Exercise‐based rehabilitation programmes for pulmonary hypertension
Abstract
Background
Individuals with pulmonary hypertension (PH) have reduced exercise capacity and quality of life. Despite initial concerns that exercise training may worsen symptoms in this group, several studies have reported improvements in functional capacity and well‐being following exercise‐based rehabilitation in PH.
Objectives
To assess the efficacy and safety of exercise‐based rehabilitation for people with PH. Primary outcomes were exercise capacity, adverse events during the intervention period and health‐related quality of life (HRQoL). Secondary outcomes included cardiopulmonary haemodynamics, functional class, clinical worsening during follow‐up, mortality and changes in B‐type natriuretic peptide.
Search methods
We searched the Cochrane Airways Specialised Register of Trials up to August 2016, which is based on regular searches of CINAHL, AMED, Embase, PubMed, MEDLINE, PsycINFO and registries of clinical trials. In addition we searched CENTRAL and the PEDro database up to August 2016 and handsearched relevant journals.
Selection criteria
All randomised controlled trials (RCTs) focusing on exercise‐based rehabilitation programmes for PH.
Data collection and analysis
Two reviewers extracted data independently. For binary outcomes, we calculated odds ratios and their 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a random‐effects model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
We included six RCTs and were able to extract data from five studies. The total number of included participants was 206. The majority of participants were Group I pulmonary artery hypertension (PAH). Study duration ranged from three to 15 weeks. Exercise programmes included both inpatient‐ and outpatient‐based rehabilitation that incorporated both upper and lower limb exercise. The mean six‐minute walk distance following exercise training was 60.12 metres higher than control (30.17 to 90.07 metres, n = 165, 5 RCTs, low‐quality evidence; minimal important difference was 30 metres), the mean peak oxygen uptake was 2.4 ml/kg/minute higher (1.4 to 3.4 ml/kg/min, n = 145, 4 RCTs, low‐quality evidence) and the mean peak power in the intervention groups was 16.4 W higher (10.9 to 22.0 higher, n = 145, 4 RCTs, low‐quality evidence). The mean change in HRQoL for the SF‐36 physical component score was 4.63 points higher (0.80 to 8.47 points, n = 33, 2 RCTs, low‐quality evidence) and for the SF‐36 mental component score was 4.17 points higher (0.01 to 8.34 points; n = 33; 2 RCTs, low‐quality evidence). One study reported a single adverse event, where a participant stopped exercise training due to lightheadedness.
Authors' conclusions
In people with PH, exercise‐based rehabilitation results in clinically relevant improvements in exercise capacity. Exercise training was not associated with any serious adverse events. Whilst most studies reported improvements in HRQoL, these may not be clinically important. Overall, we assessed the quality of the evidence to be low. The small number of studies and lack of information on participant selection makes it difficult to generalise these results across the spectrum of people with PH.
PICOs
Plain language summary
Exercise‐based rehabilitation in pulmonary hypertension
What is pulmonary hypertension? Pulmonary hypertension is a condition in which the blood pressure in the arteries that carry blood from the heart to the lungs is elevated well above normal. Often with a gradual onset, it affects individuals of all ages, significantly reduces quality of life and results in premature death.
Bottom Line. We reviewed randomised controlled trials to determine whether exercise training improved short‐ and long‐term patient outcomes in people with pulmonary hypertension. The number of participants in randomised controlled trials of exercise‐based rehabilitation for pulmonary hypertension was relatively small. These studies all reported large increases in exercise capacity as evaluated by six‐minute walk distance, maximal oxygen consumption and peak power. Health‐related quality of life was also improved, but to a lesser extent. Serious adverse events were rare with only one report of a participant being required to stop exercise training due to feeling lightheaded. There were no reports of death or other adverse events with exercise training.
What evidence did we find and how good was it? The review included six studies on 206 people with pulmonary hypertension and we could combine data from five of these studies. We could only use data for 165 participants, however not all of these data could be included in the analysis for all outcome measures. The majority of studies implemented an inpatient exercise rehabilitation programme with only a small number of studies examining an outpatient programme. The methods used to conduct these trials were of low quality. Given this low‐quality evidence, it was not possible to generalise the results of this review across the spectrum of people with pulmonary hypertension.
