Exercise‐based cardiac rehabilitation for adults with stable angina
Abstract
Background
A previous Cochrane review has shown that exercise‐based cardiac rehabilitation (CR) can benefit myocardial infarction and post‐revascularisation patients. However, the impact on stable angina remains unclear and guidance is inconsistent. Whilst recommended in the guidelines of American College of Cardiology/American Heart Association and the European Society of Cardiology, in the UK the National Institute for Health and Care Excellence (NICE) states that there is "no evidence to suggest that CR is clinically or cost‐effective for managing stable angina".
Objectives
To assess the effects of exercise‐based CR compared to usual care for adults with stable angina.
Search methods
We updated searches from the previous Cochrane review 'Exercise‐based cardiac rehabilitation for patients with coronary heart disease' by searching the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, DARE, CINAHL and Web of Science on 2 October 2017. We searched two trials registers, and performed reference checking and forward‐citation searching of all primary studies and review articles, to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) with a follow‐up period of at least six months, which compared structured exercise‐based CR with usual care for people with stable angina.
Data collection and analysis
Two review authors independently assessed the risk of bias and extracted data according to the Cochrane Handbook for Systematic Reviews of Interventions. Two review authors also independently assessed the quality of the evidence using GRADE principles and we presented this information in a 'Summary of findings' table.
Main results
Seven studies (581 participants) met our inclusion criteria. Trials had an intervention length of 6 weeks to 12 months and follow‐up length of 6 to 12 months. The comparison group in all trials was usual care (without any form of structured exercise training or advice) or a no‐exercise comparator. The mean age of participants within the trials ranged from 50 to 66 years, the majority of participants being male (range: 74% to 100%). In terms of risk of bias, the majority of studies were unclear about their generation of the randomisation sequence and concealment processes. One study was at high risk of detection bias as it did not blind its participants or outcome assessors, and two studies had a high risk of attrition bias due to the numbers of participants lost to follow‐up. Two trials were at high risk of outcome reporting bias. Given the high risk of bias, small number of trials and participants, and concerns about applicability, we downgraded our assessments of the quality of the evidence using the GRADE tool.
Due to the very low‐quality of the evidence base, we are uncertain about the effect of exercise‐based CR on all‐cause mortality (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.18 to 5.67; 195 participants; 3 studies; very low‐quality evidence), acute myocardial infarction (RR 0.33, 95% CI 0.07 to 1.63; 254 participants; 3 studies; very low‐quality evidence) and cardiovascular‐related hospital admissions (RR 0.14, 95% CI 0.02 to 1.1; 101 participants; 1 study; very low‐quality evidence). We found low‐quality evidence that exercise‐based CR may result in a small improvement in exercise capacity compared to control (standardised mean difference (SMD) 0.45, 95% CI 0.20 to 0.70; 267 participants; 5 studies, low‐quality evidence). We were unable to draw conclusions about the impact of exercise‐based CR on quality of life (angina frequency and emotional health‐related quality‐of‐life score) and CR‐related adverse events (e.g. skeletomuscular injury, cardiac arrhythmia), due to the very low quality of evidence. No data were reported on return to work.
Authors' conclusions
Due to the small number of trials and their small size, potential risk of bias and concerns about imprecision and lack of applicability, we are uncertain of the effects of exercise‐based CR compared to control on mortality, morbidity, cardiovascular hospital admissions, adverse events, return to work and health‐related quality of life in people with stable angina. Low‐quality evidence indicates that exercise‐based CR may result in a small increase in exercise capacity compared to usual care. High‐quality, well‐reported randomised trials are needed to assess the benefits and harms of exercise‐based CR for adults with stable angina. Such trials need to collect patient‐relevant outcomes, including clinical events and health‐related quality of life. They should also assess cost‐effectiveness, and recruit participants that are reflective of the real‐world population of people with angina.
PICOs
Plain language summary
Exercise‐based cardiac rehabilitation for people with stable angina
Review question
Is exercise‐based cardiac rehabilitation for people with stable angina helpful in improving their condition?
Background
Stable angina is a form of chronic heart disease associated with ill health and increased death rates. Exercise‐based cardiac rehabilitation is a programme that helps people with heart disease gain better health. It usually involves exercising and receiving advice on ways to improve health and takes place at hospitals or within the community or at home. The National Institute for Health and Care Excellence in the United Kingdom does not currently recommend cardiac rehabilitation programmes for people with angina, while European and United States guidelines do. In this review, we look at whether cardiac rehabilitation is helpful to people with stable angina. Specifically we assess whether cardiac rehabilitation is helpful in reducing death rates, the need for surgery, repeated heart attacks, healthcare usage and costs; and improving quality of life, physical fitness levels, and symptoms of angina.
Study characteristics
The evidence is current to 2 October 2017. We included seven studies that randomly allocated a total of 581 participants with stable angina to either receive cardiac rehabilitation or no exercise control. We identified that there are no ongoing randomised studies. The average age of participants ranged from 50 to 66 years. The majority of people recruited were middle‐aged men. Most studies were carried out in European countries and one study in India. Cardiac rehabilitation was most commonly delivered in a combined setting of home and centre or hospital. The length of the cardiac rehabilitation programmes ranged from six weeks to one year.
Key results
There is insufficient evidence to assess the impact of exercise‐based cardiac rehabilitation on the outcomes that matter most to patients: risks of death, heart attack, or future cardiac operation and quality of life. There may be a small improvement in physical fitness following exercise‐based cardiac rehabilitation compared to usual treatment. There was no evidence about returning to work.
Quality of the evidence
Due to the poor reporting, high risk of bias and small number of trials and participants included in this review, our assessment of the quality of the evidence ranged from low to very low across outcomes. For low‐quality evidence our confidence in the result is limited, and for very low‐quality evidence we have very little confidence in the result.
Conclusions
We need more high‐quality studies in more representative populations of people with stable angina. These studies should collect outcomes of relevance to patients and healthcare decision‐makers. Then we will be able to better assess the impact of exercise‐based cardiac rehabilitation.
