Вмешательства для намеренного изменения артериального давления при остром инсульте
Abstract
Background
It is unclear whether blood pressure should be altered actively during the acute phase of stroke. This is an update of a Cochrane review first published in 1997, and previously updated in 2001 and 2008.
Objectives
To assess the clinical effectiveness of altering blood pressure in people with acute stroke, and the effect of different vasoactive drugs on blood pressure in acute stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched in February 2014), the Cochrane Database of Systematic reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 2), MEDLINE (Ovid) (1966 to May 2014), EMBASE (Ovid) (1974 to May 2014), Science Citation Index (ISI, Web of Science, 1981 to May 2014) and the Stroke Trials Registry (searched May 2014).
Selection criteria
Randomised controlled trials of interventions that aimed to alter blood pressure compared with control in participants within one week of acute ischaemic or haemorrhagic stroke.
Data collection and analysis
Two review authors independently applied the inclusion criteria, assessed trial quality and extracted data. The review authors cross‐checked data and resolved discrepancies by discussion to reach consensus. We obtained published and unpublished data where available.
Main results
We included 26 trials involving 17,011 participants (8497 participants were assigned active therapy and 8514 participants received placebo/control). Not all trials contributed to each outcome. Most data came from trials that had a wide time window for recruitment; four trials gave treatment within six hours and one trial within eight hours. The trials tested alpha‐2 adrenergic agonists (A2AA), angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor antagonists (ARA), calcium channel blockers (CCBs), nitric oxide (NO) donors, thiazide‐like diuretics, and target‐driven blood pressure lowering. One trial tested phenylephrine.
At 24 hours after randomisation oral ACEIs reduced systolic blood pressure (SBP, mean difference (MD) ‐8 mmHg, 95% confidence interval (CI) ‐17 to 1) and diastolic blood pressure (DBP, MD ‐3 mmHg, 95% CI ‐9 to 2), sublingual ACEIs reduced SBP (MD ‐12.00 mm Hg, 95% CI ‐26 to 2) and DBP (MD ‐2, 95%CI ‐10 to 6), oral ARA reduced SBP (MD ‐1 mm Hg, 95% CI ‐3 to 2) and DBP (MD ‐1 mm Hg, 95% CI ‐3 to 1), oral beta blockers reduced SBP (MD ‐14 mm Hg; 95% CI ‐27 to ‐1) and DBP (MD ‐1 mm Hg, 95% CI ‐9 to 7), intravenous (iv) beta blockers reduced SBP (MD ‐5 mm Hg, 95% CI ‐18 to 8) and DBP (‐5 mm Hg, 95% CI ‐13 to 3), oral CCBs reduced SBP (MD ‐13 mmHg, 95% CI ‐43 to 17) and DBP (MD ‐6 mmHg, 95% CI ‐14 to 2), iv CCBs reduced SBP (MD ‐32 mmHg, 95% CI ‐65 to 1) and DBP (MD ‐13, 95% CI ‐31 to 6), NO donors reduced SBP (MD ‐12 mmHg, 95% CI ‐19 to ‐5) and DBP (MD ‐3, 95% CI ‐4 to ‐2) while phenylephrine, non‐significantly increased SBP (MD 21 mmHg, 95% CI ‐13 to 55) and DBP (MD 1 mmHg, 95% CI ‐15 to 16).
Blood pressure lowering did not reduce death or dependency either by drug class (OR 0.98, 95% CI 0.92 to 1.05), stroke type (OR 0.98, 95% CI 0.92 to 1.05) or time to treatment (OR 0.98, 95% CI 0.92 to 1.05). Treatment within six hours of stroke appeared effective in reducing death or dependency (OR 0.86, 95% CI 0.76 to 0.99) but not death (OR 0.70, 95% CI 0.38 to 1.26) at the end of the trial. Although death or dependency did not differ between people who continued pre‐stroke antihypertensive treatment versus those who stopped it temporarily (worse outcome with continuing treatment, OR 1.06, 95% CI 0.91 to 1.24), disability scores at the end of the trial were worse in participants randomised to continue treatment (Barthel Index, MD ‐3.2, 95% CI ‐5.8, ‐0.6).
Authors' conclusions
There is insufficient evidence that lowering blood pressure during the acute phase of stroke improves functional outcome. It is reasonable to withhold blood pressure‐lowering drugs until patients are medically and neurologically stable, and have suitable oral or enteral access, after which drugs can than be reintroduced. In people with acute stroke, CCBs, ACEI, ARA, beta blockers and NO donors each lower blood pressure while phenylephrine probably increases blood pressure. Further trials are needed to identify which people are most likely to benefit from early treatment, in particular whether treatment started very early is beneficial.
PICO
Резюме на простом языке
Лекарственные вмешательства для намеренного изменения артериального давления при остром инсульте
Актуальность: Для людей, которые только что перенесли инсульт (внезапный приступ либо из‐за закупорки, либо из‐за разрыва артерии в головном мозге), очень высокое и очень низкое артериальное давление может быть вредным. Таким образом, могут быть полезными лекарства, которые повышают низкое артериальное давление или понижают высокое артериальное давление. До 50% людей, поступающих в стационар с острым инсультом, принимают лекарства (таблетки), влияющие на артериальное давление, на момент госпитализации, и остается неясным, нужно ли продолжать принимать эти лекарства или следует прекратить их применение в острой ситуации. В этом обзоре рассмотрели клинические испытания, в которых намеренно влияли на артериальное давление или сравнивали продолжение применения лекарств для снижения артериального давления (которое было начато до инсульта) с прекращением их применения.
Характеристика исследований Этот обзор актуален на май 2014 года. Мы включили в обзор 26 клинических испытаний с участием 17011 людей: в 24 испытаниях оценивали снижение артериального давления, в одном испытании оценивали повышение артериального давления, и в двух испытаниях оценивали, что делать с лекарствами, которые пациенты начали принимать до инсульта. Все исследования были проведены в больницах, которые специализировались на лечении людей с инсультом. Не во всех клинических испытаниях была представлена информация по всем исходам и мы использовали данные, доступные в публикациях.
Основные результаты: Существует недостаточно доказательств, чтобы говорить о том, что снижение артериального давления спасает жизнь или уменьшает степень нарушения трудоспособности у людей с острым инсультом. Немедленное возобновление приема лекарств для снижения артериального давления, которые пациенты начали принимать до инсульта, может повысить степень нарушения трудоспособности.
Выводы: Необходимы дополнительные исследования для выявления людей, у которых наиболее вероятно будет польза от изменения артериального давления при остром инсульте, для выявления временного промежутка, при котором лечение с большей вероятностью окажется полезным, видов инсульта, при которых наиболее вероятно будет положительный ответ, и условий, в которых такое лечение можно наилучшим образом применять в обычной практике.
Authors' conclusions
Background
Description of the condition
Stroke is the third most common cause of death and the most common cause of disability in the western world. Acute stroke, whether due to infarction or haemorrhage, is associated with high blood pressure in 75% of patients, of whom 50% have a previous history of high blood pressure (Britton 1986; Oppenheimer 1992). After a stroke, blood pressure falls in most patients over a week although a third of patients remain hypertensive (Wallace 1981; Britton 1986; Harper 1994). A number of small studies have assessed the relationship between blood pressure (Marshall 1959; Adams 1965; Droller 1965; Bourestom 1967; Marquarsden 1969; Carlberg 1993) and outcome. A meta‐analysis of these studies found that elevated blood pressure was associated with a poor outcome (Willmot 2004). Data from 17,398 participants in the International Stroke Trial identified a U‐shaped relationship such that both low and high blood pressure were associated independently with increased early death and later death or dependency (Leonardi‐Bee 2002), a finding that has been replicated by others (Castillo 2004; Vemmos 2004). High blood pressure is also associated with an increased early recurrence of stroke (Leonardi‐Bee 2002; Sprigg 2006).
The mechanisms underlying high blood pressure in stroke are complex but pre‐existing hypertension, hospitalisation stress, activation of the sympathetic renin‐angiotensin‐aldosterone, cortisol and natriuretic peptide neuroendocrine systems, and the Cushing reflex (raised blood pressure secondary to raised intracranial pressure) all contribute (Myers 1982). In ischaemic stroke, high blood pressure also appears to adversely affect outcome through increasing the risk of cerebral oedema, but not haemorrhagic transformation (Leonardi‐Bee 2002). Haematoma expansion is related to high blood pressure in people with intracerebral haemorrhage (ICH) although this relationship may be confounded by stroke severity and time to presentation (Fujii 1994; Kazui 1997; Fujii 1998; Bath 2003).
Description of the intervention
Although debated more than 29 years ago, it still remains unclear whether high blood pressure should (Spence 1985) or should not (Yatsu 1985) be treated acutely following stroke. Recent guidelines recommend that acute lowering of blood pressure should be delayed for several days or even weeks unless blood pressure is greater than 220/120 mmHg, blood pressure is greater than 200/100 mmHg with end organ involvement (hypertensive encephalopathy, aortic dissection, cardiac ischaemia, pulmonary oedema, acute renal failure), or blood pressure is greater than 200/120 mmHg with primary ICH, are present (O'Connell 1994; EUSI 2004; AHA‐HS 2010; RCP 2012; AHA‐IS 2013). Though the evidence is weaker, guidelines now recommend that patients who have elevated blood pressure and are otherwise eligible for treatment with recombinant tissue plasminogen activator may have their blood pressure lowered so that systolic blood pressure (SBP) is less than or equal to 185 mmHg and diastolic blood pressure (DBP) is less than or equal to 110 mmHg before thrombolysis using intravenous labetalol, nitroprusside or nicardipine and it should be maintained below 180/105 mmHg for at least the first 24 hours after therapy (AHA‐IS 2013). Unfortunately, such guidelines are inconsistent and are based on theoretical arguments and individual case reports, and not on the results of systematic overviews or large intervention trials of blood pressure manipulation in acute stroke. Nevertheless, a number of case reports and series have suggested that active lowering of blood pressure in people with primary intracranial haemorrhage and ischaemic stroke may improve (Dandapani 1995; Chamorro 1998) or worsen (Graham 1975; Britton 1980; Fischberg 2000) outcome.
Low blood pressure is not common in acute stroke but it, like high blood pressure, is associated with a poor outcome (Leonardi‐Bee 2002). Possible reasons for low blood pressure include potentially reversible conditions such as hypovolaemia, sepsis, impaired cardiac output secondary to cardiac failure, arrhythmias or cardiac ischaemia, and aortic dissection (Sprigg 2005). Guidelines recommend that causes of hypotension in the setting of acute stroke should be sought with the view to correcting reversible causes such as hypovolaemia and cardiac arrhythmias (AHA‐IS 2013). Since cerebral autoregulation is lost following stroke (Strandgaard 1973; Burke 1986; Paulson 1990), such that cerebral blood flow becomes dependent on systemic blood pressure, some researchers have hypothesised that blood pressure should be increased to improve cerebral perfusion (Sandercock 1992) and a case series (Rordorf 1997) and a pilot randomised trial of phenylephrine (Hillis 2003) reporting this approach have been published.
How the intervention might work
Although the different drugs assessed work in a variety of ways, all lower (or elevate ‐ phenylephrine) blood pressure.
Why it is important to do this review
We are reviewing this topic in three parts (Bath 1997).
Part 1: this review
Assessment of trials in which the primary aim of the intervention was to alter blood pressure in people with acute stroke with the aim of improving clinical outcome.
A Cochrane review of blood pressure intervention in stroke published in 2001 (BASC 2001) updated the original review published in 1997. It was again updated in 2008 to include more information from 13 trials published between 2003 and 2008 including a total of 1153 participants (BASC 2009). With a relatively small amount of data, there was insufficient evidence to evaluate the effect of altering blood pressure during the acute phase of stroke.
The present review includes all new trials completed and published since 2008. The total number of participants is now 17,011, a 14‐fold increase since the review in 1997 and 2008. Although many of the data are from trials testing blood pressure alteration in the acute phase (≤ 48 hours), some recent trials have examined specific questions such as lowering blood pressure in ICH (INTERACT pilot 2008; INTERACT‐2 2013), with angiotensin receptor antagonists (SCAST 2011), or glyceryl nitrate (ENOS 2014) or in the pre‐hospital setting (PIL‐FAST 2013; RIGHT 2013). Furthermore, two trials (COSSACS 2010; ENOS 2014) have investigated whether to continue or stop temporarily pre‐stroke antihypertensive therapy. This systematic review includes these data and provides up‐to‐date evidence.
Part 2: vasoactive drugs for acute stroke
Assessment of trials where vasoactive drugs were administered to people with acute stroke and where clinical outcome was measured. Drugs include: alpha receptor antagonists, angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor inhibitors (ARA), beta receptor antagonists, calcium channel blockers (CCB), diuretics, magnesium, naftidrofuryl, nitric oxide donors (nitrates), papaverine, pentoxifylline, prostacyclin, serotonin receptor antagonists, sympathomimetics, theophylline (and mimetics), thromboxane A2 antagonists, vinpocetine, and their derivatives. Aggregated patient data are analysed separately for drugs which lower and elevate blood pressure (BASC 2000). Work on this analysis is ongoing.
Part 3: analysis of individual patient data from the trials identified in parts 1 and 2
Work on this analysis is ongoing through the international Blood pressure in Acute Stroke Collaboration. In brief, individual patient data from the trials included in Part 1 and Part 2 are being collated with the intention of extending analyses, particularly in subgroups of participants and interventions.