Authors' conclusions
Summary of findings
| Exercise compared to control for pulmonary hypertension | |||||
| Patient or population: people with pulmonary hypertension | |||||
| Outcomes | Illustrative comparative effects* (95% CI) | No of participants | Quality of the evidence | Comments | |
| Response on control | Treatment effect | ||||
| Control | Exercise | ||||
| Change in functional exercise capacity (6MWD) | Median change = 5 m | The mean exercise capacity 6MWD in the intervention groups was 60.12 higher | 165 | ⊕⊕⊝⊝ | Subgroup PAH: (2 studies, n = 36), mean 6MWD for intervention group was 33.84 m higher (0.95 to 66.73 higher); these studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component Minimal important difference was 30 metres |
| Exercise capacity: VO2peak Oxygen uptake, ml/kg/min | Median change = ‐0.25 ml/kg/min | The mean VO2peak in the intervention groups was 2.41 ml/kg/min higher | 145 | ⊕⊕⊝⊝ | Subgroup PAH (2 studies, n = 36), the mean VO2peak in the intervention groups was 1.28 ml/kg/min higher (‐0.19 to 2.75 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
| Exercise capacity: peak power watts | Median change = 1 watt | The mean exercise capacity: peak power in the intervention groups was 16.44 W higher | 145 | ⊕⊕⊝⊝ | Subgroup PAH (2 studies, n = 36), the mean peak power in the intervention groups was 14.24 watts higher (5.78 to 22.70 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
| HRQoL SF‐36: PCS units Follow‐up median 11 weeks | Median change = ‐0.49 units | The mean HRQoL SF‐36: PCS in the intervention groups was 4.63 higher (0.80 to 8.47 higher) | 33 | ⊕⊕⊝⊝ | Both studies were only PAH |
| HRQoL SF‐36: MCS units Follow‐up median 11 weeks | Median change = ‐0.31 units | The mean HRQoL SF‐36: MCS in the intervention groups was 4.17 higher (0.01 to 8.34 higher) | 33 | ⊕⊕⊝⊝ | Both studies were only PAH |
| *The basis for the response on control is the median control group response across studies | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Two studies did not report random sequence generation, no studies reported allocation concealment 3 Imprecision (2 small studies of 33 participants) and neither reported allocation concealment | |||||
Background
Description of the condition
Pulmonary hypertension (PH) is a progressive vasculopathy characterised by extensive remodelling of the pulmonary vasculature resulting in a narrowing of the arterial lumen (Casserly 2009). There is a marked increase in pulmonary vascular resistance resulting in right ventricular remodelling and eventual failure, which, in the majority of cases, results in patient death (Tuder 2013). Confirmatory diagnosis of PH is made via right heart catheterisation in which the patient has a resting mean pulmonary artery pressure of greater than 25 mmHg (Hoeper 2013). PH may arise in association with a broad range of disease states (over 40) of both known and unknown cause. International guidelines classified PH into the following five clinical groups (Simonneau 2013).
-
Group 1: pulmonary arterial hypertension (PAH)
-
Group 2: PH due to left heart disease
-
Group 3: PH due to lung diseases or hypoxia, or both
-
Group 4: chronic thromboembolic PH (CTEPH)
-
Group 5: PH with unclear multifactorial mechanisms.
Given the evolving definition of PH, the incidence and prevalence of the disease is difficult to define (Strange 2012). One recent study suggested that this varies markedly between the five clinical groups. In an observational cohort study of over 10,000 individuals from Armadale and the surrounding region in Western Australia, Strange 2012 reported the minimum indicative prevalence for all groups of PH was 326/100,000, with left heart disease associated with Group 2 being the most prevalent. Registries of prevalent and incident cases from around the world have now been published (McGoon 2013), suggesting an increased global awareness of the disease.
Regardless of aetiology, PH is characterised by limited exercise capacity and a progressive increase in breathlessness. Until recently, treatment options for PH remained limited and patient prognosis poor. One early registry of people with PH reported a median survival time of 2.8 years post diagnosis (D'Alonzo 1991). The development of PH‐specific drug therapies, targeted at the pulmonary vasculature, has significantly improved prognosis. This improved survival has been reflected in several of the more recently published registries (McGoon 2013). For example, the United States Registry to Evaluate Early and Long‐Term PAH Disease Management (REVEAL) registry of over 3500 prevalent and incident cases recorded between 2006 and 2009, reported five‐year survival rates for PAH at 57% (Benza 2010).
Advances in PH‐specific therapies have improved survival and slowed disease progression. As a result, other treatment options aimed at improving outcomes such as exercise capacity and quality of life have been explored. In people with other chronic heart and lung diseases, there is strong evidence that exercise training improves functional capacity, quality of life and even long‐term survival (Spruit 2013). However, until very recently, exercise rehabilitation has been actively discouraged in people with PH for fear it would worsen symptoms and negatively impact on cardiac function (Galie 2013). Whilst guidelines released in December 2013 recommend exercise training, the guideline authors acknowledge that gaps in the knowledge exist including knowledge of the optimal training dose, characteristics of supervision, mechanisms of adaptation and the impact of exercise training on long‐term survival (Galie 2013).