Authors' conclusions
Summary of findings
| Exercise‐based cardiac rehabilitation (CR) compared to usual care for patients with stable angina | ||||||
| Patient or population: adults with stable angina | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise‐based cardiac rehabilitation | |||||
| All‐cause mortality Follow‐up: 12 months | Study population | RR 1.01 | 195 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on all‐cause mortality compared to usual care. | |
| 20 per 1,000 | 21 per 1,000 | |||||
| Acute myocardial infarction (AMI) Follow‐up: 12 months | Study population | RR 0.33 | 254 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on AMI compared to usual care. | |
| 39 per 1,000 | 13 per 1,000 | |||||
| Exercise capacity (assessed using a variety of outcomes including VO2 max and duration of exercise) Follow‐up: range 6 to 12 months | The mean exercise capacity in the intervention groups was 0.45 standard deviations higher | 267 | ⊕⊕⊝⊝ | Using Cohen's rule of thumb a SMD of 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect between groups (Cohen 1988). Exercise‐based CR may slightly improve exercise capacity compared to usual care. | ||
| Cardiovascular‐related hospital admissions Follow‐up: 12 months | Study population | RR 0.14 (0.02 to 1.1) | 101 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on cardiovascular‐related hospital admissions compared to usual care. | |
| 140 per 1000 | 20 per 1000 (2 to 154) | |||||
| Health‐related quality of life (assessed with: Seattle Angina Questionnaire and The MacNew Questionnaire) | One study showed improvement in emotional score at 6‐week follow up, and benefits in angina frequency and social HRQL score at 6 months follow‐up. | Not estimable | 94 (1 RCT) | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on quality of life compared to usual care. | |
| Return to work | No studies were found that looked at return to work. | |||||
| Adverse events (e.g. skeletomuscular injury) Follow‐up: 12 months | Only one study looked at adverse events and reported that there were no adverse events during the exercise‐based CR. | Not estimable | 101 (1 RCT) | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on adverse events compared to usual care. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some concerns with random sequence generation, allocation concealment, blinding of outcome assessment and selective reporting; bias likely, therefore quality of evidence downgraded by one level 2 Some concern with applicability to review question as participants in all studies were limited to middle‐aged men, therefore quality of evidence downgraded by one level 3 Imprecise due to small number of participants (less than 300) and confidence intervals including potential for important harm or benefit as 95% CI crosses RR of 0.75 and 1.25, therefore quality of evidence downgraded by two levels 4 Some concerns with random sequence generation, allocation concealment, blinding of outcome assessment, selective reporting and unbalanced groups at baseline; bias likely, therefore quality of evidence downgraded by one level 5 Some concern with random sequence generation, allocation concealment, blinding of outcome assessment, high loss to follow‐up, selective reporting and unbalanced groups at baseline; serious bias likely, therefore quality of evidence downgraded by two levels 6 Imprecise due to small number of participants (less than 300) therefore quality of evidence downgraded by one level 7 Some concerns with random sequence generation, allocation concealment and selective reporting; bias likely, therefore quality of evidence downgraded by one level 8 Some concerns with blinding of outcome assessment, selective reporting and groups not receiving comparable care; bias likely, therefore quality of evidence downgraded by one level 9 Imprecise due to very small number of participants therefore quality of evidence downgraded by two levels | ||||||
Background
Description of the condition
Angina pectoris is traditionally defined as a pain, discomfort or tightness, most commonly felt in the chest, that may radiate to the neck, jaw and arms. It is typically reproducible, gradual in onset and offset and may be associated with breathlessness and nausea. Angina occurs when the coronary arteries become narrowed and myocardial oxygen demand exceeds oxygen supply. This leads to reversible myocardial ischaemia or hypoxia, particularly when oxygen demands are high, such as during exercise and stress. The complex mechanisms leading to symptoms of angina are not entirely understood. Importantly, acidosis results from myocardial ischaemia, causing the release of metabolites such as adenosine and bradykinin that stimulate the sympathetic afferent nerve pathway, eventually transmitting the painful stimuli to the brain (Crea 1990; Foreman 1999).
It was estimated that in 2013 over 1.3 million people in the UK had angina (BHF 2014) and it was thought to affect approximately 112 million people, or 1.6% of the population worldwide (Vos 2012). Data suggest an annual incidence of uncomplicated angina of 1.0% in western men aged 45 to 65 years, with a slightly higher incidence in women in this age bracket (Hemingway 2006; NHLBI 2012). Incidence increases with age in both men and women aged 75 to 84 years, reaching almost 4% (Hemingway 2006). However, age standardised angina prevalence decreased globally from 21.9 to 20.3 per 100,000 in males and from 17.7 to 15.9 in females between 1990 and 2010 (Moran 2014).
Angina is considered stable when there is no increase in frequency or severity of symptoms (NICE 2011). However, the transition from stable to unstable angina, is in reality, a continuum and without clear boundaries (Montalescot 2013). We define stable angina in this review as chest pain and associated symptoms precipitated by activity (e.g. running, walking) with minimal or non‐existent symptoms at rest. We define unstable angina as chest pain and other symptoms of cardiovascular disease which are of new onset (previous four to six weeks), worsening, becoming more frequent and/or occurring at rest or minimal exertion. Despite the term "stable", a diagnosis of stable angina is a chronic medical condition associated with a low but appreciable incidence of acute coronary events and increased mortality. Management options include lifestyle advice, drug therapy and revascularisation, which aim to minimise symptoms, and improve quality of life and long‐term morbidity and mortality.
Although it can be precipitated by a number of conditions, stable angina is considered to be a symptom of coronary heart disease (CHD), which is the single most common cause of global mortality, and accounts for approximately one‐third of all deaths worldwide, placing a major economic and resource burden on healthcare systems (WHO 2014).
Description of the intervention
As previously described (Anderson 2016), many definitions of cardiac rehabilitation (CR) have been proposed (for example, BACPR 2012; Balady 2011; WHO 1993).The following definition encompasses the key concepts of CR: "The coordinated sum of activities required to influence favourably the underlying cause of cardiovascular disease, as well as to provide the best possible physical, mental and social conditions, so that the patients may, by their own efforts, preserve or resume optimal functioning in their community and through improved health behaviour, slow or reverse progression of disease" (BACPR 2012). A complex intervention that may involve a variety of therapies, CR includes exercise, risk factor education, behaviour change, psychological support, and strategies that are aimed at targeting traditional risk factors for cardiovascular disease. Cardiac rehabilitation is an essential part of contemporary heart disease care and is considered a priority in countries with a high prevalence of CHD. Based on evidence from previous meta‐analyses and systematic reviews, exercise‐based CR following a cardiac event is a Class I recommendation from the American College of Cardiology/American Heart Association (Fihn 2012; Smith 2011) and the European Society of Cardiology (Montalescot 2013). Service provision, though predominantly hospital‐based, varies markedly, and referral, enrolment and completion are sub‐optimal, especially among women and older people (Beswick 2004; Clark 2012). Home‐based CR programmes have been increasingly introduced to widen access and participation (Taylor 2010), and interventions aimed at improving patient uptake and adherence to CR programmes have been adopted (Karmali 2014).
Exercise‐based CR in selected patient groups is remarkably safe. An observational study of more than 25,000 participants who underwent CR following cardiac surgery, recent percutaneous coronary intervention (PCI) or with other coronary and non‐coronary conditions reported one cardiac event for 50,000 hours of exercise training, equivalent to 1.3 cardiac arrests per million patient‐hours (Pavy 2006). An earlier study reported one case of ventricular fibrillation per 111,996 patient‐hours of exercise and one myocardial infarction (MI) per 294,118 patient‐hours (Van Camp 1986). However, people with unstable angina, uncontrolled ventricular arrhythmia, and severe heart failure (New York Heart Association level 4) have been considered at high risk, and careful assessment by an experienced clinician is recommended before they engage in the exercise component of CR (BACPR 2012). Historically, CR has often not been routinely offered to people with stable angina. Indeed, 20% of all CR programmes included in the 2009 UK national audit of CR, actively excluded stable angina (Lewin 2010). In the 2016 National Audit for Cardiac Rehabilitation Annual Report, it was outlined that angina referrals accounted for less than 4% of the 79,442 people receiving CR, although 24% of all participants were reported as having co‐morbid angina at the point of entry to their CR programme (NACR 2016).