Objectives
To assess the clinical effectiveness of altering blood pressure in people with acute stroke, and the effect of different vasoactive drugs on blood pressure in acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) of vasoactive drugs in acute ischaemic stroke or acute intracerebral haemorrhage (ICH) where the aim of the trial was to alter blood pressure, and drug therapy was initiated within one week of stroke onset. We excluded uncontrolled studies, confounded controlled studies where two or more active interventions were compared, and studies of people with subarachnoid haemorrhage.
Types of participants
Adults (age 18 or older) of either sex with acute ischaemic stroke or ICH who were eligible for randomisation to either active treatment, or placebo or open control.
Types of interventions
We sought RCTs evaluating single or multiple agents of deliberate blood pressure lowering or elevation in acute stroke, regardless of dosage or route of treatment, compared against placebo or open control. We also included trials with two groups receiving different doses of the same BP lowering agent, and studies assessing effects of continuing or stopping pre‐existing antihypertensive treatment.
Types of outcome measures
Primary outcomes
-
Combined death or disability/dependency at end of trial (≥ one month after stroke). We defined death or dependency as the modified Rankin Scale (mRS) > 2 (or > 3 as available).
Secondary outcomes
-
Blood pressure when first measured after randomisation.
-
Early case fatality (< one month).
-
Late case fatality (≥ one month).
-
Early neurological deterioration (< one month). As there is no consensus on how early neurological deterioration should be standardised, we used the trial‐specific definition as a decrease in the Scandinavian Stroke Scale (SSS) of > 5 points or a decrease in consciousness part of the SSS by > 2 points (ENOS 2014), increase in the National Institutes of Health Stroke Scale (NIHSS) of 2 (Koch 2008) or more (CHHIPS 2009; COSSACS 2010; INTERACT‐2 2013) or decline of 2 or more points in Glasgow Coma Scale (GCS) (INTERACT‐2 2013).
-
Late disability or dependency (Barthel Index ≥ one month).
-
Baseline and on‐treatment blood pressure and heart rate.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. Our methods comprised electronic searches and assessment of studies referenced in published systematic and non‐systematic reviews. We applied no language restrictions.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, (last searched by the Managing Editor in February 2014), the Cochrane Database of Systematic reviews (CDSR) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 2), MEDLINE (Ovid) (1966 to May 2014) (Appendix 1), EMBASE (Ovid) (1974 to May 2014) (Appendix 2), Science Citation Index (ISI, Web of Science, 1981 to May 2014) (Appendix 3) and the Stroke Trials Registry (www.strokecenter.org/trials/) (searched May 2014).
Searching other resources
We searched reviews of acute stroke relating to drugs that may alter blood pressure, including: calcium channel blockers (CCBs) (Horn 2001), nitric oxide (Bath 2002), pentoxifylline (Bath 2004a) and prostacyclin (Bath 2004b). In addition, we searched reference lists of included trials and relevant papers. We contacted principal investigators and researchers when we required additional information. For a previous version of this review (BASC 2001), we contacted the following pharmaceutical companies: Bayer (nimodipine), Napp (pentoxifylline), Novartis (isradipine), Lipha Sante (naftidrofuryl), Hoffmann la Roche (N‐methyl‐D‐aspartate), Hoechst (flunarizine) and UCB Pharma (piracetam).
Data collection and analysis
We extracted data using a standard proforma; KK entered data into Review Manager (RevMan 2012) and PB checked the data.
Selection of studies
For this update, one review author (KK) screened the records obtained from the electronic searches and excluded obviously irrelevant studies. We obtained the full paper copy of the remaining studies and both review authors (KK and PB) selected trials for inclusion criteria detailed previously. We resolved any disagreements by discussion.
Data extraction and management
We extracted data from published and unpublished material where available. We recorded information on the method of randomisation, concealment of allocation, blinding of treatment administration, analysis (intention‐to‐treat, efficacy analysis), stroke type (ischaemia, haemorrhage, or mixed), drug dose, route of administration (oral, sublingual, intravenous, transdermal) and timing, blood pressure and heart rate before and during treatment, numbers of deaths, functional disability, quality of life, and length of stay.
Assessment of risk of bias in included studies
We assessed the methodological quality of the trials using the following criteria.
-
Method of randomisation.
-
Balance of prognostic factors.
-
Allocation concealment.
-
Blinding of treatment administration.
-
Intention‐to‐treat analysis.
-
Blinding of outcome assessment.
-
Follow‐up.
We used the quality criteria to derive an overall assessment bias score as 'low risk' (all criteria met), moderate risk (one or more criteria unclear) and high risk (one or more criteria absent) (Higgins 2011).
Measures of treatment effect
We calculated the weighted estimate of the typical treatment effect across trials using RevMan 5 (RevMan 2012), odds ratios (OR) using the Mantel‐Haenszel random‐effects model for binary data, and mean difference (MD) using the inverse variance method for continuous data, each with 95% confidence intervals (CI).
Unit of analysis issues
The primary outcome was based on the modified Rankin Scale (mRS 0 to 6, where death = 6) assessed using the binary outcome of combined death or dependency (mRS > 1 or > 2 depending on trial definition). The Barthel Index (BI) (disability measure of activities of daily living) was also assessed (BI 100 to ‐5, where death = ‐5). Where functional outcome was not assessed, we excluded the trial from analysis of functional outcome.
Dealing with missing data
We attempted to collect missing data from trial investigators. In instances where on‐treatment blood pressure data were not provided or could not be obtained from study authors, we obtained data (mean, SD) from graphs in the trial publication; where the SD was not presented graphically, we used baseline data, a conservative strategy. We excluded trials from individual analyses when summary data were omitted in the trial publication.
Assessment of heterogeneity
We assessed heterogeneity between RCTs' results using the I2 statistic based on the DerSimonian‐Laird formula.
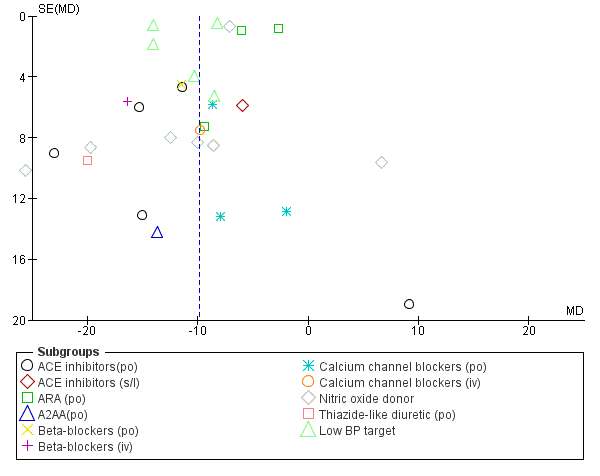
Assessment of reporting biases
We examined reporting bias using funnel plots (Figure 1; Figure 2).

Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.1 Death or dependency, end of trial by intervention.

Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.23 SBP, first after randomisation, by intervention.
Data synthesis
We performed statistical analysis using RevMan (RevMan 2012). We reported outcomes as ORs with 95% CIs for dichotomous data, and MD with 95% CI for continuous data. We used a random‐effects model to analyse individual results regardless of whether there was heterogeneity or not; this is a conservative strategy and takes account that the trials had heterogenous designs and participant populations.
Subgroup analysis and investigation of heterogeneity
We assessed the primary outcomes in the following pre‐specified subgroups.
-
Class or type of intervention.
-
Type of stroke: ischaemic stroke or ICH.
-
Stroke location: cortical or subcortical ischaemic stroke; deep or superficial ICH. (The definition of deep haemorrhage was not defined in all the trials and we therefore used the data as given in the trials.)
-
Timing of intervention: ultra‐acute (≤ four hours) and pre‐hospital, hyper‐acute (≤ six hours) and in hospital, acute (≤ 48 hours), sub‐acute (≤ 168 hours).
We considered an I2 greater than 50% to infer significant heterogeneity. If significant heterogeneity was present, we looked for potential causes, e.g. differences in trial design and study participants.
Sensitivity analysis
We based the analyses on all trials. We did not perform any sensitivity analyses.
Results
Description of studies
The 26 included trials are summarised in Characteristics of included studies, including details on baseline characteristics (Figure 3).

Results of database search
Results of the search
The quantity of outcome data varied between studies:
-
outcomes not universally collected;
-
some data still to be published.;
-
'raw data' available by personal communication.
If a trial used more than one dose of a particular drug then the trial identifier is written as author followed by year and dose of the drug. Referencing the whole trial was given by author and year. For example the Fagan 1988 trial comprises: (Fagan 1988 120 mg; Fagan 1988 240 mg).
Included studies
We identified 26 trials that fulfilled the inclusion criteria (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; ACCESS 2003; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; PIL‐FAST 2013; RIGHT 2013; ICH‐ADAPT 2013; INTERACT‐2 2013; TAST 2013; VENTURE 2013; ENOS 2014). Three trials compared more than one drug against the control group (Lisk 1993; CHHIPS 2009; ICH‐ADAPT 2013) and two studies compared different doses (Fagan 1988 120 mg/Fagan 1988 240 mg; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg).
We obtained trial protocols and group data from published material for the following studies (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Hillis 2003; ACCESS 2003; Eames 2005; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; RIGHT 2013; TAST 2013; ENOS 2014), whilst individual patient data were provided by seven sets of authors (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; ENOS 2014). We obtained unpublished SBP, DBP and heart rate data for active and control groups by contacting the authors for three trials (Hillis 2003; Eveson 2007; COSSACS 2010).
A variety of strategies and drug classes were used to lower blood pressure.
-
Alpha‐2‐adrenoceptor agonist, oral centrally‐acting (clonidine: two participants): one trial (Lisk 1993).
-
Angiotensin converting enzyme‐inhibitor (ACE‐I) (captopril, perindopril or lisinopril: 152 participants): five trials (Lisk 1993; Dyker 1997; Eveson 2007; CHHIPS 2009; PIL‐FAST 2013).
-
Angiotensin receptor antagonist (ARA), oral (candesartan or telmisartan: 4190 participants): six trials (ACCESS 2003; ACCOST 2006; PRoFESS 2009; SCAST 2011; TAST 2013; VENTURE 2013).
-
Beta‐receptor antagonist (ß‐RA) (labetalol: 56 participants): one trial (CHHIPS 2009).
-
Calcium channel blocker (CCB) (nimodipine or nicardipine: 75 participants): three trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Uzuner 1995).
-
Diuretic, oral thiazide‐like (bendrofluazide: 18 participants): one trial (Eames 2005).
-
Nitric oxide (NO) donor (transdermal glyceryl trinitrate (GTN): 4197 participants): five trials (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014).
-
Intensive versus guideline blood pressure targets (7421 participants): five trials (INTERACT pilot 2008; Koch 2008; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013).
-
Continue versus stop pre‐stroke antihypertensive drugs (2860 participants): two trials (COSSACS 2010; ENOS 2014).
One strategy was used to raise blood pressure.
-
Sympathomimetic, intravenous (phenylephrine: nine participants): one trial (Hillis 2003).
The trials recruited participants with only ischaemic stroke, mixed stroke (ischaemic stroke and ICH), or only ICH.
-
Ischaemic stroke: 12 trials (Fagan 1988 120 mg; Fagan 1988 240 mg; Lisk 1993; Dyker 1997; ACCESS 2003; Hillis 2003; Eames 2005; ACCOST 2006; Eveson 2007; PRoFESS 2009; CATIS 2013; TAST 2013; VENTURE 2013). In the Fagan study (Fagan 1988 120 mg; Fagan 1988 240 mg) participants were recruited with presumed ischaemic stroke based on history and neurological examination.
-
Mixed stroke: 10 trials (Uzuner 1995; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; CHHIPS 2009; COSSACS 2010; SCAST 2011; PIL‐FAST 2013; RIGHT 2013; ENOS 2014).
-
ICH: four trials (INTERACT pilot 2008; Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013).
Trials recruited participants at different time frames after stroke:
-
Ulta‐acute (< four hours of onset)/pre‐hospital: two trials (PIL‐FAST 2013; RIGHT 2013).
-
Hyper‐acute (< six hours)/hospital: two trials (INTERACT pilot 2008; INTERACT‐2 2013).
-
Acute (< 48 hours): 11 trials (Uzuner 1995; ACCESS 2003; CHHIPS 2009; COSSACS 2010; SCAST 2011; Eveson 2007; Koch 2008; CATIS 2013; ICH‐ADAPT 2013; VENTURE 2013; ENOS 2014).
-
Sub‐acute (< 168 hours): 10 trials (Lisk 1993; Dyker 1997; Bath 2000; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; PRoFESS 2009; TAST 2013).
-
Timing unclear: one trial (Fagan 1988 120 mg/Fagan 1988 240 mg).
Trials variously defined enrolment blood pressure levels.
-
Hypertension (SBP > 120 to 170 and ≤ 220 mm Hg): 21 trials (Lisk 1993; Dyker 1997; Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; TAST 2013; VENTURE 2013; ENOS 2014).
-
Normotension (systolic BP < 140 mmHg): two trials (Hillis 2003; ACCOST 2006).
-
No BP criteria: three trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Uzuner 1995; COSSACS 2010).
Trials treated participants for varying lengths of time.
-
For one day: one trial (ICH‐ADAPT 2013).
-
For up to two days: three trials (Uzuner 1995; Hillis 2003; Koch 2008).
-
For up to three days: one trial (Lisk 1993).
-
For seven to 12 days: 13 trials (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; ACCESS 2003; Eames 2005; Willmot 2006; INTERACT pilot 2008; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; RIGHT 2013; VENTURE 2013; ENOS 2014).
-
For 14 days: five trials (Dyker 1997; Eveson 2007; CHHIPS 2009; COSSACS 2010; CATIS 2013).
-
For 21 days: one trial (Fagan 1988 120 mg/Fagan 1988 240 mg).
-
For 28 days: one trial (ACCOST 2006).
