Antihypertensive pharmacotherapy for prevention of sudden cardiac death in hypertensive individuals
Information
- DOI:
- https://doi.org/10.1002/14651858.CD011745.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 10 March 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Hypertension Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Garry Taverny: drafted the protocol, obtained copies of studies, selected studies for inclusion, extracted data from reports, entered data into RevMan, carried out the analysis, interpreted the analysis, drafted the final review and will update the review.
Yanis Mimouni: selected studies for inclusion and extracted data from reports.
Anne LeDigarcher: selected studies for inclusion and extracted data from reports.
Pr. Philippe Chevalier: formulated the idea for the review.
Lutgarde Thijs: extracted data from studies.
Pr. James M Wright: extracted data from studies, interpreted the analysis, drafted the final review and will update the review.
Pr. François Gueyffier: interpreted the analysis, drafted the final review, extracted data from the INDANA database for related trials and will update the review.
Pr. François Gueyffier formulated the idea for the review, and every review author contributed to the draft of the protocol.
Sources of support
Internal sources
-
University of British Columbia, Vancouver, Canada.
-
Université Claude Bernard Lyon 1, France.
External sources
-
No sources of support supplied
Declarations of interest
Garry Taverny: nothing to declare.
Yanis Mimouni: nothing to declare.
Anne LeDigarcher: nothing to declare.
Pr. Philippe Chevalier: nothing to declare.
Lutgarde Thijs: nothing to declare.
Pr. James M Wright: nothing to declare.
Pr. François Gueyffier: nothing to declare.
Acknowledgements
We would like to thank the Cochrane Hypertension Group, especially Ciprian Jauca and Douglas Salzwedel, for assistance provided.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Mar 10 | Antihypertensive pharmacotherapy for prevention of sudden cardiac death in hypertensive individuals | Review | Garry Taverny, Yanis Mimouni, Anne LeDigarcher, Philippe Chevalier, Lutgarde Thijs, James M Wright, Francois Gueyffier | |
| 2015 Jun 14 | Antihypertensive pharmacotherapy for prevention of sudden cardiac death in hypertensive individuals | Protocol | Garry Taverny, Yanis Mimouni, James M Wright, Francois Gueyffier | |
Differences between protocol and review
Anne LeDigarcher, Pr. Philippe Chevalier and Lutgarde Thijs joined the team of review authors and contributed to this review.
We have made the following changes to the inclusion criteria:
-
Added the requirement that “> 50% [of] study participants had to have hypertension” (added to be more inclusive and more explicit).
-
Added the requirement that trials report primary outcome in intervention and control groups (added so that irrelevant trials could be excluded).
-
Deleted the primary outcome “specific cause of SCD when known” (changed because if cause of death were known, it would not be reported as SCD).
-
Deleted secondary outcome "change in blood pressure at study end" (judged not relevant to the objective of this review).
-
Added "withdrawals due to adverse effects" as secondary outcome (important to include harms data in all reviews if possible).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

PRISMA study flow diagram.

Funnel plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.1 Sudden cardiac death.

Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.2 Non‐fatal myocardial infarction.
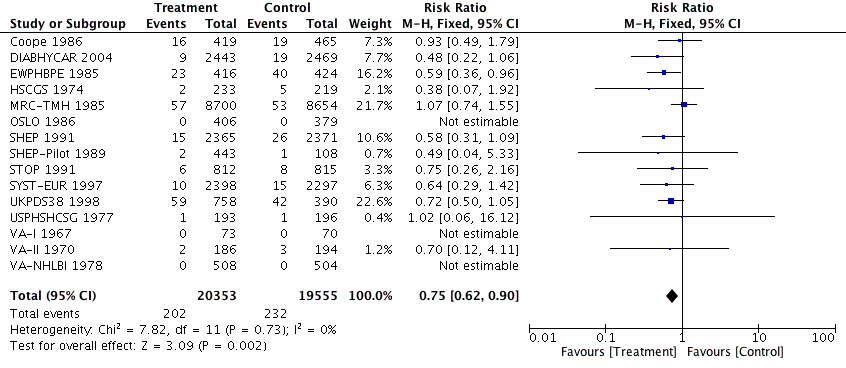
Forest plot of comparison: 1 Antihypertensive drug therapy vs control, outcome: 1.3 Fatal myocardial infarction.

Forest plot of comparison: 3 Subgroup analysis, first‐line diuretic versus others, outcome: 3.1 Sudden cardiac death.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 1 Sudden cardiac death.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 2 Non‐fatal myocardial infarction.

Comparison 1 Antihypertensive drug therapy versus control, Outcome 3 Fatal myocardial infarction.
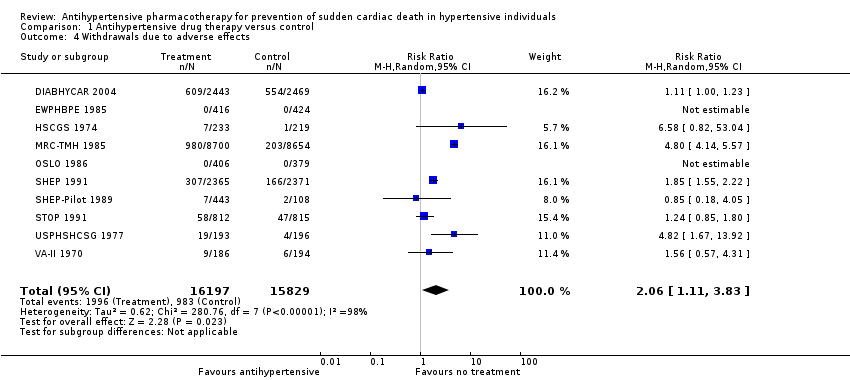
Comparison 1 Antihypertensive drug therapy versus control, Outcome 4 Withdrawals due to adverse effects.

Comparison 2 Subgroup analysis, first‐line diuretic versus others, Outcome 1 Sudden cardiac death.
| Antihypertensive pharmacotherapy versus control for prevention of sudden cardiac death in hypertensive individuals | ||||||
| Patient or population: people with hypertension (defined as baseline systolic resting blood pressure ≥ 140 mmHg and/or resting diastolic blood pressure ≥ 90 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with antihypertensive pharmacotherapy | |||||
| Sudden cardiac death (mean follow‐up: 4.2 years) | Study population | RR 0.96 | 39908 | ⊕⊕⊕⊝ | ||
| 13 per 1000 | 12 per 1000 | |||||
| Non‐fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.85 0.98) | 39908 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 17 per 1000 | |||||
| Fatal myocardial infarction (mean follow‐up: 4.2 years) | Study population | RR 0.75 0.90) | 39908 | ⊕⊕⊕⊝ | ||
| 12 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded 1 level for imprecision (wide confidence intervals) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sudden cardiac death Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.15] |
| 2 Non‐fatal myocardial infarction Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 3 Fatal myocardial infarction Show forest plot | 15 | 39908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.62, 0.90] |
| 4 Withdrawals due to adverse effects Show forest plot | 10 | 32026 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.11, 3.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sudden cardiac death Show forest plot | 15 | 38281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.19] |
| 1.1 First‐line diuretic | 10 | 17912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 1.2 First‐line other antihypertensive drugs | 5 | 20369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.74, 1.19] |