How the intervention might work
The precise mechanisms by which CR improves mortality in people with CHD has not been fully elucidated. Exercise training has been shown to have direct benefits on the heart and coronary vasculature, including myocardial oxygen demand, endothelial function, autonomic tone, coagulation and clotting factors, inflammatory markers, and the development of coronary collateral vessels (Clausen 1976; Hambrecht 2000; Lavie 2015). However, it has been suggested that approximately half of the 28% reduction in cardiac mortality in people with CHD may also be mediated via the indirect effects of exercise through improvements in the risk factors for atherosclerotic disease (i.e. total cholesterol, smoking and blood pressure) (Taylor 2006). Further reductions in mortality may be attributed to reductions in psychological stress, including depression, anxiety and hostility (Lavie 2011).
Why it is important to do this review
The American College of Cardiology/American Heart Association give a Class I recommendation that medically supervised CR programmes and physician‐directed, home‐based programs are offered to at‐risk people with stable CHD including those with stable angina, at first diagnosis (Fihn 2012). Similarly, the European Society of Cardiology recommends that people with stable CHD, including stable angina, should undergo "moderate‐to‐vigorous intensity aerobic exercise training ≥ 3 times a week and for 30 min per session" (Montalescot 2013). The British Association for Cardiovascular Prevention and Rehabilitation (BACPR), recommend CR for people following a cardiac event, with heart failure, and to those with other established forms of cardiovascular disease, including stable angina (BACPR 2012).
Despite these guidelines, the current National Institute for Health and Care Excellence (NICE) guideline for the management of stable angina (CG126) states that there is "no evidence to suggest that CR is clinically or cost‐effective for managing stable angina" (NICE 2011). NICE report that while there has been limited research on short‐term outcomes such as a change in diet or exercise levels, the effect on morbidity and mortality has not been studied, and they highlight research into CR for this patient population as one of their key research recommendations (NICE 2011).
Previous Cochrane reviews have looked at the effect of exercise‐based CR in people with CHD (Anderson 2016), heart failure (Taylor 2014) and after heart valve surgery (Sibilitz 2016). A meta‐analysis of 63 trials, which randomised 14,486 people with CHD (including those with angina) to exercise‐based CR or a no‐exercise control, showed that exercise‐based CR led to a reduction in cardiovascular mortality (risk ratio (RR) 0.74, 95% CI 0.64 to 0.86), hospital admissions (RR 0.82, 95% CI 0.70 to 0.96) and health‐related quality of life (Anderson 2016). However, many trials in this review were in a mixed population of people with CHD (Anderson 2016). Given the NICE key research recommendations, we believe there is a good case for separating out the evidence for CR in stable angina. Our scoping searches have confirmed that no systematic review has been previously conducted which has specifically assessed the impact of CR in a population in people with stable angina.
Objectives
To assess the effects of exercise‐based cardiac rehabilitation compared to usual care for adults with stable angina.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) (individual patient allocation, cluster allocation, or cross‐over) which compared exercise‐based CR with a usual care or a no exercise comparator. We only included RCTs with a follow‐up period of at least six months, in order to reflect current practice of guideline‐ and policy‐writing, which is driven by long‐term health benefits. Where studies had mixed populations, we included those in which 50% or more of participants had stable angina.
Types of participants
We included adult men and women aged 18 years or older who had been diagnosed with coronary heart disease (CHD) and had stable angina. We included participants who presented with stable or exertional angina (effort‐induced chest discomfort), who were being treated with medical anti‐anginal therapy and who may have had a previous myocardial infarction (MI), coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI). However, we excluded people in the immediate period following such an event, i.e. within three months of MI, PCI or CABG. We also excluded people with unstable angina (pain at rest) and those with refractory angina for whom revascularisation was planned.
We included studies with a mixed population of people with CHD, where the data for people with stable angina and without any confounding co‐morbidities were reported separately. We also included studies where the majority of the participant sample were reported to have stable angina, regardless of whether data for this sub‐population was reported separately.
Types of interventions
We sought exercise‐based CR interventions either alone or as an element of a comprehensive CR programme including components such as health education, behavioural and psychological interventions or surgery in addition to the exercise intervention. The exercise‐based intervention could have been supervised or unsupervised, and conducted in a hospital, community or home‐based environment.
The control group received usual care which could have included standard medical care (such as drug therapy, health education, behavioural or psychological interventions, or surgery) but without any structured exercise training or advice on structured exercise training.
Types of outcome measures
We were interested in the following outcomes with a minimum follow‐up of six months.
Primary outcomes
-
All‐cause mortality
-
Morbidity (myocardial infarction (MI); revascularisation (CABG or PCI); or all‐cause hospital admissions)
-
Health‐related quality of life (HRQL) assessed using validated instruments (e.g. 36‐Item Short Form Health Survey (SF‐36), EQ‐5D)
-
Exercise capacity assessed by validated outcome measure (e.g. VO2peak, 6‐minute walk test)
-
Cardiovascular‐related hospital admissions
Secondary outcomes
-
Severity of angina, assessed using validated instruments (e.g. Canadian Cardiovascular Society grading of angina pectoris; New York Heart Association Functional Classification of Angina)
-
Reported adverse events (clinical events relating to CR, e.g. skeletomuscular injuries or arrhythmias, or withdrawal from the intervention)
-
Return to work
-
Costs
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases up to 9 September 2016 and then updated this with a further search up to 2 October 2017:
-
CENTRAL Issue 9 of 12, 2017 (Cochrane Library)
-
Database of Abstracts of Reviews of Effects (DARE) Issue 2 of 4, 2015 (last issue, now ceased publication) (Cochrane Library)
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 2 October 2017)
-
Embase (Ovid, 1980 to 2017 Week 40)
-
CINAHL Plus (EBSCO, 1937 to 2 October 2017)
-
Conference Proceedings Citation Index—Science (CPCI‐S) in Web of Science Core Collection (Thomson Reuters, 1990 to 2 October 2017).
The search strategies were designed with reference to those of a previous related systematic review of exercise‐based CR for CHD (Anderson 2016a). The preliminary search strategy for MEDLINE (Ovid) was adapted for use in the other databases (Appendix 1). We searched databases using a strategy combining selected MeSH terms and free‐text terms relating to exercise‐based rehabilitation and stable angina, with filters applied to limit to RCTs. We used the Cochrane sensitivity‐maximising RCT filter for MEDLINE, and applied terms recommended in the Cochrane Handbook for Systematic Reviews of Interventions for Embase (Lefebvre 2011). We applied adaptations of this filter to CINAHL and Web of Science. We translated the MEDLINE search strategy for use with the other databases using the appropriate controlled vocabulary as applicable.
