암 환자의 진균감염증 관리를 위한 통상적인 항균제 투여와 선별적 항균제 투여 비교
Appendices
Appendix 1. PubMed search strategy
#1: random* OR control* OR blind*
#2: nystatin OR amphotericin OR fluconazol* OR itraconazol* OR ketoconazol* OR miconazol* OR voriconazol*
#3: bone‐marrow OR cancer* OR fungemia OR hematologic* OR malignan* OR neoplas* OR neutropeni* OR granulocytopeni* OR leukemi* OR lymphom*
#4: #1 AND #2 AND #3.
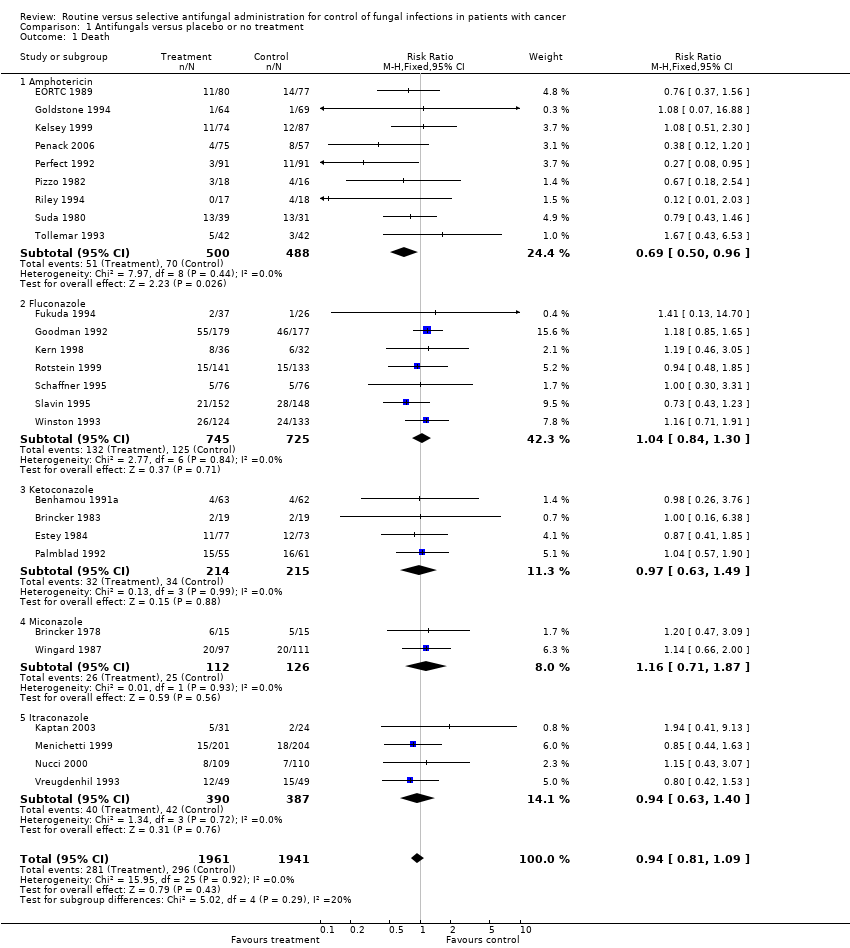
Comparison 1 Antifungals versus placebo or no treatment, Outcome 1 Death.

Comparison 1 Antifungals versus placebo or no treatment, Outcome 2 Death related to fungal infection.

Comparison 1 Antifungals versus placebo or no treatment, Outcome 3 Invasive infections.

Comparison 1 Antifungals versus placebo or no treatment, Outcome 4 Colonisation.
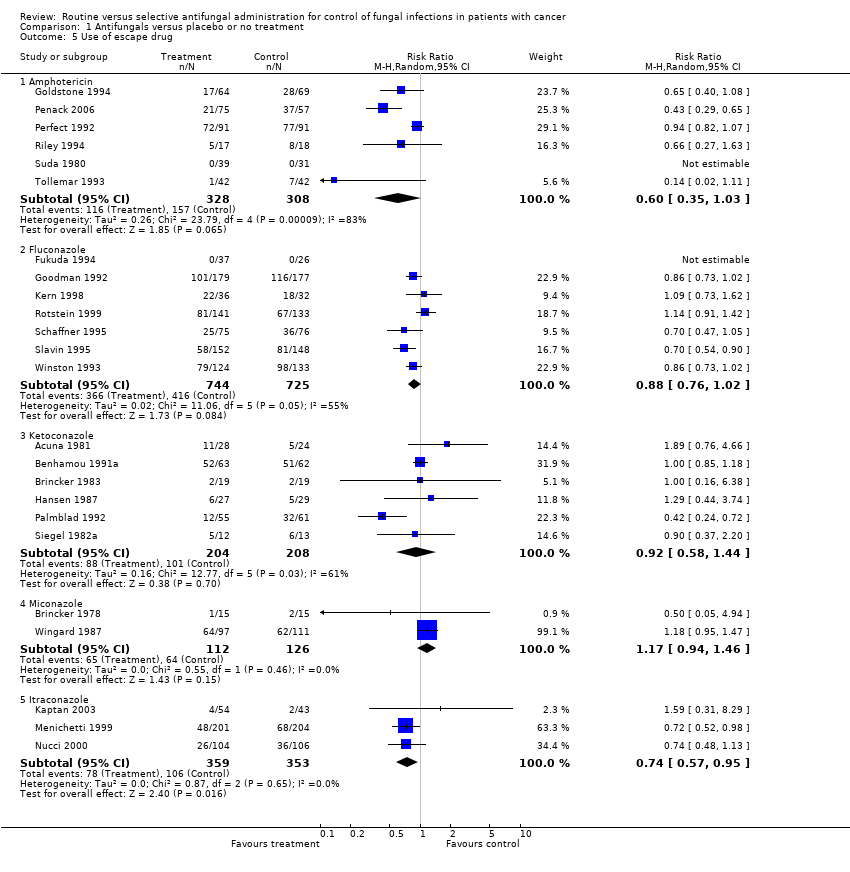
Comparison 1 Antifungals versus placebo or no treatment, Outcome 5 Use of escape drug.