-
For three months: one trial (TAST 2013).
-
For up to 2.5 years: one trial (PRoFESS 2009); outcomes at one to three months are used and longer‐term follow‐up data are ignored.
The trials recruited from one or more centres.
-
Single centre: 14 trials (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Hillis 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; Koch 2008; PIL‐FAST 2013; RIGHT 2013; TAST 2013).
-
Multicentre: 12 trials (Fagan 1988 120 mg; Fagan 1988 240 mg; ACCESS 2003; INTERACT pilot 2008; CHHIPS 2009; COSSACS 2010; PRoFESS 2009; SCAST 2011; ICH‐ADAPT 2013; CATIS 2013; INTERACT‐2 2013; VENTURE 2013; ENOS 2014).
A total of 17,011 participants received placebo or control treatment across the studies. Several trials compared two or more active treatment groups (8497 participants) with one control group (8512 participants) (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg).
One study reported on 19 participants from a larger RCT (Fagan 1988 120 mg/Fagan 1988 240 mg); further information on the main study is not available.
One trial was performed in two stages: this review includes the first phase, a double‐blind comparison of candesartan versus placebo (ACCOST 2006), and excludes the second open‐label comparison of candesartan and an ACE‐I.
One study expressly included patients with either ICH, who were given intravenous nimodipine (treatment: eight participants; placebo: three participants), or ischaemic stroke, who were given oral nimodipine (treatment: 38 participants; placebo: 39 participants) (Uzuner 1995); 10 participants (treatment: two participants; placebo: eight participants) treated with antihypertensive agents for malignant hypertension, and two participants treated with intravenous nimodipine for subarachnoid haemorrhage were excluded.
Data from two trials were only available from published abstracts (ACCOST 2006; VENTURE 2013).
Blood pressure measurements
Sixteen studies reported the method by which blood pressure was measured, including equipment (manufacturer, model) and patient posture (Lisk 1993; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; RIGHT 2013; TAST 2013; ENOS 2014). The Fagan trial only reported the average blood pressure measurements at, and for one hour after, morning dosing over seven days of treatment (Fagan 1988 120 mg/Fagan 1988 240 mg); in the absence of individual patient data, it is not possible to determine the blood pressure at selected time points during treatment. Furthermore, this trial co‐administered beta blockers to some participants, although these were always given at least two hours before or after nimodipine. In ACCESS 2003, during the first three days blood pressure measurements were performed by nurses as part of routine clinical care; on day seven, automatic 24‐hour blood pressure recording was performed. The other nine trials (Fagan 1988 120 mg/Fagan 1988 240 mg; Uzuner 1995; Hillis 2003; ACCOST 2006; INTERACT pilot 2008; INTERACT‐2 2013; ICH‐ADAPT 2013; PIL‐FAST 2013; VENTURE 2013) made no mention of patient posture or how blood pressure was measured.
Three trials recorded systolic but not diastolic BP after the first intervention: (Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013).
Outcomes
The trials reported a variety of outcomes.
-
mRS or BI or both at ≥ one month: 15 trials (Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014). Historically, trials dichotomised outcome as death or dependency, defined as mRS > 2, or mRS > 3, or BI < 60. Ordinal analysis of ordered categorical data is statistically more efficient and provides information on severity of outcome (Bath 2012) and recent trials have used ordinal analysis of mRS data (INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; SCAST 2011; CATIS 2013; INTERACT‐2 2013; TAST 2013; ENOS 2014).
-
Case fatality at ≥ one month: 15 trials (Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014).
-
Early neurological impairment (e.g. NIHSS, SSS) at < one month: 11 trials (Lisk 1993; Dyker 1997; Hillis 2003; Eames 2005; Eveson 2007; INTERACT pilot 2008; CHHIPS 2009; COSSACS 2010; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013).
-
Hospital length of stay: four trials (CHHIPS 2009; CATIS 2013; RIGHT 2013; ENOS 2014).
Excluded studies
We excluded 66 studies because they lacked randomisation, were irrelevant to the questions addressed in the current review, or failed to provide blood pressure or outcome assessments (see Characteristics of excluded studies).
Other studies
Eight studies are either awaiting assessment (MAPAS 2009; ATTACI 2010; STABLE‐ICAS 2010; ESH‐CHL‐SHOT 2013) or are ongoing (ATACH‐2 2011; ENCHANTED 2011; SETIN‐HYPERTENSION 2012; FAST‐BP 2013) (Figure 3).
Risk of bias in included studies
Computed tomography was used prior to entry in 10 trials to identify people with ICH (Uzuner 1995; INTERACT pilot 2008; Koch 2008; ICH‐ADAPT 2013; INTERACT‐2 2013) or to exclude people with ICH (Lisk 1993; Dyker 1997; ACCESS 2003; Hillis 2003; Eames 2005). Another study attempted to exclude ICH through information from the history and neurological examination (Fagan 1988 120 mg/Fagan 1988 240 mg); it may therefore have inadvertently included some participants with ICH.
Statistical analysis
Four trials compared more than one treatment against a common control group (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; CHHIPS 2009). The most appropriate analysis in this situation involves dividing the control group participants equally between treatment groups to prevent control participants being counted more than once and thereby artificially narrowing the confidence intervals.
Allocation
We classified allocation concealment as 'low risk', 'high risk' or 'unclear risk' according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Blinding
The method of randomisation was given for 23 trials (Dyker 1997; Bath 2000; ACCESS 2003; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Hillis 2003; Eames 2005; ACCOST 2006; Willmot 2006; Eveson 2007; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011; ICH‐ADAPT 2013; INTERACT‐2 2013; PIL‐FAST 2013; CATIS 2013; RIGHT 2013; TAST 2013; VENTURE 2013; ENOS 2014). Two authors were unable to describe the method of randomisation (Lisk 1993; Uzuner 1995) and one did not respond to our communication (Fagan 1988 120 mg/Fagan 1988 240 mg).
Participants and investigators were blinded to treatment as follows.
-
Double‐blind (participant and investigator blinded): 13 trials (Fagan 1988 120 mg; Lisk 1993; Dyker 1997; Bath 2000; ACCESS 2003; CHHIPS 2009; Eames 2005; SCAST 2011; ACCOST 2006; Eveson 2007; PRoFESS 2009; PIL‐FAST 2013; TAST 2013).
-
Single‐blind (participant blinded): four trials (Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014).
-
Open‐label: six trials (Rashid 2003 5 mg; Rashid 2003 5/10 mg; Rashid 2003 10 mg; Koch 2008; COSSACS 2010; ICH‐ADAPT 2013; INTERACT‐2 2013; VENTURE 2013).
Incomplete outcome data
Sixteen trials were analysed by intention‐to‐treat (ITT) (Fagan 1988 120 mg/Fagan 1988 240 mg; Lisk 1993; Dyker 1997; Bath 2000; Rashid 2003 5 mg /Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; INTERACT pilot 2008; Koch 2008; CHHIPS 2009; PRoFESS 2009; COSSACS 2010; SCAST 2011CATIS 2013; INTERACT‐2 2013; RIGHT 2013; ENOS 2014).
One study excluded 10 participants from the analysis because they had been treated with antihypertensive agents for concurrent accelerated (malignant) hypertension (Uzuner 1995).
Cardiovascular data were analysed on a per‐protocol basis and outcome data by intention‐to‐treat in one trial of lisinopril (Eveson 2007).
Selective reporting
We assessed selective reporting as low risk, high risk or unclear risk according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not see evidence of selective reporting in any of the trials.
Other potential sources of bias
One trial randomised participants before neuroimaging and those having a non‐ischaemic stroke were subsequently withdrawn from the study (Eveson 2007). Two trials did not state the method of analysis (Hillis 2003; Eames 2005). We did not find any other potential risks to the validity of the included studies.
Effects of interventions
The Results section is split into three parts.
-
Comparisons of BP lowering with control.
-
Comparisons of continuing versus stopping temporarily pre‐stroke antihypertensive drugs.
-
Comparisons of BP elevation with control.
Blood pressure lowering
Clinical outcomes
Twenty‐one trials provided data on one or more outcomes relating to treatment with:
-
ACE‐I (lisinopril): (Eveson 2007; CHHIPS 2009; PIL‐FAST 2013);
-
ARA (candesartan, telmisartan): (ACCESS 2003; PRoFESS 2009; SCAST 2011; TAST 2013; VENTURE 2013);
-
ß‐RA (labetalol): (CHHIPS 2009);
-
CCB (nimodipine): (Uzuner 1995);
-
NO donor (glyceryl trinitrate): (Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Willmot 2006; RIGHT 2013; ENOS 2014);
-
intensive blood pressure lowering: (INTERACT pilot 2008; Koch 2008; CHHIPS 2009; CATIS 2013; ICH‐ADAPT 2013; INTERACT‐2 2013);
-
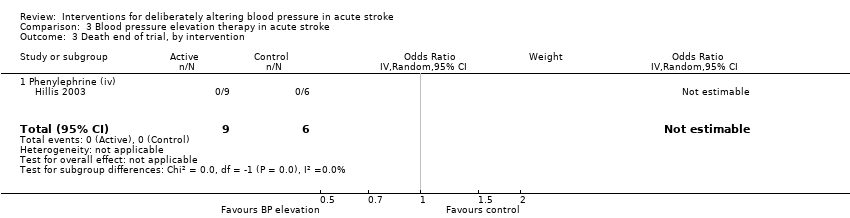
blood pressure elevation (phenylephrine): (Hillis 2003).
Death or dependency, end of trial
Drug class
Combined death or dependency was assessed using the mRS at the end of follow‐up. Data were available for 15,489 participants recruited into 14 trials (Analysis 1.1). We observed no significant difference between blood pressure lowering and control (OR 0.98; 95% CI 0.92 to 1.05), and heterogeneity was minimal. No individual comparison within drug classes or BP lowering strategies was significant (Analysis 1.1).
Stroke type
The effect of lowering blood pressure did not vary by stroke subtype (ischaemic stroke, mixed stroke, ICH) across 14 trials (Analysis 1.2).
Stroke location
The effect of lowering blood pressure did not vary by stroke location (ICH deep or not, ischaemic stroke cortical or subcortical) across six trials with 11951 participants (Analysis 1.2). Although there was insufficient evidence to assess heterogeneity, blood pressure lowering in deep ICH almost reached significance (OR 0.86; 95% CI 0.73 to 1.00, P value = 0.06) (Analysis 1.3).
Time to treatment
Data from 15 trials involving 15,520 participants were available. There was significant reduction in death or dependency if treatment was administered during the hyperacute period and in hospital (OR 0.87; 95% CI 0.76 to 0.99, P value = 0.03). Recruitment of participants later than this was associated with no benefit (Analysis 1.4).
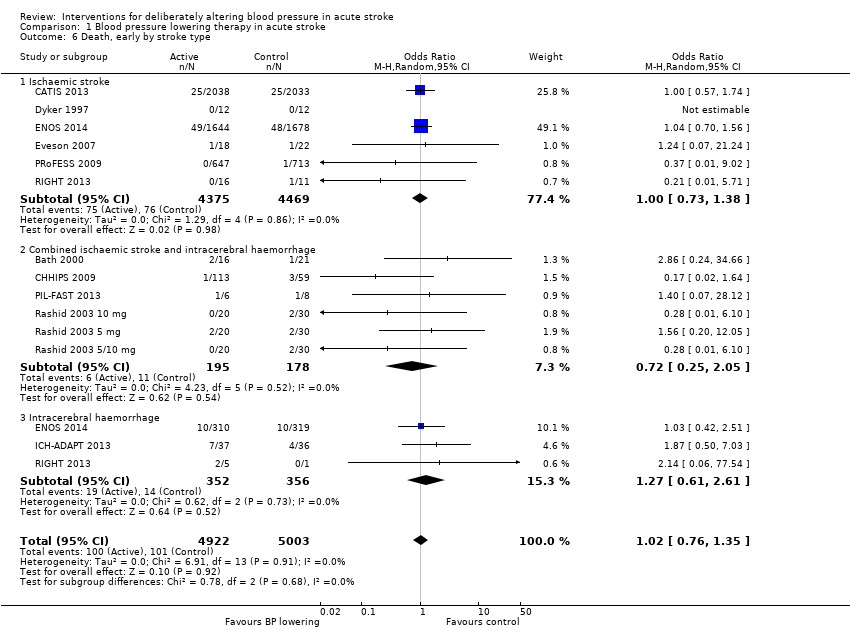
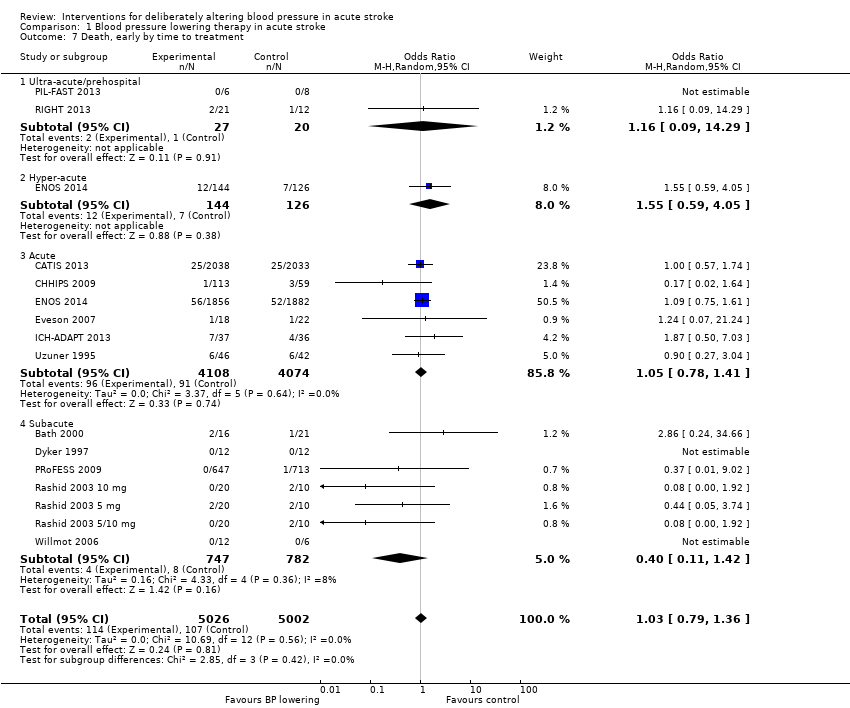
Death, early and end of trial
There was no overall effect of treatment on early death or death at end of trial (Analysis 1.5; Analysis 1.8). When considering subgroups, no differences existed when analysed by drug class (Analysis 1.5; Analysis 1.8), stroke type (Analysis 1.6; Analysis 1.9), or time to treatment (Analysis 1.7; Analysis 1.10).