We searched all databases from their inception to the present, imposed no restriction on language of publication and gave consideration to variations in terms used and spellings of terms in different countries so that the search strategy would not miss studies because of such variations.
Searching other resources
We hand‐searched reference lists, and conducted forward citation searching of all primary studies and review articles for additional references not identified by the electronic searches. We conducted a search of trial registers on 30 November 2016: World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; http://www.who.int/ictrp/en) and ClinicalTrials.gov (https://clinicaltrials.gov) for ongoing clinical trials. We also contacted experts in the field for unpublished and ongoing trials and contacted trial authors where necessary for any additional information. We also examined any relevant retraction statements and errata for included studies.
Data collection and analysis
Selection of studies
Two review authors (AD and LA) independently screened titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible, or potentially eligible/unclear) or 'do not retrieve'. If there were any disagreements, three additional authors (RST, JH and MG) were asked to arbitrate. We retrieved the full‐text study reports/publications and two review authors (AD and LA) independently screened the full‐texts and identified studies for inclusion, and recorded reasons for exclusion of the ineligible studies. If there were any disagreements, three review authors (RST, JH and MG) were asked to arbitrate. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of included studies table.
Data extraction and management
The study characteristics and outcome data from included studies were extracted independently by two reviewers (JH and shared between AD and LA), using a standardised data extraction form which had been piloted on at least one of the studies included in the review. Any disagreements were resolved by consensus or by consulting a third reviewer (RST). The following study characteristics were extracted.
-
Methods: study design, total duration of study, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, and co‐interventions.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
AD transferred data into the Review Manager (RevMan 2014) file and LL double‐checked that data were entered correctly by checking the study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
LL, JH and AD independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by consensus and decisions were independently checked by a third review author (RT). We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other (specifically sources of funding and conflicts of interest).
We also assessed two additional domains: whether the study groups were balanced at baseline, and if the study groups received comparable care (apart from the exercise component of the intervention). These criteria, agreed upon in advance by the review authors, have not been validated but have been used to assess quality in previous Cochrane reviews (Anderson 2016; Anderson 2016a; Brown 2011; Sibilitz 2016; Taylor 2014; Taylor 2015). We assessed these two domains as follows.
Groups balanced at baseline
-
Low risk of bias: the characteristics of the participants in the intervention and control groups at baseline are reported to be comparable or can be judged to be comparable (e.g. baseline data reported in a table in the study report) in terms of likely main prognostic factors.
-
Unclear risk of bias: whether the characteristics of the participants in the intervention and control groups are balanced at baseline is not reported, and reported information is inadequate to assess this (e.g. no table of baseline data in the study report).
-
High risk of bias: there is evidence of substantive imbalance in the baseline characteristics of the intervention and control groups with regard to likely major prognostic factors.
Groups received comparable treatment (except exercise)
-
Low risk of bias: all co‐interventions were delivered equally across intervention and control groups.
-
Unclear risk of bias: information to assess whether co‐interventions were delivered equally across groups was insufficient.
-
High risk of bias: the co‐interventions were not delivered equally across intervention and control groups.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it, if occurring, in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We analysed dichotomous data as a risk ratio (RR) with 95% confidence intervals (CIs) and continuous data as mean difference (MD) or standardised mean difference (SMD) with 95% CIs. For outcomes that were measured by studies in a variety of ways (for example exercise capacity), the SMD with 95% CIs was used as the summary statistic. We entered data presented as a scale with a consistent direction of effect.
Unit of analysis issues
In accordance with Section 16.4 of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), we included data from both periods of any cross‐over trials identified, assuming 1) there had been a wash‐out period considered long enough to reduce carry‐over, 2) no irreversible events such as mortality had occurred, and 3) appropriate statistical approaches had been used. If cluster trials had been included, consideration would have been given to whether the reported data analysis had appropriately taken account of the aggregate nature of the data.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (for example when a study was identified as abstract only). Where this was not possible, and the missing data were not thought to introduce serious bias, we explored the impact of including such studies on the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We explored heterogeneity amongst included studies qualitatively (by comparing the characteristics of included studies) and quantitatively (using the Chi2 test of heterogeneity and I2 statistic). We considered that an I2 between 50% and 90% may represent substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
If we had been able to pool more than 10 trials, we had intended to create and examine a funnel plot and use the Egger test (Egger 1997) to explore possible small‐study biases for the primary outcomes. However, this was not possible owing to the small number (n = 7) of included trials.
Data synthesis
We undertook a meta‐analyses only where this was meaningful, i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. Data from each study were pooled using a fixed‐effect model, except where substantial heterogeneity existed. If possible, we intended to pool the results for HRQL using a standardised mean difference. If there was evidence of substantial statistical heterogeneity (P value less than 0.10, I2 above 50%) associated with an effect estimate, we applied a random‐effects model, which provided a more conservative statistical comparison of the difference between intervention and control because a confidence interval around the effect estimate is wider than a confidence interval around a fixed‐effect estimate. If a statistically significant difference was still present using the random‐effects model, we also reported the fixed‐effect pooled estimate and 95% confidence interval because of the tendency of smaller trials, which are more susceptible to publication bias, to be over‐weighted with a random‐effects analysis (Heran 2008a; Heran 2008b).
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We completed data synthesis and analyses using Review Manager 5.3 software (RevMan 2014), and we planned to conduct a meta‐regression analysis using the 'metareg' command in Stata version 14.2 (StataCorp 2013).
'Summary of findings' table
Two reviewers (LL and RST) independently employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2011) to interpret result findings. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses and narrative summaries for the pre‐specified outcomes. Any discrepancies in judgements were resolved through discussion. One reviewer (LL) used GRADEpro GDT 2015 to import data from Review Manager to create a 'Summary of findings' table using the following pre‐specified outcomes: all‐cause mortality; myocardial infarction (MI); all‐cause hospital admissions; HRQL; return to work, exercise capacity and adverse events. We justified all decisions to downgrade the quality of evidence using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We anticipated length of follow‐up to be a driver of intervention effect, and therefore sought to stratify meta‐analysis of each outcome according to the length of trial duration, i.e. 'short‐term' follow‐up (6 to 12 months); 'medium‐term' follow‐up (13 to 36 months); and 'long‐term' follow‐up (more than 36 months). We also aimed to undertake univariate meta‐regression to explore heterogeneity and examine potential treatment effect modifiers. We sought to test the a priori hypotheses that there may be differences in the effect of exercise‐based CR on all‐cause mortality, morbidity, health related quality of life and exercise capacity across particular subgroups (Anderson 2016):
-
type of CR: exercise‐only CR versus comprehensive CR (categorical variable);
-
'dose' of exercise intervention (dose = number of weeks of exercise training x average number of sessions per week x average duration of session in minutes): dose 1000 units or more, versus dose less than 1000 units (continuous variable);
-
follow‐up period (continuous variable);
-
year of publication: pre‐1995 and post‐1995 (continuous variable)—timing reflects the introduction of modern‐day drug therapy for the management of CHD;
-
sample size (continuous variable);
-
setting: home‐ or centre‐based CR (categorical variable);
-
study location: continent (categorical variable);
-
mean age of participants (continuous variable);
-
percentage of male participants (continuous variable); and
-
percentage of post‐MI participants (continuous variable).
