High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012413.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 12 September 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Neonatal Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
All review authors developed the protocol. TA and WM screened search outputs, assessed study eligibility, and extracted and synthesised data. TA and VA assessed risk of bias across key domains and undertook GRADE assessment with WM. All review authors revised the final review.
Sources of support
Internal sources
-
University of York, UK.
-
Christian Medical College, Vellore, India.
-
Sri Ramachandra Medical College and Research Institute, Chennai, India.
External sources
-
National Institute for Health Research, UK.
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C.
Declarations of interest
Dr. Thomas was the principal investigator in the only study included in the review (Thomas 2012).
Acknowledgements
None.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Mar 12 | High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants | Review | Thangaraj Abiramalatha, Niranjan Thomas, Sivam Thanigainathan | |
| 2017 Sep 12 | High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants | Review | Thangaraj Abiramalatha, Niranjan Thomas, Vijay Gupta, Anand Viswanathan, William McGuire | |
| 2016 Oct 21 | High versus standard volumes of enteral feeds for preterm or low birth weight infants | Protocol | Thangaraj Abiramalatha, Niranjan Thomas, Vijay Gupta, Anand Viswanathan, William McGuire | |
Differences between protocol and review
None.
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for the included study.

Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.1 Weight gain (g/kg/d).
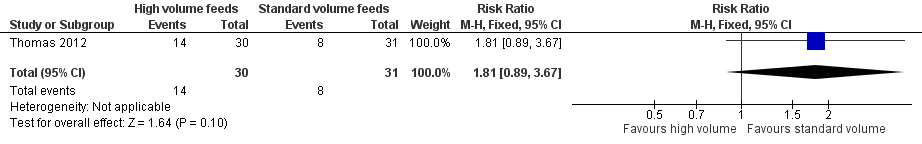
Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.2 Feed intolerance.

Forest plot of comparison: 1 High‐volume vs standard‐volume feeds, outcome: 1.3 Necrotising enterocolitis.

Comparison 1 High‐volume vs standard‐volume feeds, Outcome 1 Weight gain (g/kg/d).
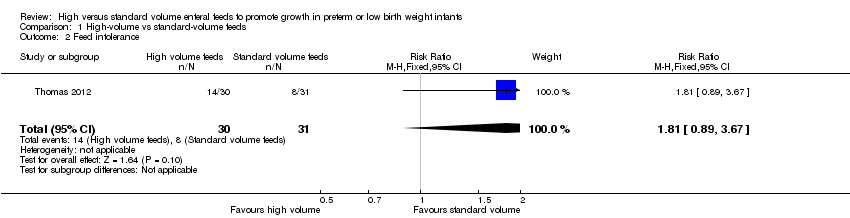
Comparison 1 High‐volume vs standard‐volume feeds, Outcome 2 Feed intolerance.
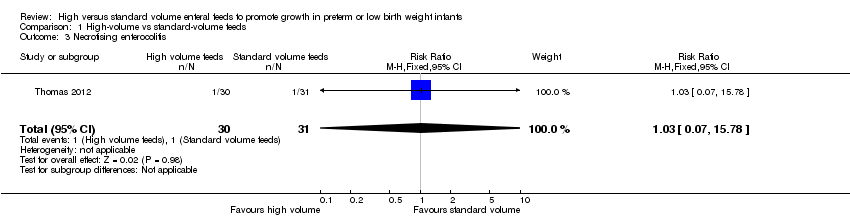
Comparison 1 High‐volume vs standard‐volume feeds, Outcome 3 Necrotising enterocolitis.
| High‐volume feeds vs standard‐volume feeds for preterm or low birth weight infants | |||||
| Patient or population: preterm or low birth weight infants | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
| Risk with standard‐volume feeds | Risk with high‐volume feeds | ||||
| Weight gain (g/kg/d) | Mean weight gain was 18.7 g/kg/d | Mean weight gain was 6.2 g/kg/d higher | ‐ | 61 | ⊕⊕⊝⊝ |
| Feed intolerance | Study population | RR 1.81 | 61 | ⊕⊕⊝⊝ | |
| 258 per 1000 | 467 per 1000 | ||||
| Necrotising enterocolitis | Study population | RR 1.03 | 61 | ⊕⊝⊝⊝ | |
| 32 per 1000 | 33 per 1000 | ||||
| *Risk in the intervention group (and its 95% CI) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). | |||||
| aDowngraded for risk of bias (lack of blinding). bDowngraded for imprecision. cDowngraded (by 2) for serious imprecision. | |||||
| per 100 mL | Expressed breast milk (EBM) | EBM + Fortifier | Term formula | Preterm formula |
| Energy (kCal) | 67 | 74 to 80 | 67 | 80 |
| Protein (g) | 1.2 to 1.7 | 2.0 to 2.5 | 1.5 | 2.4 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weight gain (g/kg/d) Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [2.71, 9.69] |
| 2 Feed intolerance Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.89, 3.67] |
| 3 Necrotising enterocolitis Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.78] |