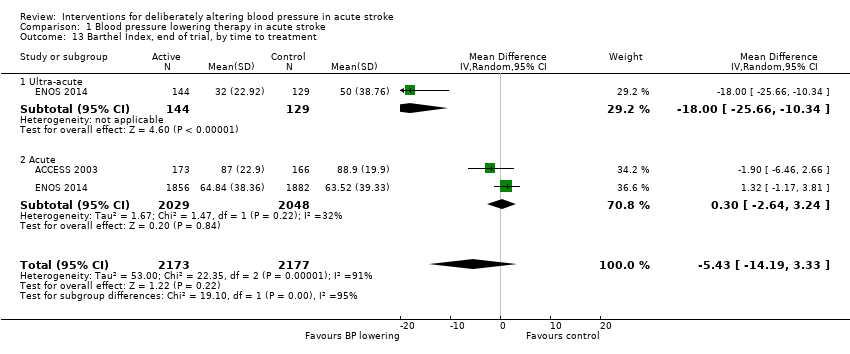
Barthel Index (disability), end of trial
We assessed the BI at the end of follow‐up in two trials with 4350 participants (Analysis 1.11). Although we observed no significant difference between blood pressure lowering and control (OR 0.63; 95% CI ‐3.28 to ‐4.54) (Analysis 1.11) and did not vary with stroke type (Analysis 1.12), BI scores were lower if treatment was started within six hours of stroke onset (Analysis 1.13).
Neurological deterioration, early
There was no overall difference in the rate of early neurological deterioration across seven trials (Analysis 1.14). Subgroup differences were not present when analysed by drug class or intensity (Analysis 1.14), or stroke type (Analysis 1.15). However, subgroup differences were apparent when assessed by time to treatment (Analysis 1.16); specifically, an increase in neurological deterioration was seen in participants with acute stroke (≤ 48 hours post stroke) (OR 1.39; 95% CI 1.07 to 1.81, P value = 0.01) but not when trials specifically treated earlier during the ultra‐acute and hyper‐acute periods.
Quality of life
Quality of life, assessed using the EQ‐5D and transformed into a Health Utility Status, was assessed in three trials (Analysis 1.17; Analysis 1.18). Health Utility scores were higher/better with blood pressure lowering (MD 0.02; 95% CI 0.01 to 0.04). However, heterogeneity was apparent between studies (I2 = 76.0%) with a significant result in INTERACT‐2 2013, but not ENOS 2014. When broken down into stroke types, participants with ICH in INTERACT‐2 2013 treated with blood pressure lowering tended to report a better quality of life (Analysis 1.18). When assessed by time to treatment, Health Utility Status scores were higher in participants treated ≤ six hours (MD 0.06; 95% CI 0.03 to 0.08), but not when treated later (Analysis 1.19).
Length of stay
Length of stay was not influenced by lowering blood pressure and there were no subgroup differences by type of intervention, stroke type or time to treatment (Analysis 1.20; Analysis 1.21; Analysis 1.22).
Haemodynamic measures
Blood pressure
The effect of different blood pressure‐lowering strategies on blood pressure after the first dose are summarised in Analysis 1.23 and Analysis 1.29, and in Table 1. Altogether, 24 trials studied 15,432 participants; most participants received an ARA, a NO donor or intensive blood pressure lowering. The focus for the following comments are on systolic rather than diastolic blood pressure. The magnitude of blood pressure reduction varied between ‐4.6/‐2.5 mmHg for oral ARA (primarily candesartan and telmisartan) and ‐13.7/‐7.9 mmHg for ACE‐Is. When assessed by stroke type, heterogeneity was present; slightly larger reductions in blood pressure were seen in participants with ICH (‐11.8/‐5.1 mmHg) with mixed stroke intermediate (‐7.9/‐3.0 mmHg) and ischaemic stroke least (‐7.0/‐3.1 mmHg) (Analysis 1.22; Analysis 1.30). Similarly, the magnitude of reduction varied by time to randomisation or treatment (I2 = 89%); a graded decrease was seen by time with the largest reduction occurring in participants treated during the ultra‐acute/pre‐hospital (‐16.0/‐15.0 mmHg), hyper‐acute/hospital (‐13.4/‐7.5 mmHg), acute (‐8.2/‐2.8 mmHg) and sub‐acute (‐7.3/‐4.9 mmHg) periods.
| Type | Trials | Participants | SBP | 95% CI | I2 % | DBP | 95% CI | I2% |
| Type of intervention | ||||||||
| ACE‐I, oral | 5 | 123 | ‐13.7 | ‐20.0 to ‐7.3 | 0 | ‐4.2 | ‐9.7 to 1.2 | 16 |
| ACE‐I, sublingual | 1 | 42 | ‐6.0 | ‐17.6 to 5.6 | ‐ | +2.2 | ‐5.5 to 9.9 | ‐ |
| ARA | 3 | 3408 | ‐4.6 | ‐8 to ‐1 | 74 | ‐2.5 | ‐4.3 to ‐0.6 | 67 |
| α‐2 adrenoceptor agonist | 1 | 4 | ‐13.7 | ‐41.5 to 14.1 | ‐ | ‐2.1 | ‐15.4 to 11.2 | ‐ |
| ß‐receptor antagonist, oral | 1 | 44 | ‐11.5 | ‐20.3 to ‐2.7 | ‐ | ‐5.4 | ‐13 to 2.2 | ‐ |
| ß‐receptor antagonist, iv | 1 | 41 | ‐16.4 | ‐27.4 to ‐5.4 | ‐ | ‐17.5 | ‐25.3 to ‐9.7 | ‐ |
| Calcium channel blocker, oral | 3 | 106 | ‐7.6 | ‐17.2 to 1.9 | 0 | ‐3.3 | ‐9.3 to 2.7 | ‐ |
| Calcium channel blocker, iv | 1 | 11 | ‐9.7 | ‐24.4 to 4.9 | ‐ | ‐12.9 | ‐31.4 to 5.6 | ‐ |
| Nitric oxide donor | 5 | 4192 | ‐9.3 | ‐14.5 to ‐4 | 26 | ‐3.6 | ‐4.4 to ‐2.8 | 0 |
| Diuretic, thiazide‐like | 1 | 40 | ‐20.0 | ‐38.6 to ‐1.4 | ‐ | ‐ | ‐ | ‐ |
| Low BP target | 5 | 7421 | ‐11.4 | ‐15.3 to ‐7.5 | 93 | ‐6.9 | ‐15.7 to 1.9 | 94 |
| Overall | 27 | 15432 | ‐11.2 | ‐13.7, ‐8.7 | 86 | ‐3.9 | ‐5.4 to ‐2.6 | 61 |
ACE‐I: angiotensin converting enzyme inhibitors
ARA: angiotensin receptor antagonist
BP: blood pressure
DBP: diastolic blood pressure
SBP: systolic blood pressure
iv: intravenous
The effect of the various types of intervention over the first week are summarised in Table 2.
| SBP | First | Day 1 | Day 7 |
| Type of intervention | |||
| ACE‐I, oral | ‐21.0 | ‐7.9 | ‐26 |
| ACE‐I, sublingual | ‐6.0 | ‐12 | ‐ |
| Angiotensin receptor antagonist | ‐4.6 | ‐0.5 | ‐6 |
| Alpha‐2 adrenoceptor agonist | ‐13.7 | ‐21.8 | ‐ |
| Beta‐receptor antagonist, oral | ‐11.5 | ‐14 | ‐ |
| Beta‐receptor antagonist, iv | ‐16.4 | ‐5 | ‐ |
| Calcium channel blocker, oral | ‐7.6 | ‐13.2 | ‐ |
| Calcium channel blocker, iv | ‐9.8 | ‐31.9 | ‐ |
| Nitric oxide donor | ‐9.3 | ‐7 | ‐1 |
| Diuretic, thiazide‐like | ‐20.0 | ‐ | ‐15 |
| Low BP target | ‐11.4 | ‐10 | ‐8 |
| Overall | ‐11.2 | ‐7.7 | ‐7 |
ACE‐I: angiotensin converting enzyme inhibitors
BP: blood pressure
SBP: systolic blood pressure
iv: intravenous
Heart rate
Thirteen trials reported heart rate measurements (Lisk 1993; Uzuner 1995; Dyker 1997; Bath 2000; Rashid 2003 5 mg/Rashid 2003 5/10 mg/Rashid 2003 10 mg; Eames 2005; Willmot 2006; Eveson 2007; PRoFESS 2009; ICH‐ADAPT 2013; RIGHT 2013; TAST 2013; ENOS 2014). The heart rate for glyceryl trinitrate increased at day one (MD 4.5 bpm; 95% CI 2 to 8) (Analysis 1.37) (Table 3). Intravenous CCBs reduced heart rate at day one (Uzuner 1995) (Analysis 1.37).
| Type of intervention | Trials | Participants | Day 1 | 95% CI |
| ACE (oral) | 2 | 39 | 0.03 | ‐40.4 to 40.5 |
| Alpha‐2 adrenoceptor agonist | 1 | 3 | ‐ | ‐ |
| Calcium channel blocker, oral | 2 | 83 | ‐2.6 | ‐7.7 to 2.6 |
| Calcium channel blocker, iv | 1 | 11 | ‐16.9 | ‐27.4 to ‐6.5 |
| Nitric oxide donor | 5 | 4197 | 4.5 | 1.5 to 7.6 |
ACE: angiotensin converting enzyme
iv: intravenous
Continuing versus stopping pre‐stroke antihypertensive drugs
Two trials tested whether pre‐stroke antihypertensives should be continued in the immediate post‐stroke period, or stopped temporarily (COSSACS 2010; ENOS 2014). The total number of participants numbered 2860.
Clinical outcomes
Death or dependency, end of trial
There was no significant difference in mRS at day 90 between those participants assigned to continue or stop antihypertensives (OR 1.06; 95% CI 0.91 to 1.24) (Analysis 2.1). Within subgroups, the effect of continuing versus stopping antihypertensives did not differ by stroke types or time to treatment across both trials (Analysis 2.2 and Analysis 2.3).
Death, early and at the end of trial
The rates of death at the end of treatment, and at the end of trial, did not differ between the treatment groups (Analysis 2.4 and Analysis 2.7). No significant differences were observed by stroke types or time to treatment (Analysis 2.5; Analysis 2.6; Analysis 2.8; Analysis 2.9).
Barthel Index
Barthel Index scores were lower in participants assigned to continue treatment (MD ‐3.18; 95% CI ‐0.55 to ‐5.80, P value = 0.02) (Analysis 2.10).
Neurological deterioration, early
The rate of death at the end of treatment did not differ between the treatment groups (Analysis 2.11).
Quality of life
Quality of life, assessed using the EQ‐5D and transformed into a Health Utility Status, was lower (i.e. worse) in participants randomised to continue pre‐stroke antihypertensive drugs (MD ‐0.03; 95% CI ‐0.05 to ‐0.01, P value = 0.008) (Analysis 2.12).
Haemodynamic measures
Blood pressure
Blood pressure was lower by ‐7.9/‐1.2 mmHg at the first measurement after randomisation in participants randomised to continue treatment with the reduction in systolic BP much greater in COSSACS 2010 than in ENOS 2014 (Analysis 2.14). By the end of treatment, blood pressure was lower by ‐11.3/‐6.4 mmHg in the continue group.
Heart rate
Data were only available for ENOS 2014. Heart rate was significantly lower by 3.2 bpm by end of treatment in those who were randomised to continue treatment (Analysis 2.21).
Blood pressure elevation therapy in acute stroke
Phenylephrine
Phenylephrine non‐significantly increased systolic blood pressure at 24 hours (MD 21 mmHg; 95% CI ‐13 to 55 mmHg), but had no significant effect on diastolic blood pressure (Hillis 2003) (Analysis 3.4). Insufficient data were available on clinical outcomes.
Discussion
Twenty‐six trials, involving 17,011 participants with stroke, assessed the effects of deliberate blood pressure alteration.
Blood pressure lowering
The results come from 24 trials that studied 15,432 participants.
Clinical outcomes
Overall, lowering blood pressure did not improve outcome, whether assessed as death, combined death or dependency, neurological deterioration or quality of life. These findings were maintained irrespective of type of intervention (drug class, intensity of lowering) or type of stroke. However, when assessed by time to treatment, very early blood pressure lowering (before hospital presentation or within six hours of stroke onset) was associated with reduced death or dependency, and improved quality of life (INTERACT‐2 2013; RIGHT 2013).