We sought to extract results of subgroup analyses, including participant‐level subgroup analyses, if reported by individual included studies, for example if a trial reports whether there was a difference in the effectiveness of CR between males and females. Given the anticipated small ratio of trials to covariates, we had anticipated a meta‐regression limited to univariate analysis (Higgins 2011). However, given the small number of trials (N = 7) included in this review, neither meta‐regression or a stratified meta‐analysis were deemed appropriate (Higgins 2011).
Sensitivity analysis
We had planned to compare meta‐analysis results including all studies versus only including those studies judged to have overall low risk of bias (low risk in four or more domains). We had also intended to conduct a sensitivity analysis excluding studies at high risk of bias and to produce funnel plots and tests of asymmetry to assess possible publication bias (Egger 1997). However, due to the small number of trials included in this review (N = 7) neither the sensitivity analysis or funnel plots were undertaken.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggested priorities for future research and outlined what the remaining uncertainties were in the area.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The electronic search yielded 3,841 titles and abstracts (3,585 for September 2016; 256 for October 2017 update). Following screening, 24 studies were evaluated for formal inclusion or exclusion by retrieving the full‐text publications. A total of seven randomised controlled trials (RCTs) were included for review. Backwards and forwards searching of the reference lists of the eligible publications did not detect any further publications for inclusion. One ongoing trial protocol was identified (NCT01147952). The study selection process is summarised in the flow diagram (Figure 1).
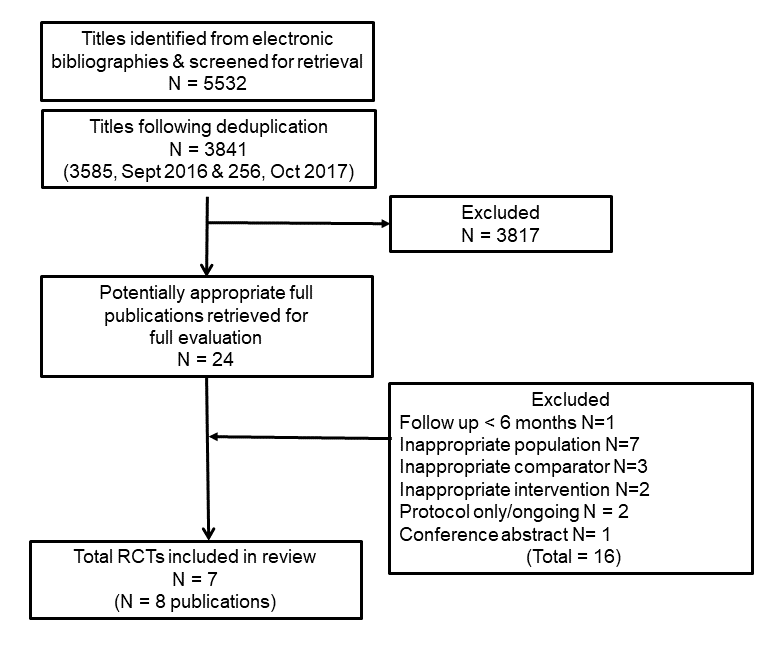
PRISMA flow diagram of trial selection
Included studies
Overall, we included seven trials (eight publications) (Devi 2014; Hambrecht 2004; Jiang 2007; Manchanda 2000; Raffo 1980; Schuler 1992; Todd 1991) with 581 participants. One trial (Schuler 1992) reported results from two publications in the same trial population. The original study (Schuler 1992) was included and analysed in this review. Although included, one trial provided no outcome data relevant to this review (Jiang 2007). Detailed study characteristics and risks of bias assessment for the seven included studies are provided in the Characteristics of included studies table.
Four studies (Devi 2014; Hambrecht 2004; Raffo 1980; Todd 1991) were exercise‐only intervention studies and three studies (Jiang 2007; Manchanda 2000; Schuler 1992) were comprehensive cardiac rehabilitation (CR), i.e. exercise plus education interventions. Five studies were performed in European countries, one in China (Jiang 2007) and one in India (Manchanda 2000). All studies were relatively small in size ranging from 24 to 113 participants with a median of 86 participants. The median intervention duration was 12 months (range: 6 weeks to 12 months). Mean age of participants within the trials ranged from 50 to 66 years. All studies recruited a majority of males (range: 74% to 100%), with four recruiting men only.
One study was based solely on a hospital‐based CR programme (Todd 1991). Three studies initiated CR in the hospital followed by home‐based delivery (Hambrecht 2004; Manchanda 2000; Raffo 1980). Two studies were entirely home‐based (Devi 2014; Jiang 2007) and used an on‐line exercise goal setting intervention. In all the included studies the primary mode of exercise was aerobic exercise ‐ typically walking and cycling. The dose of exercise varied considerably across studies, in terms of overall duration (range: 6 weeks to 12 months), frequency (daily), session length (range: 11 to 90 minutes per session) and intensity (defined as 'moderate intensity' or 70% to 75% maximal heart rate). Intervention adherence and fidelity were either poorly reported or not reported at all so we were not able to assess the actual amounts of exercise or other CR that the CR participants undertook. Six studies compared CR to usual care which included medication, education and advice about diet and risk factors. One trial (Hambrecht 2004) compared exercise training to percutaneous coronary intervention (PCI).
Excluded studies
Seventeen studies identified in the search were excluded for reasons listed in the Characteristics of excluded studies table. The most common reasons for exclusion were a failure to include the appropriate population of people with stable angina.
Risk of bias in included studies
Figure 2 and Figure 3 provide summaries of the risk of bias judgements presented as percentiles across all studies, and for each included study, respectively. We were unable to fully assess the potential for risk of bias in many instances due to there being insufficient details reported in the studies. The reporting of details tended to be poorer in studies published prior to 2000.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In five studies there was an unclear risk of bias in the method used to generate randomisation sequence (Hambrecht 2004; Manchanda 2000; Raffo 1980; Schuler 1992; Todd 1991), with all five studies reporting that the study was ‘randomised’ but not providing adequate details for us to assess whether their method was appropriate. In the same five studies there was also an unclear risk of bias in the methods used to conceal participant allocation, as the studies did not describe the measures taken to ensure concealment of group allocation.
Blinding
One study stated participants were not blinded (Devi 2014) and we judged this study to be at high risk of bias as the outcome assessments are likely to be influenced by the lack of blinding. Blinding of participants were not reported in the remaining five studies (Hambrecht 2004; Manchanda 2000; Raffo 1980; Schuler 1992; Todd 1991). One study blinded outcome assessors to group allocation (Hambrecht 2004) while another used procedures to ensure that assessment of exercise capacity was blinded (Raffo 1980); both studies were judged to be at low risk of bias. The outcome assessor was not blinded in one study and therefore we judged it to be at high risk of bias (Devi 2014). Blinding of outcome assessors was not reported in five studies (Jiang 2007; Manchanda 2000; Raffo 1980; Schuler 1992; Todd 1991), four of which were judged to be of unclear risk of bias (Jiang 2007; Manchanda 2000; Schuler 1992; Todd 1991).