Description of the intervention
Exercise‐based rehabilitation programmes include aerobic and strength training elements designed to improve both aerobic capacity and muscle strength. Aerobic training involves the activation of a large skeletal muscle mass through an extended period of cycling or walking exercise that is between 20 and 40 minutes in duration. Strength training programmes involve upper and lower body muscle groups with the participant completing a number of sets of exercises at a fixed percentage of a repetition maximum (RM) Spruit 2013. Programmes are typically offered in an outpatient or inpatient setting, involving two to three sessions per week typically over at least a four‐week period.
How the intervention might work
In healthy young and older patients, exercise training results in improved oxygen transport and uptake at peak exercise through both central and peripheral adaptations. Central adaptations include an increase in maximal cardiac output, through an increase in stroke volume (Ogawa 1992). Central adaptations are the result of volume overload mediated cardiac remodelling that leads to improved cardiac function at rest and during exercise (Ogawa 1992; Pluim 2000). In the periphery, greater skeletal muscle oxidative capacity occurs with an increase in enzymes associated with cellular respiration, in particular those involved in the citric acid cycle (the Krebs cycle) and oxidative phosphorylation (Gollnick 1973; Coggan 1992). In addition, there is an increase in the capillary density per myofibril (Gollnick 1973; Coggan 1992). As a result of these central and peripheral adaptations, there is not only an increased delivery of oxygen to the exercising myofibril, there is also increased capacity to metabolise oxygen for the production of adenosine triphosphate. Transition between myofibril types typically occurs with an increase in the fast twitch oxidative and a decrease in fast twitch glycolytic fibres following exercise training (Gollnick 1973; Coggan 1992; Ennion 1995). Moreover, there is an increase in the cross sectional area of slow twitch (Type I) and Type IIa fibres in trained individuals (Gollnick 1973; Coggan 1992).
In PH, the factors which contribute to exercise limitation are complex (Fowler 2012; Panagiotou 2015; Babu 2016b). The changes in the pulmonary vasculature associated with PH results in a significant increase in pulmonary artery pressure and right ventricular afterload during exercise (Riley 2000; Provencher 2008). Right ventricular contractility is decreased and there is a reduced capacity for stroke volume and therefore for cardiac output to increase during exercise (Fowler 2012). Moreover, people with PH have a reduced heart rate response to exercise (chronotropic incompetence), which further decreases the ability for cardiac output to increase during exercise (Provencher 2006). As a result, people with PH have a blunted increase in cardiac output during exercise that significantly reduces peak oxygen transport. In the periphery, people with PH appear to have marked skeletal muscle dysfunction consistent with a reduced oxidative capacity (Mainguy 2010a). Compared to controls, people with PH had a lower percentage of Type I fibres and increased concentrations of enzymes associated with glycolytic (anaerobic) metabolism (Mainguy 2010a). These central and peripheral changes would result in a substantial reduction in the ability to transport and utilise oxygen during exercise.
In people with chronic lung disease, lower limb exercise training and strength training have both been demonstrated to increase exercise capacity and quality of life (Spruit 2013). The primary site of adaptation appears to be the skeletal muscle, with little change in cardiac function following exercise training in people with chronic heart and lung disease (Vogiatzis 2013). For example, in people with chronic obstructive pulmonary disease there is evidence that exercise training results in improved skeletal muscle structure and function with little change in cardiac function (Whittom 1998; Vogiatzis 2013). Whilst preliminary evidence in a small number of people suggests that there is some improvement in skeletal muscle function following exercise training in PH (de Man 2009; Mainguy 2010b), it remains unclear if these changes result in improved exercise capacity or if they relate to improved long‐term outcomes. Currently there is limited evidence for any central changes following exercise training in PH.
Why it is important to do this review
The objective of this review was to assess the efficacy and safety of exercise‐based rehabilitation for people with PH. In other chronic lung disease populations, for example chronic obstructive pulmonary disease, this form of rehabilitation is safe and has demonstrable benefits in terms of improvement in exercise capacity, lower limb muscle strength and quality of life (Spruit 2013). Until recently there had been a reluctance to recommend exercise‐based rehabilitation for PH due to the fact that it may worsen the patient's long‐term health outcomes (Galie 2009). Given international guidelines recommending exercise training in PH (Galie 2013, Galie 2015), it is important that the current state of the evidence regarding the efficacy and safety of exercise‐based rehabilitation is established. The results of this review will provide essential information to clinicians who may consider referring people with PH for exercise‐based rehabilitation, and help guide decisions on which PH patients may be suitable.
Objectives
To assess the efficacy and safety of exercise‐based rehabilitation for people with PH.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported in full or abstract form as well as any relevant, unpublished data.