Haemodynamic effects
All the studied antihypertensive drug classes lowered blood pressure during the period of treatment. Reductions in blood pressure after the first treatment varied between ‐4.6/‐2.5 mmHg for oral ARA and ‐21.0/‐7.9 mmHg for ACE‐I. Slightly larger reductions in blood pressure were seen in participants with ICH (‐11.8/‐5.1 mmHg) than in those with ischaemic stroke (‐7.0/‐3.1 mmHg). The largest reductions were seen if treatment was started very early before hospital presentation (‐16.0/‐15.0 mmHg); smaller reductions occurred if treatment was started beyond 48 hours after stroke onset (‐7.3/‐4.9 mmHg).
Discussion
A variety of hypotheses can be postulated for why functional outcome was better if blood pressure lowering was started very early after stroke. First, the magnitude of blood pressure lowering may be important since the greatest improvement in outcome occurred when treatment was started early. Second, the type of intervention may be important since improved outcome was seen with early intensive blood pressure lowering (INTERACT‐2 2013), and early nitrate administration (RIGHT 2013; ENOS 2014). Conversely, apparent hazard was seen with ARA drugs (SCAST 2011; Jusufovic 2014).
Perhaps surprisingly, stroke type may not be particularly relevant since differential effects on outcome were not seen for ischaemic stroke versus ICH.
In summary, very early treatment with an appropriate agent or target blood pressure may be the most important strategy to test in the future, irrespective of stroke type. Importantly, systolic blood pressure should not be lowered excessively (> 20%), at least in ischaemic stroke, since trials of intravenous CCBs found that these worsened outcome (Bridgers 1991; Wahlgren 1994).
Continue versus stop pre‐stroke antihypertensive drugs
The results come from two trials that studied 2860 participants (COSSACS 2010; ENOS 2014).
Clinical outcomes
The findings were mixed with some comparisons, in particular dependency (mRS), death, and neurological deterioration, neutral for the comparison of continue versus stop pre‐stroke antihypertensive drugs. In contrast, measures of disability (BI) and quality of life (EQ‐5D, transformed into a Health Utility Status) were worse in participants randomised to continue treatment immediately.
Haemodynamic effects
Immediately continuing antihypertensive drugs taken before stroke was associated with a lower blood pressure by ‐7.9/‐1.2 mmHg at the first measurement after randomisation, and ‐11.3/‐6.4 mmHg by end‐of‐treatment.
Discussion
The discrepancy in findings for two measures of functional outcome, mRS and BI, is challenging to explain. First, it may represent chance such that no difference exists between the interventions. Second, it could reflect outcome bias since it is not possible to test this question in a double‐blind placebo‐controlled design. Nevertheless, both trials used blinded outcome assessment for both mRS and BI. Further, since a majority of stroke physicians tend to continue treatment in routine practice (Bath 2000b), the result seen across the two results is counter‐intuitive. Last, the difference may be real in which case mRS, usually considered to be the optimal functional outcome in stroke trials (Lees 2012), failed to detect a difference in contrast to a comparison of BI scores.
If continuing drugs immediately is, indeed, hazardous, then the two trials do not identify the cause. Drugs that attenuate stress hormones, in particular that down‐regulate the renin‐angiotensin‐aldosterone‐system (RAAS) were commonly taken before stroke, e.g. ACE‐I, ARA and ß‐receptor antagonists. Initiating these drugs in the acute phase of stroke has been associated with harm (BEST 1988; SCAST 2011), so it can be postulated that continuing these during the acute phase of stroke is potentially harmful. Alternatively, continuing drugs in people who are dysphagic and who do not have safe enteral access for feeding may be hazardous through aspiration of these drugs and then the development of pneumonia. The ENOS trial gives some support for this hypothesis (ENOS 2014).
The main implication for clinicians is that it is reasonable to withhold BP‐lowering drugs until patients are medically and neurologically stable, and have suitable oral or enteral access, after which drugs can then be reintroduced.
General
An important problem with some of the trials was the absence of detailed information on how blood pressure was measured. Hence, the quality of blood pressure readings is unknown. It is essential that future trials describe in detail how blood pressure is measured by including the following information.
-
Equipment: manufacturer, model, measurement method (mercury, anaeroid or oscillometry, and manual or automatic), and whether the equipment has been independently validated, and if so by whom.
-
Measurer: who measured blood pressure, and how they were trained, assessed, re‐trained and re‐assessed.
-
Measurements: the number of readings at each time point, site of measurement (brachial, finger, etc) and what position the person was in (supine, sitting, standing).
Little is known about the effect of blood pressure altering in older people with acute stroke, who comprise the largest group of patients, including those with ischaemic stroke who need thrombolysis. Of the trials, only two had mean age over 75 years contributing to a total of 98 participants (CHHIPS 2009; RIGHT 2013). The number and proportion of older people is likely to increase with population ageing. As the variation in response by individuals to blood pressure modulating agents increases with age, e.g. related to concurrent isolated systolic hypertension or cardiac dysfunction, it may be inappropriate to assume that the effects of changing blood pressure seen in younger populations will necessarily be the same in older ones.
This review is Part 1 of the Blood pressure in Acute Stroke Collaboration and reports only those trials that specifically set out to alter blood pressure in people with acute stroke. Part 2 of the project assesses all RCTs in acute stroke where vasoactive drugs were administered and includes all those studies covered in Part 1. Although progress has been made in the number and quality of stroke trials in the last few years, a substantial number of questions remain. The number of participants included in this review is very small in comparison to the global burden of stroke (about 15,000,000 per year worldwide). At present, any benefit of treatment is small, and additional data are required to recommend changes to routine clinical practice. The centres that took part in the trials were interested and familiar with the management of acute stroke. To extrapolate these results in routine clinical practice to less specialist centres could result in greater hazard or completely negate any potential benefit. Therefore, there is a need for new centres to participate in trials. Further evidence is needed on:
-
how to select participants;
-
the influence of age, time of onset, stroke subtype, severity, choice of drug, dose, route of administration and blood pressure variability, on response to active changes in blood pressure.
Recent guidelines based on the non‐systematic analysis of (largely) observational data recommend that hypertension should not be treated for up to two weeks after an ischaemic stroke unless severe hypertension, hypertensive encephalopathy, heart failure, cardiac ischaemia, aortic dissection, or continued intracerebral bleeding are present (O'Connell 1994; EUSI 2004; AHA‐HS 2010; AHA‐IS 2013). Persistent hypertension after two weeks should be treated since the risk of stroke in people with cerebrovascular disease is dependent on systolic and diastolic blood pressure (Rodgers 1996). In PROGRESS 2001, perindopril (with or without indapamide) reduced the risk of stroke among both hypertensive and non‐hypertensive participants with a history of stroke or transient ischaemic attack. Further, evidence from the Heart Outcomes Prevention Evaluation (HOPE) trial suggests that an ACE‐I may reduce stroke and other vascular events in people with prior cerebrovascular disease (HOPE 2000). Overall, lowering blood pressure in people with chronic stroke reduces the subsequent risk of recurrence (Rashid 2003).
Summary of main results
In this updated review, blood pressure lowering did not improve functional outcome. However, early initiation of treatment might be beneficial, and further studies are required to test this specific question. Immediately continuing pre‐stroke antihypertensive drugs appears to be associated with a worse functional outcome and lower quality of life; hence, it is reasonable to delay treatment until patients are stable and have oral or enteral access to allow safe administration of drugs.
Overall completeness and applicability of evidence
The included trials are an excellent start to answering the question of optimal blood pressure management in acute stroke. The lack of a definitive result based on data from more than 16,000 participants confirms that the question is complex and future trials need to refine trial design, especially focusing on very early treatment.
Quality of the evidence
The quality of the included studies was variable. Methods of randomisation and allocation concealment were not always clear. Not all trials contributed to each outcome. Details of blood pressure recording including equipment, number of readings and patient positioning were not provided in some studies. Trials in the last decade were more standardised compared with earlier ones when reporting baseline stroke characteristics, primary and secondary outcomes. Outcome assessment was blinded in recent large RCTs and clearly reported in trial protocols. Trials were largely not representative of unselected stroke populations around the world ‐ participants tended to be younger, have fewer comorbidities and be conscious, so that poor outcomes were less common than might be expected.
Potential biases in the review process
This review follows an extensive literature search by both the Cochrane Stroke Review Group, and the authors, and without any language restrictions. Hence the risk of study inclusion bias is low.
Agreements and disagreements with other studies or reviews
The limited data mean that we can draw no firm conclusions, as shown in other reviews.

Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.1 Death or dependency, end of trial by intervention.
Funnel plot of comparison: 1 Blood pressure lowering therapy in acute stroke, outcome: 1.23 SBP, first after randomisation, by intervention.

Results of database search

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 1 Death or dependency, end of trial by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 2 Death or dependency, end of trial by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 3 Death or dependency, end of trial by stroke location.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 4 Death or dependency, end of trial by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 5 Death, early by intervention.
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 6 Death, early by stroke type.
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 7 Death, early by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 8 Death, end of trial by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 9 Death, end of trial by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 10 Death, end of trial by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 11 Barthel Index, end of trial, by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 12 Barthel Index, end of trial, by stroke type.
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 13 Barthel Index, end of trial, by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 14 Early neurological deterioration, by intervention.
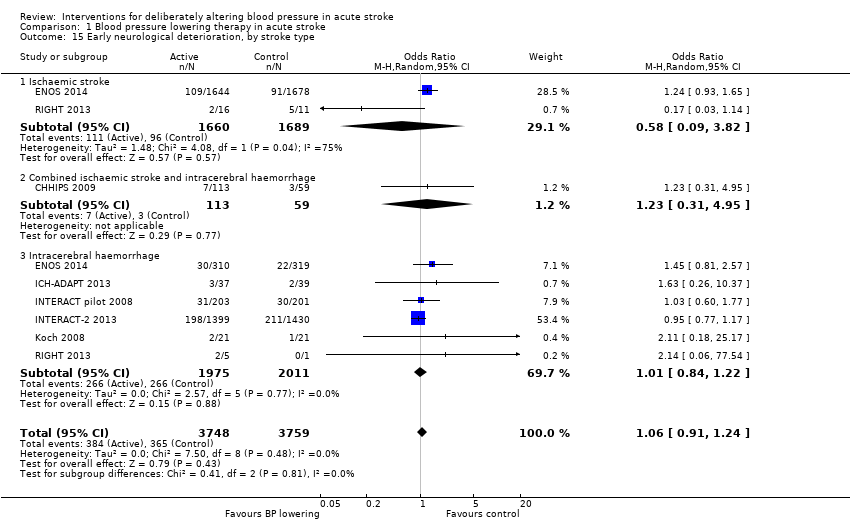
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 15 Early neurological deterioration, by stroke type.
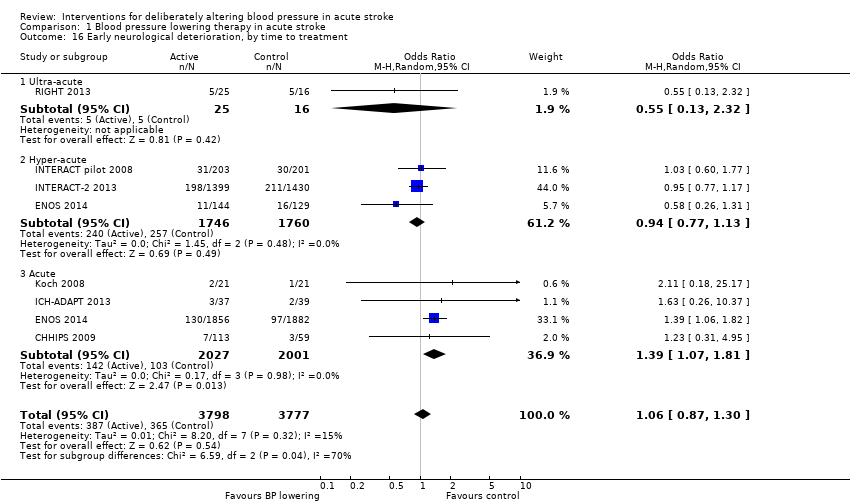
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 16 Early neurological deterioration, by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 17 Quality of life (EuroQol) at end of trial, by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 18 Quality of life (EuroQoL) at end of trial, by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 19 Quality of life (EuroQoL) at end of trial, by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 20 Length of stay, by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 21 Length of stay, by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 22 Length of stay, by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 23 SBP, first after randomisation, by intervention.
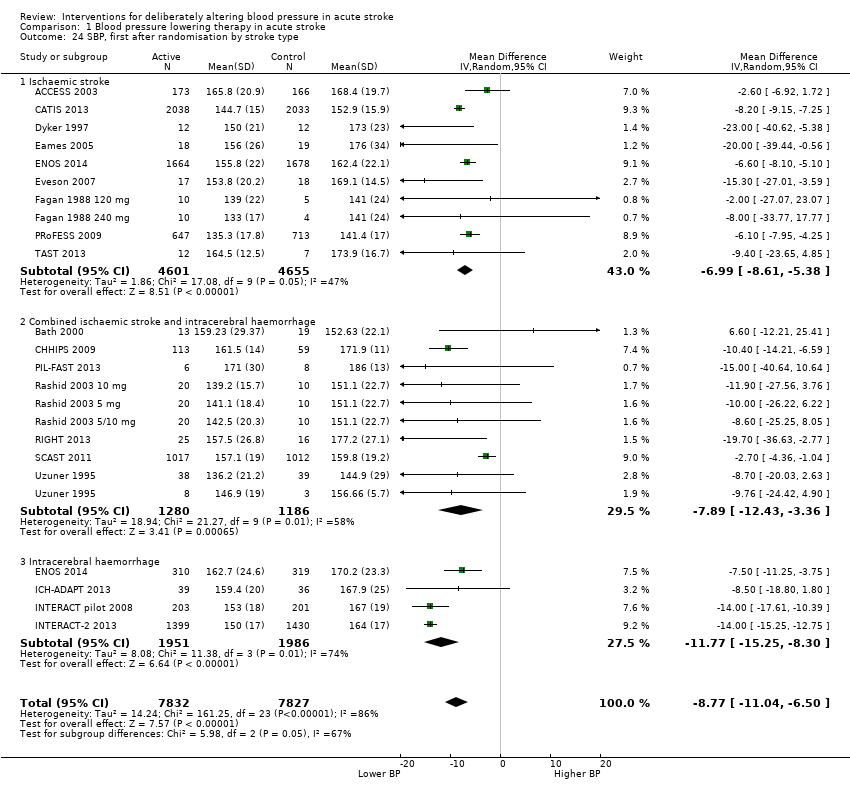
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 24 SBP, first after randomisation by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 25 SBP, first after randomisation by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 26 SBP, at day 1.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 27 SBP, at day 7.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 28 SBP, at end of treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 29 DBP, first after randomisation by intervention.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 30 DBP, first after randomisation by stroke type.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 31 DBP, first after randomisation by time to treatment.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 32 DBP, at day 1.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 33 DBP, at day 7.