Incomplete outcome data
Where reported, losses to follow‐up and dropout were relatively high, ranging from 15% to 58% across studies. We judged four studies (Devi 2014; Hambrecht 2004; Jiang 2007; Todd 1991) to be of low risk of bias as they described the number of and reasons for dropouts, which were balanced across groups. We judged two studies to be at high risk of bias (Raffo 1980; Schuler 1992). Raffo 1980 had a high rate of dropout (58%). In Schuler 1992, dropouts were high in the intervention group (29%) due to patients experiencing adverse clinical events during exercise. One study (Manchanda 2000) was judged have unclear risk of bias as reporting was insufficient.
Selective reporting
We judged the risk of selective reporting to be unclear in five studies where the study protocol was not available (Hambrecht 2004; Jiang 2007; Raffo 1980; Schuler 1992; Todd 1991). Risk of bias was considered high in one further study where the protocol was not available; in the methods section of the study paper, participants are described as being assessed monthly but only results at 12 months are reported (Manchanda 2000). We judged one study to be at high risk of bias because the primary outcome and two secondary outcomes described in the trial protocol were not reported (Devi 2014).
Other potential sources of bias
Groups balanced at baseline
We considered the risk of bias associated with groups being unbalanced at baseline as low for five studies (Devi 2014; Hambrecht 2004; Jiang 2007; Schuler 1992; Todd 1991). The risk associated with groups being unbalanced at baseline was unclear in one study (Raffo 1980). We judged one study (Manchanda 2000) to be at high risk of bias as there was evidence of substantial imbalance in the baseline characteristics of the intervention and control groups with regard to a likely prognostic factor (angina episodes per week).
Groups received comparable care (except the intervention)
We judged three studies (Hambrecht 2004; Todd 1991; Raffo 1980) to be at low risk of bias for this domain, because both groups received comparable care except for the CR intervention. Three studies were judged to be at high risk of bias because groups did not receive comparable care (Devi 2014; Manchanda 2000; Schuler 1992) and one study to be unclear risk of bias (Jiang 2007).
Effects of interventions
Exercise‐based cardiac rehabilitation compared to usual care for adults with stable angina
See summary of findings Table for the main comparison. None of the included studies reported on the following outcome measures: all‐cause hospital admissions or return to work.
Primary Outcomes
Mortality
Three studies (Manchanda 2000; Schuler 1992; Todd 1991) (total of 195 participants) reported a total of four all‐cause deaths with a pooled risk ratio (RR) of 1.01 (95% confidence interval (CI) 0.18 to 5.67, I2 = 0%, fixed‐effect) (Analysis 1.1). We assessed the evidence to be of very low‐quality using GRADE, because of concerns about risk of bias (random sequence generation, allocation concealment, blinding of outcome assessment and selective reporting), concerns about applicability to review question (participants in all studies were limited to middle‐aged men) and concerns about imprecision (small number of participants and confidence intervals including potential for important harm or benefit); see summary of findings Table for the main comparison. One study (Schuler 1992) reported cardiovascular‐related mortality in two participants in the CR group and none in the control.
Morbidity
Myocardial infarction (MI)
Three studies (Hambrecht 2004; Schuler 1992; Todd 1991) (total of 254 participants) reported on the incidence of MI with a total of six events (Analysis 1.2). There was a pooled RR of 0.33 in favour of CR (95% CI 0.07 to 1.63, I2 = 0%, fixed‐effect). We assessed the evidence to be of very low‐quality using GRADE, because of concerns about risk of bias (sequence generation, allocation concealment, blinding of outcome assessment, selective reporting, high loss to follow‐up and unbalanced groups at baseline), concerns about applicability to review question (participants in all studies were limited to middle‐aged men), and concerns about imprecision (small number of participants and confidence intervals including potential for important harm or benefit) (summary of findings Table for the main comparison).
Revascularisations (coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI))
Three studies (Hambrecht 2004; Manchanda 2000; Schuler 1992) (total of 256 participants) reported on the incidence of revascularisations with a total of 28 events (Analysis 1.3). In total, six revascularisations were reported among the CR groups in the three studies, and 22 in the control groups, with a pooled RR for risk of revascularisations of 0.27 in favour of CR (95% CI 0.11 to 0.64, I2 = 0%, fixed‐effect). We assessed the evidence to be of very low‐quality using GRADE, because of concerns about risk of bias (random sequence generation, allocation concealment, selective reporting and high losses to follow‐up), concerns about applicability to review question (participants in all studies were limited to middle‐aged men) and concerns about imprecision (small number of participants).
All‐cause hospital admissions
No studies reported this outcome.
Health‐related quality of life (HRQL)
One study (Devi 2014, 94 participants) reported HRQL using validated instruments (Seattle angina questionnaire and MacNew questionnaire). Compared to control, improvements with CR at the six‐week follow‐up were seen in emotional score (P = 0 .04) and angina frequency (P = 0.002). Benefits in favour of CR in angina frequency (P = 0.02) and social HRQL score (P = 0.02) were also observed at the six‐month follow‐up. We assessed the evidence for this outcome as very low‐quality using GRADE because of concerns about risk of bias (blinding of outcome assessment, high losses to follow‐up, selective reporting and unbalanced groups at baseline) and concerns about imprecision (small number of participants). Given the variation of outcomes it is not possible to consistently comment on the clinical importance of these results (summary of findings Table for the main comparison).
Exercise capacity
Five studies (Hambrecht 2004; Manchanda 2000; Raffo 1980; Schuler 1992; Todd 1991) (total of 267 participants) reported exercise capacity with a range of validated measures (VO2 peak and exercise duration) (Analysis 1.4). There was a small improvement in exercise capacity with CR compared to control (standardised mean difference (SMD) 0.45, 95% CI 0.20 to 0.70; I2 = 16%, fixed‐effect). We assessed the evidence to be of low quality using GRADE because of concerns about risk of bias (random sequence generation, allocation concealment, blinding of outcome assessment and selective reporting, high losses to follow‐up and unbalanced groups at baseline) and concerns with imprecision due to small number of participants (summary of findings Table for the main comparison).
Cardiovascular‐related hospital admissions
One study (Hambrecht 2004) (101 participants) reported that one CR participant and seven control participants experienced cardiovascular‐related hospital admissions (RR 0.14, 95% CI: 0.02 to 1.10 (Analysis 1.5)), with very low‐quality evidence as assessed using GRADE because of concerns about risk of bias (random sequence generation, allocation concealment, high losses to follow‐up and selective reporting), concerns about applicability to review question (participants in all studies were limited to middle‐aged men) and concerns about imprecision (small number of participants). See summary of findings Table for the main comparison.