Types of participants
We included adults with a diagnosis of PH. We included all five clinical groups of PH (Simonneau 2013), independent of whether the patients were stable on therapy (i.e. change of therapy over the past three months).
Types of interventions
We included trials comparing exercise‐based rehabilitation with usual care or no exercise‐based rehabilitation. Exercise‐based rehabilitation of any frequency and duration was eligible for inclusion, including inpatient, outpatient or home‐based settings. We included exercise programmes of any length; however, we only included trials in which exercise training was supervised. We excluded exercise programmes that only provided exercise advice. We included exercise‐based programmes prescribing aerobic or strength training, or both.
We planned to analyse exercise‐rehabilitation that only included a strength‐training programme separately, however no such trials were found. The control group included individuals randomised to a programme of education which had no specific exercise prescription component.
Types of outcome measures
Primary outcomes
-
Exercise capacity
-
Measures of exercise capacity included but were not confined to outcomes such as the six‐minute walk distance (6MWD), peak exercise capacity (VO2peak), peak power (Wpeak) and measures derived during the assessment of exercise capacity such as breathing efficiency (VE/VCO2 slope) and anaerobic threshold
-
-
Serious adverse events during the intervention period
-
We used this measure to assess the short‐term safety of exercise training in PH. We defined adverse events as:
-
-
-
mortality;
-
disease progression, defined according to the investigators' definition;
-
symptoms precluding training, such as illness, lightheadedness, syncope or presyncope; and
-
discontinuation of the study
-
-
Health‐related quality of life measured by any validated generic or disease‐specific quality‐of‐life measure
Secondary outcomes
-
Cardiopulmonary haemodynamics
-
These included measures made using echocardiographic, right heart catheter or magnetic resonance imaging techniques
-
Outcome measures included, but were not confined to indices such as mean pulmonary artery pressure (mPAP), mean pulmonary vascular resistance, right ventricular systolic pressure, tricuspid annular plane systolic excursion, ventricular ejection fraction, ventricular end diastolic volume and ventricular end systolic volume
-
-
Functional Class measured by the New York Heart Association (NYHA) Classification/ (NYHA 1994) World Health Organisation (WHO) Functional Classification Rubin 2004
-
Clinical worsening during the follow‐up period.
-
The impact of exercise training on clinical worsening was assessed using the investigators definition
-
Typically clinical worsening is defined using a combination of outcomes including survival, hospitalisation due to PH, transplantation, requirement for additional pharmacological therapy, a reduction in functional class and or a reduction in the six‐minute walk test (Frost 2013)
-
For the purpose of this study, we treated mortality during the follow‐up period as a separate secondary outcome measure
-
-
Mortality during the follow‐up period
-
We recorded all deaths reported following the exercise intervention
-
We treated these deaths separately to those that occurred during the exercise training period, which were recorded by the primary outcome measure, serious adverse events
-
-
B‐type natriuretic peptide
-
A commonly used marker of right ventricular dysfunction in PH that is correlated with survival (Casserly 2009)
-
We examined changes in B‐type natriuretic peptide following exercise‐based rehabilitation
-
Reporting one of more of the outcomes listed here was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from searches of the following databases.
-
The Cochrane Airways Register of Trials: all years to 23 August 2016
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, issue 8) (via the Cochrane Register of Studies (CRS‐Web): searched 23 August 2016
-
MEDLINE (Ovid): 1950 to August week 1 2016
-
Embase (Ovid): 1974 to week 33 2016
-
Physiotherapy Evidence Database (PEDro): all years to 23 August 2016
The database search strategies are listed in Appendix 1. We searched all databases from their inception to August 2016, with no restriction on language or type of publication. We identified handsearched conference abstracts and grey literature from the CENTRAL database. We also conducted a search of ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/).
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched for errata or retractions from included studies published in full‐text on PubMed on 16 August 2016.
Data collection and analysis
Selection of studies
Two review authors (NM and AH) independently screened titles and abstracts for inclusion and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publication, and two review authors (NM and AH) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We used Covidence (Covidence 2016) to manage the selection process. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
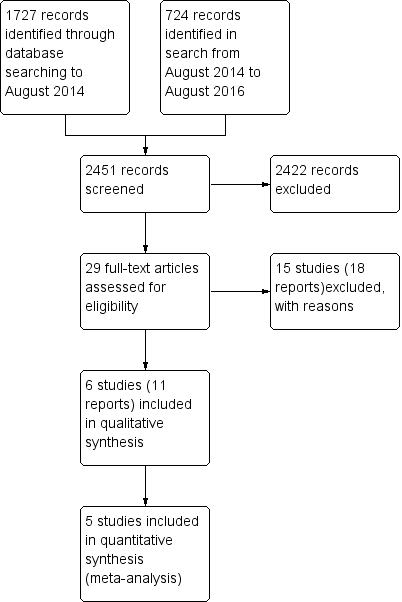
Study flow diagram
Data extraction and management
We used a data collection form for study characteristics and outcome data which was piloted on one study in the review. Two review authors (NM and AH) extracted study characteristics from included studies in Covidence.
We extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number enrolled, mean age, age range, gender, severity of condition, diagnostic criteria, baseline echocardiography and right heart catheter data, baseline lung function, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, training dose (intensity, frequency and duration of exercise training), comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus. One review author (NM) transferred data into the Cochrane Collaboration's statistical software, Review Manager (RevMan) (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (AH) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (NM and AH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreements by discussion.
We assessed the risk of bias according to the following domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective outcome reporting;
-
other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in a 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a HRQoL scale).
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in thDifferences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs). For continuous data, we used mean differences (MDs) or standardised mean differences (SMDs). Where it was reported, we used the change from baseline. Where the change from baseline was not reported, we used the adjusted results or final score. We did not combine data expressed as change from baseline with that reported as other metrics. We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We narratively described skewed data reported as medians and interquartile ranges.
Where multiple trial arms were reported in a single trial, we planned to include only the relevant arms, however no trials of this nature were identified.
Unit of analysis issues
Where studies randomly allocated the participants to either the exercise‐based rehabilitation or control, we considered the participant as the unit of analysis. We excluded cross‐over trials due to the potential carry‐over effects of exercise training.
Dealing with missing data
We contacted trial investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we identified substantial heterogeneity, we explored possible causes by prespecified subgroup analysis (Deeks 2011).
Assessment of reporting biases
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small study and publication biases, however insufficient numbers of trials were identified.
Data synthesis
We performed a pooled quantitative synthesis where the trials were clinically homogeneous. We pooled data using a random‐effects model to incorporate between‐study heterogeneity into the meta‐analysis. Data from an intention‐to‐treat analysis were used where available. Where the trials were clinically heterogeneous, we performed a narrative synthesis. We used RevMan HAL, developed by the Cochrane Schizophrenia Group (http://szg.cochrane.org/revman‐hal), to construct a first draft of the results section.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: exercise capacity, serious adverse events, cardiopulmonary haemodynamics, quality of life, functional class, mortality and clinical worsening during follow‐up. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions(Schünemann 2011) using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies in the Footnotes section of summary of findings Table for the main comparison, and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
-
Type of PH:
-
we analysed data separately for people with PAH only (Group 1).
-
-
Severity of PH:
-
we planned to compare the outcomes of less severe disease classification (NYHA Class I/II) with those with more severe disease classification (NYHA Class III/IV), however insufficient data were available.
-
We used the following outcomes in subgroup analyses:
-
exercise capacity;
-
serious adverse events;
-
health‐related quality of life.
We used the formal test for subgroup interactions in RevMan 2014.
Sensitivity analysis
We performed sensitivity analyses to examine the effects of methodological quality on the pooled estimate by removing studies that were at high or unclear risk of bias for the domains of blinding and incomplete outcome data.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification for complete details.
Results of the search
The PRISMA table shows results of our searchFigure 1
In the original search to August 2014, we found 1727 papers that were potentially relevant. We conducted an additional search from August 2014 to August 2016 and identified an additional 724 papers. After removing duplicates and the clearly irrelevant material we selected 29 full‐text papers to be further assessed for inclusion. Of these, we excluded 15 studies (18 reports) because they did not meet our inclusion criteria. Finally, after careful scrutiny, we were left with six studies (11 reports).
Included studies
Refer to Characteristics of included studies. The total number of participants from included participants was 206. Sample sizes ranged from 10 to 87 participants. Most participants had PAH (Group 1 PH) or chronic thromboembolic PH. The mean age of participants ranged from 47 to 56 years, and the mPAP on right heart catheterisation ranged from 40 to 52 mmHg. All participants were stable on medical therapy.
Excluded studies
From the 29 full‐text papers reviewed, we excluded 15 studies (18 reports). Reasons for exclusion were that studies were not randomised controlled trials (RCTs, n = 8), did not include exercise training (n = 3), was a review (n = 1), included the wrong population (n = 1) or used the wrong intervention (n = 2). Full details of the reasons for exclusion are included in the Characteristics of excluded studies section.
Risk of bias in included studies
Details on our judgements on the potential risks of bias are summarised in Figure 2 and Figure 3, with full details in the Characteristics of included studies table.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
None of the studies provided details on how allocation was concealed and we therefore judged them to be at unclear risk of bias in this domain. Three of the studies (Mereles 2006; Ganderton 2013; Ley 2013) provided details on how the randomisation sequence was generated and we judged them to be at low risk. For the remaining studies we were unable to ascertain details of random sequence generation.