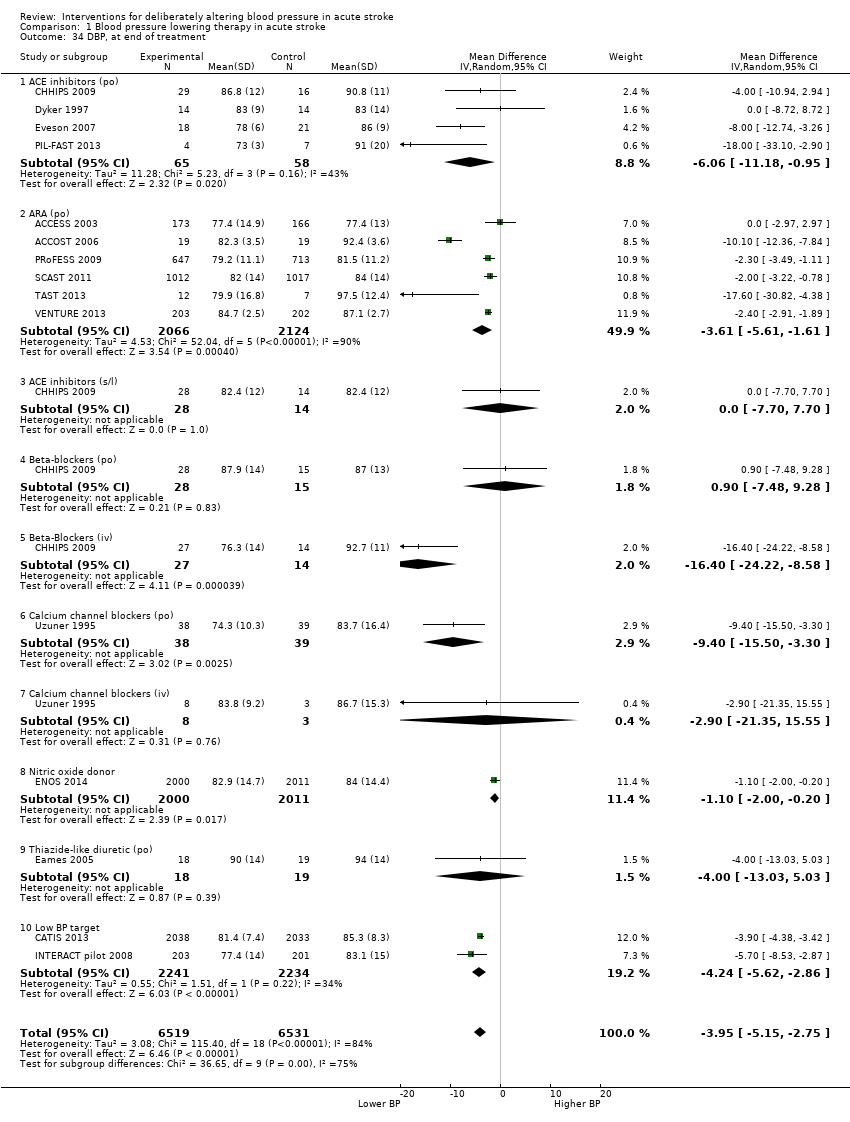
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 34 DBP, at end of treatment.
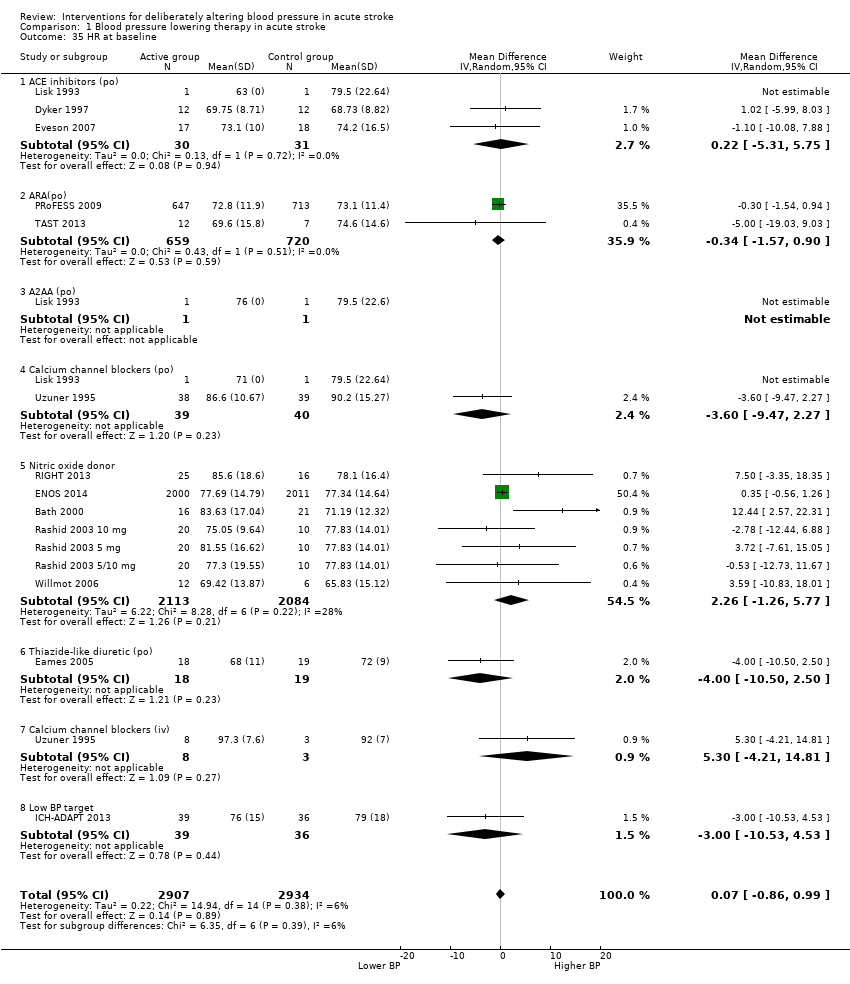
Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 35 HR at baseline.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 36 HR, first after randomisation.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 37 HR, at day 1.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 38 HR, at day 7.

Comparison 1 Blood pressure lowering therapy in acute stroke, Outcome 39 HR, at end of treatment.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 1 Death or dependency, end of trial by C/S.
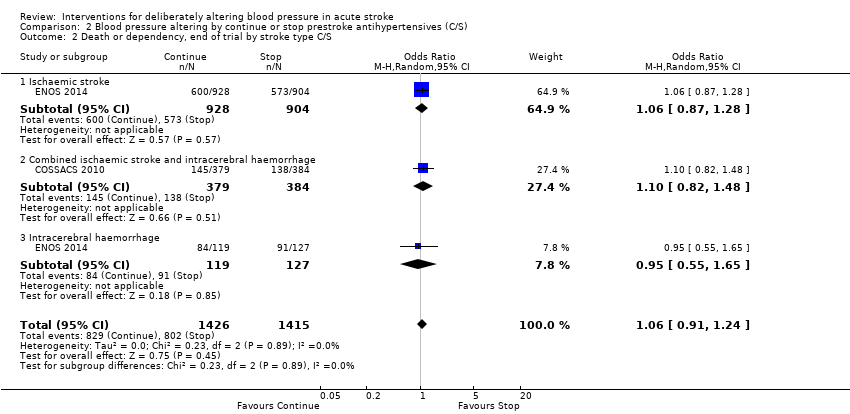
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 2 Death or dependency, end of trial by stroke type C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 3 Death or dependency, end of trial by time to treatment.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 4 Death early, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 5 Death early, by stroke type C/S.
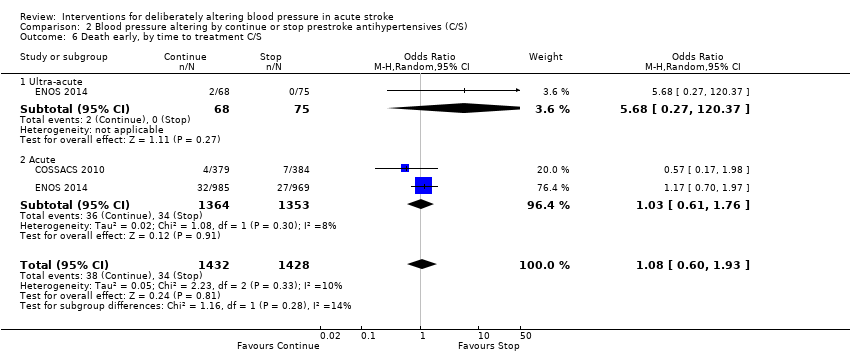
Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 6 Death early, by time to treatment C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 7 Death, end of trial by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 8 Death end of trial, by stroke type C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 9 Death, end of trial by time to treatment C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 10 Barthel Index, end of trial, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 11 Early neurological deterioration, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 12 Quality of life (EuroQol) at end of trial, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 13 Length of stay, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 14 SBP, first after randomisation, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 15 SBP, at day 1 by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 16 SBP, at end of treatment by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 17 DBP, at baseline by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 18 DBP at day 1, by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 19 DBP, at end of treatment by C/S.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 20 HR, at day 1.

Comparison 2 Blood pressure altering by continue or stop prestroke antihypertensives (C/S), Outcome 21 HR, at end of treatment.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 1 Death early, by intervention.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 2 Death early, by stroke type.
Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 3 Death end of trial, by intervention.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 4 SBP, at baseline.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 5 SBP, first after randomisation.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 6 SBP, at day 1.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 7 DBP, at baseline.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 8 DBP, first after randomisation.

Comparison 3 Blood pressure elevation therapy in acute stroke, Outcome 9 DBP, at day 1.