| Trial | Trial drug | Number of patients | Harms |
| EORTC 1989 | amphotericin B | 80 versus 77 | Treatment discontinuations: 6 pts on trial drug (infusion‐related toxicity, allergic reactions); Nephrotoxicity: 8 versus 3, none required dialysis and renal function restored later; Hypokalaemia: 33 versus 16 |
| Goldstone 1994 | amphotericin B | 64 versus 69 | Treatment discontinuations: 4 on trial drug (chills, rigour, hypotension, rash and bronchospasm); Elevated liver function tests: 26 versus 32; Nephrotoxicity: 1 on trial drug that did not require withdrawal of therapy |
| Kelsey 1999 | amphotericin B | 74 versus 87 | Treatment discontinuations: 5 on trial drug, 1 on placebo because of immediate reactions; Nephrotoxicity: 9 versus 6; Hypokalaemia: 1 versus 0; Clinical adverse events were very similar in the two groups |
| Penack 2006. | amphotericin B | 75 versus 57 | Treatment discontinuations: 2 (1 skin rash, 1 chills) versus 0; Other harms: "no differences in ... liver function tests, renal function parameters and hypokalaemia" |
| Perfect 1992 | amphotericin B | 91 versus 91 | More infusion‐related harms on active drug, but no data provided; No significant differences in renal function, hepatic enzymes or electrolytes (no data provided) |
| Pizzo 1982 | amphotericin B | 18 versus 16 | Treatment discontinuations: none; Rash: 2 versus 1; Azotaemia: 1 versus 0; liver enzyme elevations: 2 versus 3; Electrolyte abnormalities: 18 versus 16 |
| Riley 1994 | amphotericin B | 17 versus 18 | Treatment discontinuations: none; Renal function: no difference (P value 0.82 for blood urea, P value 0.63 for creatinine); Potassium supplements: no difference; Infusion reactions: none |
| Suda 1980 | amphotericin B | 39 versus 31 | Article is in Japanese |
| Tollemar 1993 | amphotericin B | 42 versus 42 | Treatment discontinuations: 4 versus 0 for infusion reactions; Potassium supplementation: no difference; Renal function: no useful data, but only small changes reported, compared to normal ranges |
| Fukuda 1994 | fluconazole | 37 versus 26 | Article is in Japanese |
| Goodman 1992 | fluconazole | 179 versus 177 | Treatment discontinuations: 1 on trial drug, 2 on placebo for clinical side effects, and 17 versus 11 for elevated liver function tests; in 7 versus 3, hepatic dysfunction contributed to death; Graft‐versus‐host disease or organ failure: 44 versus 24 deaths; Nausea: 13 versus 9; Skin rash: 9 versus 9; Eosinophilia in 6 versus 0 |
| Kern 1998 | fluconazole | 36 versus 32 | Bacteriaemia: 15 versus 7; Other harms similar: 5 versus 6 elevations in transaminases, 19 versus 17 nausea or vomiting, 3 versus 0 allergy |
| Rotstein 1999 | fluconazole | 141 versus 133 | Treatment discontinuations: none reported; Elevated liver enzymes: 17 versus 19; Rash: 51 versus 59; Nausea: 106 versus 95; Vomiting: 68 versus 85 |
| Schaffner 1995 | fluconazole | 76 versus 76 episodes | Treatment discontinuations: none reported; No data on harms ("no significant differences were found") |
| Slavin 1995 | fluconazole | 152 versus 148 | Treatment discontinuations: 32 versus 31 for abnormal liver function; Graft‐versus‐host disease: 102 versus 85; Nausea: 33 versus 22; Seizures: 8 versus 9; Liver enzymes: no differences |
| Winston 1993 | fluconazole | 124 versus 133 | Treatment discontinuations: none reported; Elevated liver enzymes: 25 versus 14; Nausea and vomiting: 9 versus 5; Rash: 13 versus 7; Other harms were similarly distributed |
| Yamac 1995 | fluconazole | 41 versus 29 | Treatment discontinuations: none reported; Other harms: no data |
| Acuna 1981 | ketoconazole | 28 versus 24 | No data |
| Benhamou 1991 | ketoconazole | 63 versus 62 | Treatment discontinuations: 38 versus 14 (among them 2 patients with veno‐occlusive disease, 1 hepatitis, 3 skin rash in active group; none in placebo group); Severe gastrointestinal intolerance: 4 versus 7; Three‐fold greater values than normal for transaminases: 13 versus 8 |
| Brincker 1983 | ketoconazole | 19 versus 19 | One patient on trial drug stopped treatment because of universal exanthema |
| Estey 1984 | ketoconazole | 77 versus 73 | Bacterial infections: 33 versus 24 |
| Hansen 1987 | ketoconazole | 27 versus 29 | Treatment discontinuations: 2 on trial drug (1 skin rash and 1 elevated liver function tests); Bacterial infections: 20% versus 15% of neutropenic episodes |