Secondary Outcomes
Severity of angina
Manchanda 2000 (42 participants) reported a reduction in mean New York Heart Association (NYHA) score from baseline to one year follow‐up (2.6 to 1.4, P < 0.0001) with CR and an increase in mean NYHA in control (2.3 to 2.9, P = 0.004). Hambrecht 2004 (101 participants) reported an improvement in angina severity assessed by mean Canadian Cardiovascular Society (CCS) score in both CR (1.5 to 0.4, P < 0.001) and control (1.7 to 0.7, P < 0.001) We assessed the evidence as very low‐quality using GRADE because of concerns about risk of bias (random sequence generation, allocation concealment, blinding of outcome assessment, selective reporting, high losses to follow‐up, and unbalanced groups at baseline), concerns about applicability to review question (participants in all studies were limited to middle‐aged men) and concerns about imprecision (small number of participants). Data could not be pooled in a meta‐analysis because of scales used to report outcome measures (i.e. NYHA is categorical whilst CCS is continuous).
Reported adverse events
Adverse events were only commented upon by one study (Hambrecht 2004, 101 participants). The authors reported "no adverse events" during the exercise training programme in the CR group. We assessed the evidence as very low quality using GRADE because of concerns about risk of bias (random sequence generation, allocation concealment and selective reporting), concerns about applicability to the review question (participants in the study were limited to middle‐aged men) and concerns about imprecision (small number of participants).
Costs
One study (Hambrecht 2004) (101 participants) reported a difference in mean participant healthcare costs in favour of CR (CR: USD 3708 versus control: USD 6086, P < 0.0001). These costs included hospitalisations, repeat vascularisations, any other cardiovascular events plus the costs of the provision of the CR exercise training programme. We assessed this evidence as very low‐quality using GRADE because of concerns about risk of bias (random sequence generation, allocation concealment, high losses to follow‐up and selective reporting), concerns about applicability to review question (participants in all studies were limited to middle‐aged men) and concerns about imprecision (small number of participants).
Return to work
No studies reported this outcome.
Discussion
Summary of main results
This systematic review identified seven randomised trials involving a total of 581 participants with a confirmed diagnosis of stable angina. Five trials compared exercise‐based cardiac rehabilitation (CR) with a no exercise control, with one trial including percutaneous coronary intervention (PCI) as a comparator (Hambrecht 2004). Four studies used exercise‐only interventions (Devi 2014; Hambrecht 2004; Raffo 1980; Schuler 1992) and three studies used comprehensive CR programmes including dietary and risk‐factor advice alongside exercise training. All exercise programmes were based on aerobic exercise, typically walking and cycling.
Trials had an intervention length of 6 weeks to 12 months and follow‐up length of 6 to 12 months. The comparison group in all trials was usual care (without any form of structured exercise training or advice) or a no‐exercise comparator. The mean age of participants within the trials ranged from 50 to 66.2 years, the majority of participants being male (range: 74% to 100%).
Due to concerns about the small number of trials and their small size, potential risk of bias and lack of applicability, we assessed the body of evidence for all but one outcome where data were reported to be of very low quality (all‐cause mortality, myocardial infarction, revascularisations, cardiovascular‐related hospital admissions, health‐related quality of life, adverse events and costs). We are therefore uncertain about the impact of exercise‐based CR on these outcomes compared to usual care in people with stable angina. Based on low‐quality evidence, there may be a small improvement in exercise capacity following exercise‐based CR compared to usual care. No studies reported the outcomes of return to work or all‐cause hospital admissions. High‐quality, well‐reported randomised trials are needed to assess the benefits and harms of exercise‐based CR for adults with stable angina. Such trials need to collect patient‐relevant outcomes, including clinical events and health‐related quality of life. They should also assess cost‐effectiveness, and recruit participants that are reflective of the real‐world population of people with angina.
Overall completeness and applicability of evidence
The generalisability of this review is limited by the small number of randomised clinical trials, the low number of people with angina and few observed events. Furthermore, included trials generally recruited primarily middle‐aged men who were willing to participate in an exercise‐based training programme and therefore the evidence lacks applicability to older and female populations. Adequately powered, high‐quality, multi‐centre randomised trials in a broader, more representative population of people with stable angina are needed. Only one study reported cardiovascular‐related hospital admissions, adverse events and costs, whilst a different single study looked at health‐related quality of life. None of the included trials measured all‐cause hospital admissions or return to work. Future trials should collect patient‐relevant outcomes and also assess cost‐effectiveness.
Quality of the evidence
Using GRADE methodology we assessed the quality of the evidence for all outcomes where data were reported to be of very low‐quality, with the exception of exercise capacity, which we assessed as being low quality (see summary of findings Table for the main comparison).
In terms of risk of bias, the majority of studies reported unclear randomisation processes and unclear concealment of randomisation. A lack of blinding resulted in a high risk of detection bias in one trial, and high numbers of participants lost to follow‐up resulted in a risk of attrition bias in two trials. Two trials were at high risk of outcome reporting bias. One trial was at high risk of imbalance between groups at baseline and three were at high risk of bias due to not receiving comparable care in addition to the intervention. The reporting of details was poorer in the older studies (from the year 2000 and earlier); this meant we had to assign assessments of unclear risk of bias in many domains.
Six outcomes (mortality, myocardial infarction, revascularisations, cardiovascular‐related hospital admissions, severity of angina and costs) were assessed in trials consisting of middle‐aged men (over 50 years old), which raises concerns about indirectness and whether the review findings are applicable to women and older populations.
We also had concerns about imprecision, either due to the low number of participants, or due to wide confidence intervals which included potential for important harm or benefit (or both) for eight outcomes (mortality, myocardial infarction, revascularisations, health‐related quality of life, exercise capacity, cardiovascular hospital admissions, severity of angina and costs).
Potential biases in the review process
We conducted the review according to the methods provided in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). We followed our peer‐reviewed and pre‐published protocol in order to avoid biases during the conduct and write‐up of the review. In addition, we performed a comprehensive literature search to identify published and unpublished trials. We contacted study authors for further information.
During the screening process, we found studies that included a mixed population of people with coronary heart disease, but did not provide a separate reporting and analysis of the participants with stable angina. We were therefore unable to include these studies. In many studies, we found clinical event data (e.g. mortality data) in the trial descriptions of losses to follow‐up, rather than being formally stated as outcomes.
If in updated versions of this review we include data from studies where the majority of a mixed population had stable angina, we will exclude these studies in a sensitivity analysis to explore their impact on the main analyses.
An additional issue is that morbidity is considered as more than one primary outcome in our analyses (MI, CABG/PCI, all‐cause hospital admissions). Likewise, two different dimensions of quality of life are reported. Therefore there are a total of eight measures of our primary outcomes. There is a strong risk of introducing multiplicity arising from the multiple measures of effects.