| Type | Trials | Participants | SBP | 95% CI | I2 % | DBP | 95% CI | I2% |
| Type of intervention | ||||||||
| ACE‐I, oral | 5 | 123 | ‐13.7 | ‐20.0 to ‐7.3 | 0 | ‐4.2 | ‐9.7 to 1.2 | 16 |
| ACE‐I, sublingual | 1 | 42 | ‐6.0 | ‐17.6 to 5.6 | ‐ | +2.2 | ‐5.5 to 9.9 | ‐ |
| ARA | 3 | 3408 | ‐4.6 | ‐8 to ‐1 | 74 | ‐2.5 | ‐4.3 to ‐0.6 | 67 |
| α‐2 adrenoceptor agonist | 1 | 4 | ‐13.7 | ‐41.5 to 14.1 | ‐ | ‐2.1 | ‐15.4 to 11.2 | ‐ |
| ß‐receptor antagonist, oral | 1 | 44 | ‐11.5 | ‐20.3 to ‐2.7 | ‐ | ‐5.4 | ‐13 to 2.2 | ‐ |
| ß‐receptor antagonist, iv | 1 | 41 | ‐16.4 | ‐27.4 to ‐5.4 | ‐ | ‐17.5 | ‐25.3 to ‐9.7 | ‐ |
| Calcium channel blocker, oral | 3 | 106 | ‐7.6 | ‐17.2 to 1.9 | 0 | ‐3.3 | ‐9.3 to 2.7 | ‐ |
| Calcium channel blocker, iv | 1 | 11 | ‐9.7 | ‐24.4 to 4.9 | ‐ | ‐12.9 | ‐31.4 to 5.6 | ‐ |
| Nitric oxide donor | 5 | 4192 | ‐9.3 | ‐14.5 to ‐4 | 26 | ‐3.6 | ‐4.4 to ‐2.8 | 0 |
| Diuretic, thiazide‐like | 1 | 40 | ‐20.0 | ‐38.6 to ‐1.4 | ‐ | ‐ | ‐ | ‐ |
| Low BP target | 5 | 7421 | ‐11.4 | ‐15.3 to ‐7.5 | 93 | ‐6.9 | ‐15.7 to 1.9 | 94 |
| Overall | 27 | 15432 | ‐11.2 | ‐13.7, ‐8.7 | 86 | ‐3.9 | ‐5.4 to ‐2.6 | 61 |
| ACE‐I: angiotensin converting enzyme inhibitors | ||||||||
| SBP | First | Day 1 | Day 7 |
| Type of intervention | |||
| ACE‐I, oral | ‐21.0 | ‐7.9 | ‐26 |
| ACE‐I, sublingual | ‐6.0 | ‐12 | ‐ |
| Angiotensin receptor antagonist | ‐4.6 | ‐0.5 | ‐6 |
| Alpha‐2 adrenoceptor agonist | ‐13.7 | ‐21.8 | ‐ |
| Beta‐receptor antagonist, oral | ‐11.5 | ‐14 | ‐ |
| Beta‐receptor antagonist, iv | ‐16.4 | ‐5 | ‐ |
| Calcium channel blocker, oral | ‐7.6 | ‐13.2 | ‐ |
| Calcium channel blocker, iv | ‐9.8 | ‐31.9 | ‐ |
| Nitric oxide donor | ‐9.3 | ‐7 | ‐1 |
| Diuretic, thiazide‐like | ‐20.0 | ‐ | ‐15 |
| Low BP target | ‐11.4 | ‐10 | ‐8 |
| Overall | ‐11.2 | ‐7.7 | ‐7 |
| ACE‐I: angiotensin converting enzyme inhibitors | |||
| Type of intervention | Trials | Participants | Day 1 | 95% CI |
| ACE (oral) | 2 | 39 | 0.03 | ‐40.4 to 40.5 |
| Alpha‐2 adrenoceptor agonist | 1 | 3 | ‐ | ‐ |
| Calcium channel blocker, oral | 2 | 83 | ‐2.6 | ‐7.7 to 2.6 |
| Calcium channel blocker, iv | 1 | 11 | ‐16.9 | ‐27.4 to ‐6.5 |
| Nitric oxide donor | 5 | 4197 | 4.5 | 1.5 to 7.6 |
| ACE: angiotensin converting enzyme | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency, end of trial by intervention Show forest plot | 16 | 15489 | Odds Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 1.1 ACE inhibitors | 2 | 126 | Odds Ratio (IV, Random, 95% CI) | 1.12 [0.53, 2.36] |
| 1.2 ARA(po) | 3 | 3737 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.93, 1.23] |
| 1.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 1.03 [0.42, 2.55] |
| 1.4 Nitric oxide donor | 7 | 4194 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 1.5 Low BP target | 4 | 7346 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.86, 1.04] |
| 2 Death or dependency, end of trial by stroke type Show forest plot | 16 | 15366 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2.1 Ischaemic stroke | 8 | 11015 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.92, 1.08] |
| 2.2 Combined Ischaemic stroke and Intracerebral haemorrhage | 5 | 142 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.64, 2.65] |
| 2.3 Intracerebral haemorrhage | 7 | 4209 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.21] |
| 3 Death or dependency, end of trial by stroke location Show forest plot | 6 | 11950 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.87, 1.01] |
| 3.1 Intracerebral haemorrhage, deep | 3 | 2536 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.00] |
| 3.2 Intracerebral haemorrhage, superficial | 3 | 851 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.77, 1.35] |
| 3.3 Ischaemic stroke, cortical | 4 | 6180 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.81, 1.09] |
| 3.4 Ischaemic stroke, subcortical | 4 | 2383 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.76, 1.27] |
| 4 Death or dependency, end of trial by time to treatment Show forest plot | 16 | 15489 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.1 Ultra‐acute/pre‐hospital | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.09, 1.43] |
| 4.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 0.99] |
| 4.3 Acute | 7 | 10440 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.94, 1.11] |
| 4.4 Subacute | 6 | 1502 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.31] |
| 5 Death, early by intervention Show forest plot | 16 | 10050 | Odds Ratio (IV, Random, 95% CI) | 0.97 [0.74, 1.28] |
| 5.1 ACE inhibitors (po) | 4 | 164 | Odds Ratio (IV, Random, 95% CI) | 0.51 [0.06, 4.34] |
| 5.2 ARA (po) | 2 | 1379 | Odds Ratio (IV, Random, 95% CI) | 0.45 [0.05, 3.97] |
| 5.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 0.25 [0.02, 2.93] |
| 5.4 Calcium channel blockers (po) | 1 | 77 | Odds Ratio (IV, Random, 95% CI) | 0.65 [0.17, 2.50] |
| 5.5 Calcium channel blockers (iv) | 1 | 11 | Odds Ratio (IV, Random, 95% CI) | 2.69 [0.10, 73.20] |
| 5.6 Nitric oxide donor | 7 | 4189 | Odds Ratio (IV, Random, 95% CI) | 0.79 [0.37, 1.72] |
| 5.7 Low BP target | 2 | 4144 | Odds Ratio (IV, Random, 95% CI) | 1.10 [0.66, 1.83] |
| 6 Death, early by stroke type Show forest plot | 13 | 9925 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.76, 1.35] |
| 6.1 Ischaemic stroke | 6 | 8844 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.73, 1.38] |
| 6.2 Combined ischaemic stroke and intracerebral haemorrhage | 6 | 373 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.25, 2.05] |
| 6.3 Intracerebral haemorrhage | 3 | 708 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.61, 2.61] |
| 7 Death, early by time to treatment Show forest plot | 15 | 10028 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.36] |
| 7.1 Ultra‐acute/prehospital | 2 | 47 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.09, 14.29] |
| 7.2 Hyper‐acute | 1 | 270 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.59, 4.05] |
| 7.3 Acute | 6 | 8182 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.41] |
| 7.4 Subacute | 7 | 1529 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.11, 1.42] |
| 8 Death, end of trial by intervention Show forest plot | 20 | 15818 | Odds Ratio (IV, Random, 95% CI) | 0.95 [0.84, 1.06] |
| 8.1 ACE inhibitors (po) | 4 | 165 | Odds Ratio (IV, Random, 95% CI) | 0.47 [0.15, 1.48] |
| 8.2 ARA (po) | 5 | 4120 | Odds Ratio (IV, Random, 95% CI) | 0.92 [0.59, 1.44] |
| 8.3 Beta‐blockers | 1 | 86 | Odds Ratio (IV, Random, 95% CI) | 0.57 [0.17, 1.89] |
| 8.4 Nitric oxide donor | 7 | 4197 | Odds Ratio (IV, Random, 95% CI) | 0.86 [0.72, 1.04] |
| 8.5 Low BP target | 4 | 7250 | Odds Ratio (IV, Random, 95% CI) | 1.04 [0.87, 1.25] |
| 9 Death, end of trial by stroke type Show forest plot | 20 | 15750 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
| 9.1 Ischaemic stroke | 10 | 11238 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.16] |
| 9.2 Combined ischaemic stroke and intracerebral haemorrhage | 7 | 328 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.22] |
| 9.3 Intracerebral haemorrhage | 6 | 4184 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 10 Death, end of trial by time to treatment Show forest plot | 20 | 15818 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.75, 1.08] |
| 10.1 Ultra‐acute/pre‐hospital | 2 | 52 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.11, 2.07] |
| 10.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.38, 1.26] |
| 10.3 Acute | 8 | 10708 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 10.4 Subacute | 8 | 1552 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.15] |
| 11 Barthel Index, end of trial, by intervention Show forest plot | 2 | 4350 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐3.28, 4.54] |
| 11.1 ARA | 1 | 339 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐6.46, 2.66] |
| 11.2 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐0.20, 4.60] |
| 12 Barthel Index, end of trial, by stroke type Show forest plot | 2 | 4310 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐1.04, 3.69] |
| 12.1 Ischaemic stroke | 2 | 3681 | Mean Difference (IV, Random, 95% CI) | 0.84 [‐3.21, 4.89] |
| 12.2 Intracerebral haemorrhage | 1 | 629 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐5.18, 6.98] |
| 13 Barthel Index, end of trial, by time to treatment Show forest plot | 2 | 4350 | Mean Difference (IV, Random, 95% CI) | ‐5.43 [‐14.19, 3.33] |
| 13.1 Ultra‐acute | 1 | 273 | Mean Difference (IV, Random, 95% CI) | ‐18.0 [‐25.66, ‐10.34] |
| 13.2 Acute | 2 | 4077 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐2.64, 3.24] |
| 14 Early neurological deterioration, by intervention Show forest plot | 7 | 7575 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.92, 1.24] |
| 14.1 ACE inhibitors | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.30, 8.41] |
| 14.2 Beta‐blockers | 1 | 86 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.03, 8.74] |
| 14.3 Nitric oxide donor | 2 | 4052 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.66, 1.98] |
| 14.4 Low BP target | 4 | 3351 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 15 Early neurological deterioration, by stroke type Show forest plot | 7 | 7507 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 15.1 Ischaemic stroke | 2 | 3349 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.09, 3.82] |
| 15.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.31, 4.95] |
| 15.3 Intracerebral haemorrhage | 6 | 3986 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.22] |
| 16 Early neurological deterioration, by time to treatment Show forest plot | 7 | 7575 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.30] |
| 16.1 Ultra‐acute | 1 | 41 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.13, 2.32] |
| 16.2 Hyper‐acute | 3 | 3506 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.77, 1.13] |
| 16.3 Acute | 4 | 4028 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [1.07, 1.81] |
| 17 Quality of life (EuroQol) at end of trial, by intervention Show forest plot | 3 | 6881 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [0.01, 0.04] |
| 17.1 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.03] |
| 17.2 Low BP target | 1 | 2829 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.02, 0.08] |
| 18 Quality of life (EuroQoL) at end of trial, by stroke type Show forest plot | 3 | 7502 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 18.1 Ischaemic stroke | 2 | 4038 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.14, 0.40] |
| 18.2 Intracerebral haemorrhage | 3 | 3464 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.03, 0.08] |
| 19 Quality of life (EuroQoL) at end of trial, by time to treatment Show forest plot | 3 | 6867 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.00, 0.11] |
| 19.1 Ultra‐acute | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.29 [0.07, 0.51] |
| 19.2 Hyperacute | 2 | 3102 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.03, 0.08] |
| 19.3 Acute | 1 | 3738 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 20 Length of stay, by intervention Show forest plot | 4 | 8295 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.30, 0.28] |
| 20.1 ACE inhibitors | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐2.11, 1.85] |
| 20.2 Beta‐blockers | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐1.76, 2.18] |
| 20.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.76, 1.14] |
| 20.4 Low BP target | 1 | 4071 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.31, 0.31] |
| 21 Length of stay, by stroke type Show forest plot | 3 | 8194 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.29] |
| 21.1 Ischaemic stroke | 2 | 7393 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.28] |
| 21.2 Combined ischaemic and Intracerebral haemorrhage | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.20, 1.60] |
| 21.3 Intracerebral haemorrhage | 1 | 629 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐1.49, 6.69] |
| 22 Length of stay, by time to treatment Show forest plot | 4 | 8295 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.30, 0.29] |
| 22.1 Ultra‐acute | 2 | 314 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐6.58, 2.83] |
| 22.2 Acute | 3 | 7981 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.29, 0.30] |
| 23 SBP, first after randomisation, by intervention Show forest plot | 24 | 15432 | Mean Difference (IV, Random, 95% CI) | ‐9.83 [‐12.11, ‐7.56] |
| 23.1 ACE inhibitors(po) | 5 | 123 | Mean Difference (IV, Random, 95% CI) | ‐13.68 [‐20.03, ‐7.32] |
| 23.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐17.55, 5.55] |
| 23.3 ARA (po) | 3 | 3408 | Mean Difference (IV, Random, 95% CI) | ‐4.59 [‐7.71, ‐1.48] |
| 23.4 A2AA(po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐13.67 [‐41.48, 14.14] |
| 23.5 Beta‐blockers (po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐11.5 [‐20.29, ‐2.71] |
| 23.6 Beta‐blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐16.40 [‐27.40, ‐5.40] |
| 23.7 Calcium channel blockers (po) | 3 | 106 | Mean Difference (IV, Random, 95% CI) | ‐7.62 [‐17.21, 1.96] |
| 23.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐9.76 [‐24.42, 4.90] |
| 23.9 Nitric oxide donor | 7 | 4192 | Mean Difference (IV, Random, 95% CI) | ‐9.25 [‐14.54, ‐3.96] |
| 23.10 Thiazide‐like diuretic (po) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐20.0 [‐38.60, ‐1.40] |
| 23.11 Low BP target | 5 | 7421 | Mean Difference (IV, Random, 95% CI) | ‐11.39 [‐15.30, ‐7.49] |
| 24 SBP, first after randomisation by stroke type Show forest plot | 22 | 15659 | Mean Difference (IV, Random, 95% CI) | ‐8.77 [‐11.04, ‐6.50] |
| 24.1 Ischaemic stroke | 10 | 9256 | Mean Difference (IV, Random, 95% CI) | ‐6.99 [‐8.61, ‐5.38] |
| 24.2 Combined ischaemic stroke and intracerebral haemorrhage | 9 | 2466 | Mean Difference (IV, Random, 95% CI) | ‐7.89 [‐12.43, ‐3.36] |
| 24.3 Intracerebral haemorrhage | 4 | 3937 | Mean Difference (IV, Random, 95% CI) | ‐11.77 [‐15.25, ‐8.30] |
| 25 SBP, first after randomisation by time to treatment Show forest plot | 17 | 15211 | Mean Difference (IV, Random, 95% CI) | ‐9.67 [‐12.14, ‐7.20] |
| 25.1 Ultra‐acute/prehospital | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐15.98 [‐30.43, ‐1.53] |
| 25.2 Hyper‐acute | 3 | 3506 | Mean Difference (IV, Random, 95% CI) | ‐13.38 [‐15.41, ‐11.35] |
| 25.3 Acute | 6 | 10120 | Mean Difference (IV, Random, 95% CI) | ‐7.23 [‐9.83, ‐4.63] |
| 25.4 Subacute | 7 | 1530 | Mean Difference (IV, Random, 95% CI) | ‐7.26 [‐10.02, ‐4.50] |
| 26 SBP, at day 1 Show forest plot | 18 | 14203 | Mean Difference (IV, Random, 95% CI) | ‐8.33 [‐10.97, ‐5.69] |
| 26.1 ACE inhibitors (po) | 5 | 120 | Mean Difference (IV, Random, 95% CI) | ‐7.90 [‐16.83, 1.03] |
| 26.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐25.60, 1.60] |
