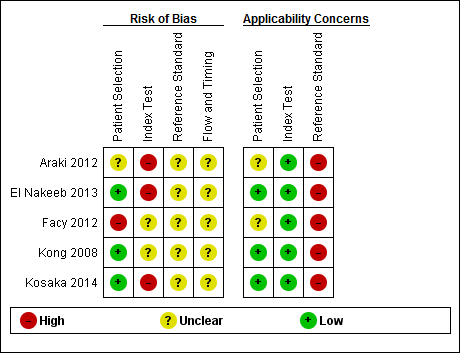
| Domain 1: Patient selection | Patient sampling | Patients who have undergone pancreatic resection with drain fluid at least 48 hours after pancreatic resection irrespective of the volume of the drain fluid. |
| Was a consecutive or random sample of patients enrolled? | Yes: if a consecutive sample or a random sample of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was included in the study.
No: if a consecutive sample or a random sample of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was not included in the study.
Unclear: if this information was not available. |
| Was a case‐control design avoided? | Yes: if a cohort of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was studied.
No: if patients with pancreatic leak were compared with patients without pancreatic leak (controls). We planned to exclude such studies.
Unclear: as anticipated, we were able to determine whether the design was case‐control. There were no case‐control studies. Hence, as anticipated, all studies included in the review were classified as 'yes' for this item. |
| Did the study avoid inappropriate exclusions? | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included.
No: if the study excluded patients based on high or low probability of pancreatic leak (for example, those with high volume in the drain).
Unclear: if this information was not available. |
| Could the selection of patients have introduced bias? | Low risk of bias: if 'yes' classification for all of the above 3 questions. High risk of bias: if 'no' classification for any of the above 3 questions. Unclear risk of bias: if 'unclear' classification for any of the above 3 questions but without a 'no' classification for any of the above 3 questions. |
| Patient characteristics and setting | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included.
No: if some patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were excluded on the basis of the results of drain fluid volume.
Unclear: if it was not clear whether the patients had been included on the basis of the results of drain fluid volume. |
| Are there concerns that the included patients and setting do not match the review question? | Low concern: if the patient characteristics and setting were classified as 'yes'. Unclear concern: if the patient characteristics and setting were classified as 'unclear'. High concern: if the patient characteristics and setting were classified as 'no'. |
| Domain 2: Index test | Index test(s) | Amylase in drain fluid. |
| Were the index test results interpreted without knowledge of the results of the reference standard? | The index test would always be conducted though not interpreted before the reference standard. Yes: if the index test was conducted and interpreted without the knowledge of the results of the reference standard.
No: if the index test was interpreted with the knowledge of the results of the reference standard.
Unclear: if it was not clear whether the index test was interpreted without the knowledge of the results of the reference standard. |
| If a threshold was used, was it pre‐specified? | Yes: if a pre‐specified threshold was used. No: if a pre‐specified threshold was not used. Unclear: if it was not clear whether the threshold used was pre‐specified. |
| Could the conduct or interpretation of the index test have introduced bias? | Low risk of bias: if 'yes' classification for both of the above questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: if the criteria for positive index test was clearly stated. High concern: if the criteria for positive index test was not stated. |
| Domain 3: Target condition and reference standard | Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak (requiring clinical intervention). Planned reference standards (see below). -
Pancreatic leak confirmed at surgery. -
Pancreatic leak confirmed at surgery for patients with elevated amylase and clinical follow‐up for a minimum period of 6 weeks (to ensure that they do not have complications due to pancreatic leak such as abdominal collections requiring drainage, intra‐abdominal sepsis, or generalised sepsis) in people with negative amylase. |
| Is the reference standard(s) likely to correctly classify the target condition? | Yes: if pancreatic leak was confirmed at reoperation.
No: if the reference standard was a combination of pancreatic leak and clinical follow‐up for a minimum period of 6 weeks (to ensure that they do not have complications due to pancreatic leak such as abdominal collections requiring drainage, intra‐abdominal sepsis, or generalised sepsis) in people with negative amylase. Unclear: although we planned to exclude studies if the reference standard was not described adequately or was not one of the above planned reference standards, this would have meant that there would have been no studies included in the review. So, we accepted the ISGPF grades B and C as an appropriate references standard and classified the answer to this signalling question as unclear. |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes: if the reference standard was interpreted without the knowledge of the results of the index test.
No: if the reference standard was interpreted with the knowledge of the results of the index test.
Unclear: it is not clear if the reference standard was interpreted without the knowledge of the results of the index test. |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk of bias: if 'yes' classification for both of the above 2 questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. |
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Although we anticipated that all of the included studies would be classified as 'low concern' because of the reference standards we planned to use, we have classified all the studies as 'high concern' because of the reference standards that we accepted. |
| Domain 4: Flow and timing | Flow and timing | Patients may have progression or resolution of pancreatic leak if there is a long delay between index test and reference standard. An arbitrary 2 weeks was chosen as an acceptable delay between index test and reference standard. |
| Was there an appropriate interval between index test and reference standard? | Yes: if the time interval between index test and reference standard was less than 2 weeks.
No: if the time interval between index test and reference standard was more than 2 weeks.
Unclear: if the time interval between index test and reference standard was unclear. |
| Did all patients receive a reference standard? | Yes: if all patients received a reference standard.
No: if some of the patients did not receive a reference standard. Such studies were excluded.
Unclear: if it was not clear whether all patients received a reference standard. Such studies were excluded. As anticipated, all studies included in the review were classified as 'yes' for this item. |
| Did all patients receive the same reference standard? | Yes: if all the patients received the same reference standard.
No: if different patients received different reference standards. Unclear: if this information was not clear. Because of the inclusion criteria, all the studies in this review were classified as 'yes' for this signalling question. |
| Were all patients included in the analysis? | Yes: if all the patients are included in the analysis irrespective of whether the results were interpretable.
No: if some patients are excluded from the analysis because of uninterpretable results.
Unclear: if this information is not clear. |
| Could the patient flow have introduced bias? | Low risk of bias: if 'yes' classification for all the above 4 questions. High risk of bias: if 'no' classification for any of the above 4 questions. Unclear risk of bias: if 'unclear' classification for any of the above 4 questions but without a 'no' classification for any of the above 4 questions. |