinding (performance bias and detection bias) | Low risk | "ampoules were prepared by MCA Chemical, Italy and contained either dexamethasone or distilled water ... these were indistinguishable and kept in identically numbered boxes; one box for each patient" |
| Incomplete outcome data (attrition bias) | Unclear risk | Authors do not state if there were any patients excluded subsequent to randomisation. None were lost to follow‐up |
| Methods | Double‐blind, placebo controlled | |
| Participants | USA 17 dexamethasone | |
| Interventions | Dexamethasone versus placebo Intervention: dexamethasone 10 mg i.v. stat, 4 mg i.m. 6 hourly for 10 days, dose gradually decreased to 0 over 7 days: total dose: unknown Control: placebo All patients: oral antacid Duration: 17 days | |
| Outcomes | Death at day 17 | |
| Notes | Exclusions: subarachnoid haemorrhage Follow‐up: 17 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Low risk | randomisation not broken until end of trial |
| Blinding (performance bias and detection bias) | Low risk | "the material was supplied in identical appearing vials bearing the patients number in the study" |
| Incomplete outcome data (attrition bias) | Unclear risk | Number lost to follow‐up not stated. The authors provide outcome data on those randomised and subsequently excluded, so that an ITT analysis can be performed |
| Selective reporting (reporting bias) | Unclear risk | Patient excluded from the analysis due to alternative mechanism of death despite meeting inclusion criteria and receiving treatment |
CT: computerised tomography
i.m.: intramuscular
ITT: intention‐to‐treat
i.v.: intravenous
p.o.: per os (by mouth)
stat: immediately
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Glycerol treatment compared with dexamethasone and not placebo | |
| Not randomised ‐ alternate allocation | |
| Alternate allocation | |
| No data on deaths in each treatment group given. Contained haemorrhagic stroke. Dexamethasone plus mannitol versus placebo | |
| Glycerol compared with dexamethasone and not placebo | |
| Glycerol treatment compared with dexamethasone and not with placebo | |
| Subarachnoid haemorrhage only | |
| No information on randomisation procedure or blinding | |
| Dexamethasone plus dextran versus placebo | |
| Uneven allocation between treatment (25 participants) and control (15 participants) and confounded by the administration of antacids to the treatment group only | |
| Dexamethasone plus dextran plus aspirin versus placebo | |
| Haemorrhagic stroke only | |
| No data available | |
| Not randomised ‐ unequal allocation between treatment (21 participants) and placebo (6 participants) | |
| Open, uncontrolled study | |
| No data provided on number of deaths | |
| Haemorraghic stroke only | |
| No randomised allocation |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All deaths Show forest plot | 8 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.57, 1.34] |
| Analysis 1.1  Comparison 1 Corticosteroids versus placebo, Outcome 1 All deaths. | ||||
| 2 Deaths within one month Show forest plot | 8 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.63, 1.47] |
| Analysis 1.2  Comparison 1 Corticosteroids versus placebo, Outcome 2 Deaths within one month. | ||||
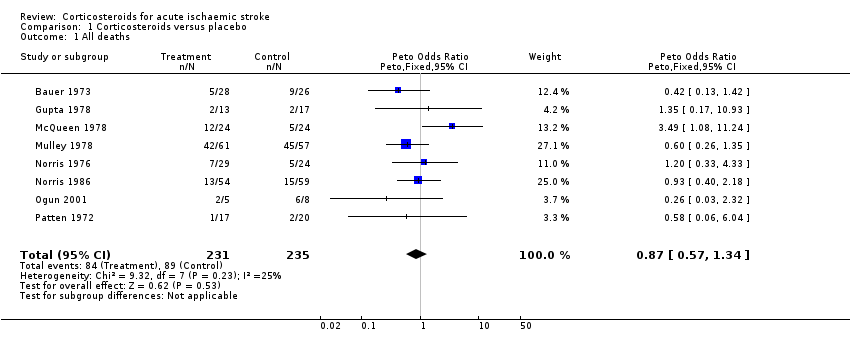
Comparison 1 Corticosteroids versus placebo, Outcome 1 All deaths.

Comparison 1 Corticosteroids versus placebo, Outcome 2 Deaths within one month.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All deaths Show forest plot | 8 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.57, 1.34] |
| 2 Deaths within one month Show forest plot | 8 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.63, 1.47] |