| 26.3 ARA (po) | 2 | 2368 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.52, 1.51] |
| 26.4 A2AA (po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐21.83 [‐66.12, 22.46] |
| 26.5 Beta‐blockers(po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐14.00 [‐27.28, ‐0.72] |
| 26.6 Beta‐blockers (iv) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐18.44, 8.44] |
| 26.7 Calcium channel blockers (po) | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐13.23 [‐43.36, 16.91] |
| 26.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐31.90 [‐64.73, 0.93] |
| 26.9 Nitric oxide donor | 7 | 4183 | Mean Difference (IV, Random, 95% CI) | ‐12.10 [‐19.06, ‐5.14] |
| 26.10 Low BP target | 3 | 7304 | Mean Difference (IV, Random, 95% CI) | ‐10.04 [‐12.47, ‐7.61] |
| 27 SBP, at day 7 Show forest plot | 11 | 15151 | Mean Difference (IV, Random, 95% CI) | ‐6.74 [‐9.39, ‐4.10] |
| 27.1 ACE inhibitors | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐26.0 [‐43.00, ‐7.00] |
| 27.2 ARA(po) | 4 | 3747 | Mean Difference (IV, Random, 95% CI) | ‐5.74 [‐7.44, ‐4.03] |
| 27.3 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐15.0 [‐34.25, 4.25] |
| 27.4 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.63, 0.31] |
| 27.5 Low BP target | 3 | 7304 | Mean Difference (IV, Random, 95% CI) | ‐7.62 [‐11.69, ‐3.56] |
| 28 SBP, at end of treatment Show forest plot | 20 | 15684 | Mean Difference (IV, Random, 95% CI) | ‐8.02 [‐10.07, ‐5.97] |
| 28.1 ACE inhibitors (po) | 4 | 123 | Mean Difference (IV, Random, 95% CI) | ‐17.37 [‐23.42, ‐11.31] |
| 28.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐12.20, 9.20] |
| 28.3 ARA (po) | 5 | 3785 | Mean Difference (IV, Random, 95% CI) | ‐7.37 [‐10.19, ‐4.56] |
| 28.4 A2AA (po) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 28.5 Beta‐blockers (po) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐3.20 [‐13.17, 6.77] |
| 28.6 Beta‐blockers (iv) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐13.00 [‐25.21, ‐4.79] |
| 28.7 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐17.12, 1.52] |
| 28.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐9.20 [‐25.73, 7.33] |
| 28.9 Nitric oxide donor | 4 | 4101 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐2.50, 0.39] |
| 28.10 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 3.0 [‐11.74, 17.74] |
| 28.11 Low BP target | 5 | 7421 | Mean Difference (IV, Random, 95% CI) | ‐10.06 [‐13.58, ‐6.55] |
| 29 DBP, first after randomisation by intervention Show forest plot | 17 | 12397 | Mean Difference (IV, Random, 95% CI) | ‐3.86 [‐5.07, ‐2.64] |
| 29.1 ACE inhibitors(po) | 5 | 123 | Mean Difference (IV, Random, 95% CI) | ‐4.23 [‐9.68, 1.21] |
| 29.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐5.50, 9.90] |
| 29.3 ARA (po) | 3 | 3408 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐4.35, ‐0.61] |
| 29.4 A2AA | 1 | 4 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐15.43, 11.23] |
| 29.5 Beta‐Blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐13.01, 2.21] |
| 29.6 Beta‐Blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐17.5 [‐25.32, ‐9.68] |
| 29.7 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐3.30 [‐9.28, 2.68] |
| 29.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐31.35, 5.55] |
| 29.9 Nitric oxide donor | 6 | 4173 | Mean Difference (IV, Random, 95% CI) | ‐3.39 [‐4.25, ‐2.52] |
| 29.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐6.43 [‐12.10, ‐0.75] |
| 30 DBP, first after randomisation by stroke type Show forest plot | 16 | 14952 | Mean Difference (IV, Random, 95% CI) | ‐3.73 [‐4.69, ‐2.76] |
| 30.1 Ischaemic stroke | 6 | 7500 | Mean Difference (IV, Random, 95% CI) | ‐3.53 [‐4.20, ‐2.87] |
| 30.2 Combined ischaemic stroke and Intracerebral haemorrhage | 10 | 6419 | Mean Difference (IV, Random, 95% CI) | ‐2.85 [‐4.26, ‐1.44] |
| 30.3 Intracerebral haemorrhage | 2 | 1033 | Mean Difference (IV, Random, 95% CI) | ‐6.80 [‐11.99, ‐1.60] |
| 31 DBP, first after randomisation by time to treatment Show forest plot | 16 | 10977 | Mean Difference (IV, Random, 95% CI) | ‐3.80 [‐5.06, ‐2.54] |
| 31.1 Ultra‐acute/prehospital | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 1.39 [‐7.71, 10.48] |
| 31.2 Hyper‐acute | 2 | 677 | Mean Difference (IV, Random, 95% CI) | ‐6.48 [‐12.55, ‐0.41] |
| 31.3 Acute | 6 | 10076 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.26, ‐1.97] |
| 31.4 Subacute | 7 | 169 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐7.98, 0.22] |
| 32 DBP, at day 1 Show forest plot | 16 | 11361 | Mean Difference (IV, Random, 95% CI) | ‐3.05 [‐4.20, ‐1.91] |
| 32.1 ACE inhibitors (po) | 4 | 96 | Mean Difference (IV, Random, 95% CI) | ‐3.24 [‐8.95, 2.47] |
| 32.2 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐9.70, 5.70] |
| 32.3 ARA (po) | 2 | 2368 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐3.47, 1.48] |
| 32.4 A2AA (po) | 1 | 4 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐26.77, 27.11] |
| 32.5 Beta‐blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐8.99, 6.99] |
| 32.6 Beta‐blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐13.21, 3.21] |
| 32.7 Calcium channel blockers (po) | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐6.10 [‐14.08, 1.89] |
| 32.8 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐12.90 [‐31.35, 5.55] |
| 32.9 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | ‐3.31 [‐4.17, ‐2.44] |
| 32.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐5.34 [‐9.02, ‐1.65] |
| 33 DBP, at day 7 Show forest plot | 10 | 12686 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐3.96, ‐1.83] |
| 33.1 ACE inhibitors | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐18.0 [‐33.10, ‐2.90] |
| 33.2 ARA(po) | 5 | 4152 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐3.24, ‐1.70] |
| 33.3 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.00, ‐0.20] |
| 33.4 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐16.00, 6.00] |
| 33.5 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐4.49 [‐6.18, ‐2.80] |
| 34 DBP, at end of treatment Show forest plot | 15 | 13050 | Mean Difference (IV, Random, 95% CI) | ‐3.95 [‐5.15, ‐2.75] |
| 34.1 ACE inhibitors (po) | 4 | 123 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐11.18, ‐0.95] |
| 34.2 ARA (po) | 6 | 4190 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐5.61, ‐1.61] |
| 34.3 ACE inhibitors (s/l) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐7.70, 7.70] |
| 34.4 Beta‐blockers (po) | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐7.48, 9.28] |
| 34.5 Beta‐Blockers (iv) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐16.40 [‐24.22, ‐8.58] |
| 34.6 Calcium channel blockers (po) | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐9.40 [‐15.50, ‐3.30] |
| 34.7 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐2.90 [‐21.35, 15.55] |
| 34.8 Nitric oxide donor | 1 | 4011 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.00, ‐0.20] |
| 34.9 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐13.03, 5.03] |
| 34.10 Low BP target | 2 | 4475 | Mean Difference (IV, Random, 95% CI) | ‐4.24 [‐5.62, ‐2.86] |
| 35 HR at baseline Show forest plot | 15 | 5841 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.86, 0.99] |
| 35.1 ACE inhibitors (po) | 3 | 61 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐5.31, 5.75] |
| 35.2 ARA(po) | 2 | 1379 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.57, 0.90] |
| 35.3 A2AA (po) | 1 | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 35.4 Calcium channel blockers (po) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐3.60 [‐9.47, 2.27] |
| 35.5 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐1.26, 5.77] |
| 35.6 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐10.50, 2.50] |
| 35.7 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 5.30 [‐4.21, 14.81] |
| 35.8 Low BP target | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐10.53, 4.53] |
| 36 HR, first after randomisation Show forest plot | 6 | 4196 | Mean Difference (IV, Random, 95% CI) | 2.74 [‐1.13, 6.61] |
| 36.1 ACE inhibitors (po) | 2 | 39 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐9.89, 8.72] |
| 36.2 ARA(po) | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.3 Calcium channel blockers (po) | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 1.28 [‐21.89, 24.46] |
| 36.4 A2AA (po) | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 36.5 Nitric oxide donor | 3 | 4061 | Mean Difference (IV, Random, 95% CI) | 3.95 [‐1.03, 8.93] |
| 36.6 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | 10.0 [0.71, 19.29] |
| 37 HR, at day 1 Show forest plot | 10 | 4333 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.06, 5.76] |
| 37.1 ACE inhibitors (po) | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐40.45, 40.51] |
| 37.2 Calcium channel blockers (po) | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.56 [‐7.71, 2.59] |
| 37.3 A2AA (po) | 1 | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 37.4 Nitric oxide donor | 7 | 4197 | Mean Difference (IV, Random, 95% CI) | 4.53 [1.46, 7.60] |
| 37.5 Calcium channel blockers (iv) | 1 | 11 | Mean Difference (IV, Random, 95% CI) | ‐16.90 [‐27.35, ‐6.45] |
| 38 HR, at day 7 Show forest plot | 4 | 5449 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.24, 1.19] |
| 38.1 Thiazide‐like diuretic (po) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.14, 6.14] |
| 38.2 ARA(po) | 1 | 1360 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.07, 1.27] |
| 38.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.20, 1.63] |
| 39 HR, at end of treatment Show forest plot | 4 | 4124 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.21, 1.61] |
| 39.1 ACE inhibitors | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐62.22, 67.02] |
| 39.2 Thiazide‐ like diuretic | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐6.14, 6.14] |
| 39.3 Nitric oxide donor | 2 | 4052 | Mean Difference (IV, Random, 95% CI) | 0.72 [‐0.20, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependency, end of trial by C/S Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 2 Death or dependency, end of trial by stroke type C/S Show forest plot | 2 | 2841 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 2.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.87, 1.28] |
| 2.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.48] |
| 2.3 Intracerebral haemorrhage | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.55, 1.65] |
| 3 Death or dependency, end of trial by time to treatment Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.91, 1.24] |
| 3.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.69, 2.61] |
| 3.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 4 Death early, by C/S Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.55, 2.00] |
| 5 Death early, by stroke type C/S Show forest plot | 2 | 2841 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.56, 1.87] |
| 5.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.37 [0.79, 2.36] |
| 5.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.98] |
| 5.3 Intracerebral haemorrhage | 1 | 246 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.09, 2.92] |
| 6 Death early, by time to treatment C/S Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.60, 1.93] |
| 6.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 5.68 [0.27, 120.37] |
| 6.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.61, 1.76] |
| 7 Death, end of trial by C/S Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.92, 1.43] |
| 8 Death end of trial, by stroke type C/S Show forest plot | 2 | 2839 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.47] |
| 8.1 Ischaemic stroke | 1 | 1832 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.96, 1.61] |
| 8.2 Combined ischaemic stroke and intracerebral haemorrhage | 1 | 763 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.67, 1.91] |
| 8.3 Intracerebral haemorrhage | 1 | 244 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.45, 1.71] |
| 9 Death, end of trial by time to treatment C/S Show forest plot | 2 | 2860 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.92, 1.42] |
| 9.1 Ultra‐acute | 1 | 143 | Odds Ratio (M‐H, Random, 95% CI) | 1.87 [0.68, 5.15] |
| 9.2 Acute | 2 | 2717 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.89, 1.40] |
| 10 Barthel Index, end of trial, by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐5.80, ‐0.55] |
| 11 Early neurological deterioration, by C/S Show forest plot | 1 | 2097 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.82] |
| 12 Quality of life (EuroQol) at end of trial, by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 13 Length of stay, by C/S Show forest plot | 1 | 2097 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.87, 3.27] |
| 14 SBP, first after randomisation, by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.75, ‐1.46] |
| 15 SBP, at day 1 by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐3.11 [‐4.75, ‐1.46] |
| 16 SBP, at end of treatment by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐11.30 [‐15.20, ‐7.40] |
| 17 DBP, at baseline by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.65, 0.27] |
| 18 DBP at day 1, by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.24, ‐0.08] |
| 19 DBP, at end of treatment by C/S Show forest plot | 2 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐9.28, ‐3.61] |
| 20 HR, at day 1 Show forest plot | 1 | 2097 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.70, 1.90] |
| 21 HR, at end of treatment Show forest plot | 1 | 2097 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐4.51, ‐1.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death early, by intervention Show forest plot | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Death early, by stroke type Show forest plot | 1 | 15 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Ischaemic stroke | 1 | 15 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death end of trial, by intervention Show forest plot | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Phenylephrine (iv) | 1 | 15 | Odds Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 SBP, at baseline Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐27.5 [‐50.83, ‐4.17] |
| 4.1 Phenylephrine (iv) | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐27.5 [‐50.83, ‐4.17] |
| 5 SBP, first after randomisation Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 20.60 [‐13.31, 54.51] |
| 6 SBP, at day 1 Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 20.60 [‐13.31, 54.51] |
| 7 DBP, at baseline Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐8.30 [‐19.13, 2.53] |
| 8 DBP, first after randomisation Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐14.86, 15.86] |
| 9 DBP, at day 1 Show forest plot | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐14.86, 15.86] |