Blinding
We rated all six of the studies as having a high risk of bias for blinding of participants and personnel. Given the nature of the intervention (exercise training) it was not possible to blind participants or personnel to the intervention. Five out of six studies reported blinding of outcome assessors.
Incomplete outcome data
Based on our review, we rated four of the studies as low risk with regards to attrition bias (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013) with each of these studies reporting no or very small numbers of dropouts. We rated the largest study, Ehlken 2016, as high risk as there was a differential rate of attrition with 17% dropout in the intervention group as opposed to 0% dropout in the control.
Selective reporting
We found three studies to have low risk of reporting bias (Mereles 2006; Ganderton 2013; Ley 2013). Two studies did not provide complete results when compared to those provided on the trial registry (Chan 2013; Ehlken 2016). The final study of Wilkinson 2007 was only in abstract form, making it difficult to ascertain if the data reported were complete.
Other potential sources of bias
We found three of the studies to be of low risk with regards to other sources of bias (Chan 2013; Ganderton 2013; Ley 2013). We were unable to rule out some selection bias in the three other studies (Mereles 2006; Wilkinson 2007; Ehlken 2016). Neither of the studies by Mereles 2006 and Wilkinson 2007 provided a CONSORT diagram and hence there is no detail how many participants they screened to achieve the enrolment target (Schulz 2010). The study by Ehlken 2016 , whilst providing a CONSORT diagram, did not provide any detail on how many patients were screened and how they applied the inclusion/exclusion criteria to achieve the target enrolment of 95 participants.
Effects of interventions
See: Summary of findings for the main comparison Exercise compared to control for pulmonary hypertension
Studies in this review compared exercise‐based rehabilitation to no intervention, education alone or usual care. In total there were six relevant randomised studies. We extracted data for meta‐analyses from five of the studies (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013; Ehlken 2016), allowing for comparison between exercise‐based intervention and control. We were unable to obtain data for analysis from the study by Wilkinson 2007 (published only as an abstract) despite several attempts to contact the study authors. See summary of findings Table for the main comparison for the main comparisons between the intervention and control groups. In total there were 21 outcomes evaluated including primary outcomes of exercise capacity, adverse events and health‐related quality of life.
Primary outcomes
Exercise capacity
Five studies (n = 165 PH participants) reported changes in the 6MWD (Mereles 2006; Chan 2013; Ganderton 2013; Ley 2013; Ehlken 2016) or changes in exercise capacity derived from an incremental exercise test (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). The mean increase in 6MWD of 60.12 m (MD 30.17 to 90.07 higher, Analysis 1.1, Figure 4) was well in excess of the minimal important difference of 30 metres (Mathai 2012; Holland 2014). However there was marked heterogeneity across studies (I2= 64%).

Forest plot of comparison: 1 Exercise vs control, outcome: 1.1 Exercise capacity: 6MWD
Four studies reported the impact of exercise‐based rehabilitation on peak exercise capacity determined from a cardiopulmonary exercise testing (CPET) (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). There were significant increases in both VO2peak with exercise‐based rehabilitation compared to control (MD 2.4 ml/kg/min, 95% CI 1.4 to 3.4, Analysis 1.2) with no significant heterogeneity across studies (I2= 37%). Similarly, increases in peak power favoured exercise rehabilitation (MD 16.4 W, 95% CI 10.9 to 22.0, Analysis 1.3) with no significant heterogeneity (I2= 0%). Three studies reported changes in the anaerobic threshold, one of which was reported as time to anaerobic threshold (Chan 2013), whilst the other two reported this in ml/min (Mereles 2006; Ganderton 2013). Pooled analysis showed an increase in the standardised mean difference favouring the exercise rehabilitation group (SMD 1.05, 95% CI 0.53 to 1.58, I2= 0%, Analysis 1.4). Whilst there is no reported minimal important difference (MID) for CPET‐derived measures of exercise capacity in PH, the increase in peak power is in excess of the MID reported for chronic obstructive pulmonary disease of 5 to 10 W (Sutherland 2005).
To date few studies have examined possible mechanisms for improved exercise capacity following exercise training in PH. In their study Ley 2013 reported improved pulmonary perfusion using magnetic resonance imaging (MRI). These authors suggested that exercise training may improve perfusion of the lungs or contractile function, or both. However it was noted that none of the changes in cardiac function correlated with changes in 6MWD. In their study Ehlken 2016 completed a right heart catheterisation (RHC) in a subgroup of exercise and control subjects. They reported improved pulmonary haemodynamics with a lowering of mean pulmonary artery pressure in the exercise group compared to the control group. For the exercise group there was an improvement in submaximal and maximal cardiac output. The authors hypothesised that exercise training may improve right ventricular (RV) function, however RV function was not directly measured.