| Hughes 1983 | ketoconazole | 42 versus 22 | Treatment discontinuations:1 on trial drug (nausea and anorexia); Other harms: 2 nausea and anorexia, 1 abdominal pain, 1 transient rash, all on trial drug |
| Palmblad 1992 | ketoconazole | 55 versus 61 | Treatment discontinuations: 2 (elevated transaminases) versus 6 (1 elevated transaminases, 5 exanthema); Bacteriaemias: 37 versus 21 |
| Siegel 1982a | ketoconazole | 12 versus 13 | No data |
| Brincker 1978 | miconazole | 15 versus 15 | None ascribable to trial drug |
| Wingard 1987 | miconazole | 97 versus 111 | Treatment discontinuations: 1 (pruritis and flushing) versus 2 (1 rash, 1 nausea); Severe hypotension: 2 on trial drug |
| Caselli 1990 | itraconazole, amphotericin B, ketoconazole | 30 versus 10 | No data |
| Kaptan 2003 | itraconazole | 31 versus 24 | Treatment discontinuations: 2 on trial drug (cardiac arrhytmia and gastric irritation); "there was a clinical impression that hypokalaemia occurred at a greater rate in patients with itraconazole" |
| Menichetti 1999 | itraconazole | 201 versus 204 | Treatment discontinuations: 37 versus 27; Bacteriaemia: 47 versus 31; Elevated transaminases: 5 versus 3 |
| Nucci 2000 | itraconazole | 104 versus 106 | Treatment discontinuations: 3 versus 4; Skin rash: 3 versus 1; Elevated liver enzymes: 3 versus 4; Nausea: 2 on placebo |
| Vreugdenhil 1993 | itraconazole | 49 versus 49 | Treatment discontinuations: 1 (nausea) versus 1 (liver function deterioration); Liver function deterioration: 28 versus 22 episodes; Renal function deterioration: 4 versus 2 episodes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 26 | 3902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.09] |
| 1.1 Amphotericin | 9 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.96] |
| 1.2 Fluconazole | 7 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.30] |
| 1.3 Ketoconazole | 4 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 1.4 Miconazole | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.71, 1.87] |
| 1.5 Itraconazole | 4 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.63, 1.40] |
| 2 Death related to fungal infection Show forest plot | 23 | 3490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 2.1 Amphotericin | 9 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.26, 0.76] |
| 2.2 Fluconazole | 6 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.73] |
| 2.3 Ketoconazole | 3 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.55, 4.04] |
| 2.4 Miconazole | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.33] |
| 2.5 Itraconazole | 4 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.31, 1.56] |
| 3 Invasive infections Show forest plot | 30 | 4044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.39, 0.64] |
| 3.1 Amphotericin | 8 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.24, 0.73] |
| 3.2 Fluconazole | 8 | 1539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
| 3.3 Ketoconazole | 7 | 562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.68, 2.54] |
| 3.4 Miconazole | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.31] |
| 3.5 Itraconazole | 4 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.29, 0.97] |
| 3.6 Itraconazole/ketoconazole/amphotericin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Colonisation Show forest plot | 22 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Amphotericin | 3 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.33, 0.77] |
| 4.2 Fluconazole | 6 | 1393 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.33, 0.90] |
| 4.3 Ketoconazole | 8 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.51, 0.87] |
| 4.4 Miconazole | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.37, 2.24] |
| 4.5 Itraconazole | 2 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.45] |
| 4.6 Itraconazole/ketoconazole/amphotericin | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.31, 1.04] |
| 5 Use of escape drug Show forest plot | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Amphotericin | 6 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.03] |
| 5.2 Fluconazole | 7 | 1469 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
| 5.3 Ketoconazole | 6 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.58, 1.44] |
| 5.4 Miconazole | 2 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.94, 1.46] |
| 5.5 Itraconazole | 3 | 712 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.57, 0.95] |