Agreements and disagreements with other studies or reviews
Our scoping searches confirmed that no systematic review has been conducted specifically assessing the impact of exercise‐based CR in a population of people with stable angina. A recent Cochrane Review and meta‐analysis of 63 trials, which randomised 14,486 participants with coronary heart disease (including angina) to exercise‐based CR or a no‐exercise control, showed that exercise‐based CR led to a reduction in cardiovascular mortality (risk ratio (RR) 0.74, 95% CI 0.64 to 0.86), hospital admissions (RR 0.82, 95% CI 0.70 to 0.96) and improved health‐related quality of life (Anderson 2016). The five trials of people with stable angina included in this present Cochrane Review were included in this previous review.

PRISMA flow diagram of trial selection

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
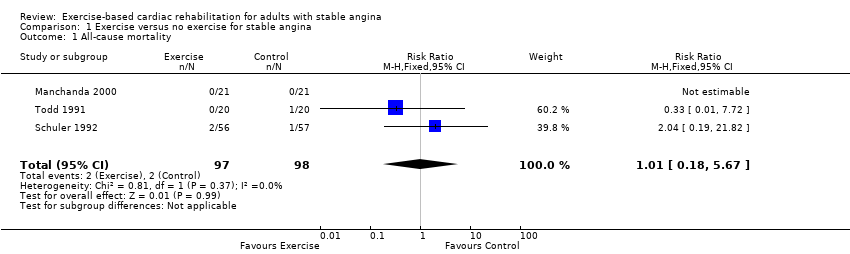
Comparison 1 Exercise versus no exercise for stable angina, Outcome 1 All‐cause mortality.
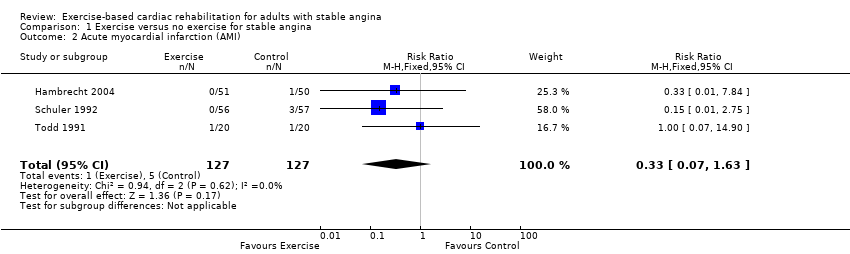
Comparison 1 Exercise versus no exercise for stable angina, Outcome 2 Acute myocardial infarction (AMI).

Comparison 1 Exercise versus no exercise for stable angina, Outcome 3 Revascularisation procedure (CABG or PCI).

Comparison 1 Exercise versus no exercise for stable angina, Outcome 4 Exercise capacity.
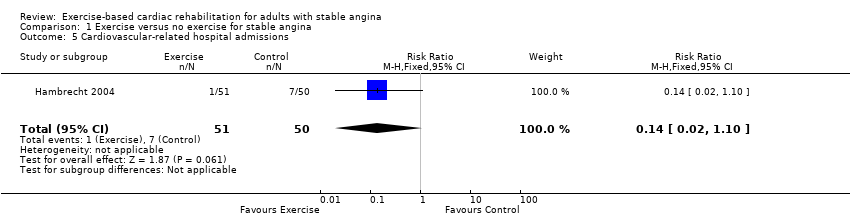
Comparison 1 Exercise versus no exercise for stable angina, Outcome 5 Cardiovascular‐related hospital admissions.
| Exercise‐based cardiac rehabilitation (CR) compared to usual care for patients with stable angina | ||||||
| Patient or population: adults with stable angina | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with exercise‐based cardiac rehabilitation | |||||
| All‐cause mortality Follow‐up: 12 months | Study population | RR 1.01 | 195 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on all‐cause mortality compared to usual care. | |
| 20 per 1,000 | 21 per 1,000 | |||||
| Acute myocardial infarction (AMI) Follow‐up: 12 months | Study population | RR 0.33 | 254 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on AMI compared to usual care. | |
| 39 per 1,000 | 13 per 1,000 | |||||
| Exercise capacity (assessed using a variety of outcomes including VO2 max and duration of exercise) Follow‐up: range 6 to 12 months | The mean exercise capacity in the intervention groups was 0.45 standard deviations higher | 267 | ⊕⊕⊝⊝ | Using Cohen's rule of thumb a SMD of 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect between groups (Cohen 1988). Exercise‐based CR may slightly improve exercise capacity compared to usual care. | ||
| Cardiovascular‐related hospital admissions Follow‐up: 12 months | Study population | RR 0.14 (0.02 to 1.1) | 101 | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on cardiovascular‐related hospital admissions compared to usual care. | |
| 140 per 1000 | 20 per 1000 (2 to 154) | |||||
| Health‐related quality of life (assessed with: Seattle Angina Questionnaire and The MacNew Questionnaire) | One study showed improvement in emotional score at 6‐week follow up, and benefits in angina frequency and social HRQL score at 6 months follow‐up. | Not estimable | 94 (1 RCT) | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on quality of life compared to usual care. | |
| Return to work | No studies were found that looked at return to work. | |||||
| Adverse events (e.g. skeletomuscular injury) Follow‐up: 12 months | Only one study looked at adverse events and reported that there were no adverse events during the exercise‐based CR. | Not estimable | 101 (1 RCT) | ⊕⊝⊝⊝ | We are uncertain about the effect of exercise‐based CR on adverse events compared to usual care. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some concerns with random sequence generation, allocation concealment, blinding of outcome assessment and selective reporting; bias likely, therefore quality of evidence downgraded by one level 2 Some concern with applicability to review question as participants in all studies were limited to middle‐aged men, therefore quality of evidence downgraded by one level 3 Imprecise due to small number of participants (less than 300) and confidence intervals including potential for important harm or benefit as 95% CI crosses RR of 0.75 and 1.25, therefore quality of evidence downgraded by two levels 4 Some concerns with random sequence generation, allocation concealment, blinding of outcome assessment, selective reporting and unbalanced groups at baseline; bias likely, therefore quality of evidence downgraded by one level 5 Some concern with random sequence generation, allocation concealment, blinding of outcome assessment, high loss to follow‐up, selective reporting and unbalanced groups at baseline; serious bias likely, therefore quality of evidence downgraded by two levels 6 Imprecise due to small number of participants (less than 300) therefore quality of evidence downgraded by one level 7 Some concerns with random sequence generation, allocation concealment and selective reporting; bias likely, therefore quality of evidence downgraded by one level 8 Some concerns with blinding of outcome assessment, selective reporting and groups not receiving comparable care; bias likely, therefore quality of evidence downgraded by one level 9 Imprecise due to very small number of participants therefore quality of evidence downgraded by two levels | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.18, 5.67] |
| 2 Acute myocardial infarction (AMI) Show forest plot | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.63] |
| 3 Revascularisation procedure (CABG or PCI) Show forest plot | 3 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.64] |
| 4 Exercise capacity Show forest plot | 5 | 267 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.45 [0.20, 0.70] |
| 5 Cardiovascular‐related hospital admissions Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.10] |