Apart from these central changes there is some evidence that exercise training improves skeletal muscle oxidative capacity, similar to what is seen with exercise training in other chronic lung disease populations. Small observational studies by Mainguy 2010b (n = 5) and de Man 2009 (n = 19) both reported improvements in skeletal muscle oxidative capacity and capillary density. These preliminary results would suggest that the mechanism for adaptation to exercise training may be the result of improved skeletal muscle oxidative capacity and capillarisation and potentially improved oxygen delivery through improved cardiac function.
Overall the quality of evidence for changes in exercise capacity was rated as low due to imprecision and selective reporting. For details see summary of findings Table for the main comparison.
Serious adverse events
Only one study reported any adverse event that precluded a participant from training in a single session (Ganderton 2013). In this study one subject was reported to have stopped training for a single session due to extreme lightheadedness. No other studies reported any serious adverse events as we defined them in the protocol, that is, mortality, disease progression, symptoms that precluded training or discontinuation of the study.
Health‐related quality of life
Quality of life was reported using either the Short‐Form 36 (SF‐36) questionnaire (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016) or using the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), a PH‐specific questionnaire (Chan 2013; Ganderton 2013).
We have reported the changes in the physical component scores (PCS) and mental component score (MCS) of the SF‐36 in summary of findings Table for the main comparison and Analysis 1.5 and Analysis 1.6 as these provide us with a summary of the global improvement in both physical and emotional aspects of quality of life. Changes in PCS and MCS were reported in two of the smaller studies (Chan 2013; Ganderton 2013). Analysis showed that exercise‐based interventions favoured improved outcomes for PCS (MD 4.63, 95% CI 0.80 to 8.47, Analysis 1.5 ) and MCS (MD 4.17, 95% CI 0.01 to 8.34, Analysis 1.6) with no significant heterogeneity between studies. Both of these studies also examined changes in health‐related quality of life using the CAMPHOR and reported greater improvement in the exercise‐based rehabilitation group in each of the subscores for activities (MD‐1.33, 95% CI ‐3.56 to 0.90, Analysis 1.16), symptoms (MD ‐3.08, 95% CI ‐7.78 to 1.62, Analysis 1.17) and overall quality of life (MD ‐5.42, 95% CI ‐8.03 to ‐2.81, Analysis 1.18), although there was marked heterogeneity for the activities and symptoms domains (I2 = 67% and 88% respectively).
Four of the studies (n = 118 randomised) reported changes in quality of life using the domains of the Short‐Form 36 (SF‐36) questionnaire (Mereles 2006; Chan 2013; Ganderton 2013; Ehlken 2016). Exercise‐based rehabilitation resulted in a substantial improvement in outcome scores for 'Role physical' (MD 21.8, 95% CI 14.40 to 29.23, Analysis 1.9), 'Vitality' (MD 13.47, 95% CI 7.55 to 19.40, Analysis 1.14), and 'Social function' (MD 14.01, 95% CI 9.82 to 18.21, Analysis 1.15). Pooled analysis found there was no improvement in 'Physical function' (MD 6.13, 95% CI ‐3.73 to 16.00, Analysis 1.8), 'Bodily pain' (MD 5.64, 95% CI ‐3.09 to 14.36, Analysis 1.10,) 'General health' (MD 5.76, 95% CI ‐0.80 to 12.32, Analysis 1.11), 'Mental health' (MD 6.21, 95% CI ‐1.85 to 14.27, Analysis 1.12) and 'Role emotional' (MD 2.79, 95% CI ‐7.43 to 13.01, Analysis 1.13).
Secondary outcomes
Cardiopulmonary haemodynamics
Only the study by Ehlken 2016 reported changes in cardiopulmonary haemodynamics measured using (RHC) following exercise‐based rehabilitation. In a subset of the study participants (31 exercise and 28 control) the authors reported a significant decrease (P < 0.01) in mPAP following exercise training (MD‐9.00, 95% CI ‐13.60 to ‐4.40, Analysis 1.19).
Functional class
Only two studies reported changes in functional class for exercise and control groups Mereles 2006; Ganderton 2013. Improvement in functional class favoured exercise rehabilitation (MD ‐0.60, 95% CI ‐0.85 to ‐0.35, Analysis 1.20).
Clinical worsening during follow‐up period
No data were available for analysis.
Mortality during follow‐up period
No data were available for analysis.
B‐type natriuretic peptide
Only the study of Ehlken 2016 reported changes in B‐type natriuretic peptide for exercise and control groups. These authors reported a non significant (P = 0.36) improvement in B‐type natriuretic peptide with exercise rehabilitation (MD ‐236.00, 95% CI ‐744.48 to 272.48, Analysis 1.21).
Sensitivity analysis
For our sensitivity analysis we removed studies that did not specify blinding of outcome measurements or had incomplete outcome data (attrition bias). As a result two studies were removed from the analysis of exercise outcomes (Wilkinson 2007; Ehlken 2016). We did not undertake sensitivity analysis for changes in health‐related quality of life as the studies of Wilkinson 2007 and Ehlken 2016 were not included in the original HRQoL analysis. Sensitivity analysis did not change the pattern of findings, with the exercise group showing improvements in 6MWD (MD 67.91 metres, 95% CI 27.12 to 108.69, Analysis 1.22), VO2peak (MD 1.94 ml/kg/m2, 95% CI 0.86 to 3.01, Analysis 1.23), and peak power (MD 15.27 Watts, 95% CI 8.57 to 21.97, Analysis 1.24) compared to control.
Subgroup analysis
Type of PH
We compared the outcomes for different subgroups of PH using the classification outlined by Hoeper 2013. Three of the studies included a mixed group of PH participants, including both those with PAH (i.e. those from Group I, Hoeper 2013) and chronic thromboembolic pulmonary hypertension (i.e. Group 4, CTEPH, Hoeper 2013) (Mereles 2006; Ley 2013; Ehlken 2016). We were unable to extract data separately for the subgroups in these studies. We performed a subgroup analysis for the two studies that only included PAH (Group 1) participants (Chan 2013; Ganderton 2013). The increase in 6MWD, whilst much lower than the group as a whole, still exceeded the MID (MD 33.84 metres, 95% CI 0.95 to 66.73, Analysis 1.25). Likewise the increases in VO2peak (MD 1.28 ml/kg/min, 95% CI ‐0.19 to 2.75, Analysis 1.26) and peak power (MD 14.24 Watts, 95% CI 5.78 to 22.70, Analysis 1.27) were lower in the subgroup of participants with PAH. However these studies also differed in the setting and nature of the exercise rehabilitation programme delivered (see 'Setting of exercise rehabilitation programme' below) and it is therefore not possible to attribute these differences solely to diagnosis.
Severity of PH
Insufficient data were available to perform subgroup analysis according to disease severity.
Setting of exercise rehabilitation programme
We identified an additional source of potential heterogeneity whilst exploring the heterogeneity in 6MWD responses (Analysis 1.1). Three studies used inpatient programmes of three weeks' duration (training seven days per week) (Mereles 2006; Ley 2013; Ehlken 2016), in some cases followed by a 12‐week, home‐based programme (Mereles 2006; Ehlken 2016), whilst the remaining studies used outpatient training programmes. Because of the observed heterogeneity we chose to examine results for programmes that included inpatient training components in the exercise‐based rehabilitation intervention separately to those that only included outpatient programmes, as inpatient programmes may allow closer supervision and greater intensity of exercise prescription. Note for the studies of Mereles 2006 and Ehlken 2016 we reported outcomes following the three‐week inpatient plus 12‐week home‐based programme (i.e. 15 weeks) whereas for Ley 2013 we have reported outcomes following the three‐week inpatient programme.
Studies that incorporated an inpatient model of exercise rehabilitation (Mereles 2006; Ley 2013; Ehlken 2016) reported very large improvements in 6MWD, however marked heterogeneity was still present across these three studies (mean improvement 72.79 metres, 95% CI 28.09 to 117.49, I2 = 78%). The studies that relied totally on outpatient‐based exercise programmes (Chan 2013; Ganderton 2013) randomised only 36 people with PH (24% of total subject sample) and reported a smaller mean difference in 6MWD favouring the exercise group of 33.84 metres (0.95 to 66.73 metres higher) but with no evidence of statistical heterogeneity (I2 = 0%). The test for subgroup differences was not significant (P = 0.17, Analysis 1.29). It should be noted that both these studies only included participants with PAH, so these subgroup analyses by setting give rise to the same results as those for the subgroup analysis according to type of PH.
Discussion
Summary of main results
The aim of this review was to examine the efficacy of exercise‐based rehabilitation in people with PH. The included studies reported large and clinically significant improvements in exercise capacity, measured using both the 6MWD and CPET. However there was marked heterogeneity across trials for 6MWD; we were unable to determine whether this was due to differences in study populations (PAH versus other), settings (inpatient versus outpatient) or the severity of the disease (where there was insufficient evidence to assess). There were also improvements in quality of life, measured using both PH‐specific and non‐specific tools, although the magnitude of these changes may not be clinically important. There was only a single reportable adverse event. These results are based on a relatively small number of participants (there were 206 participants in the trials, but data from only 165 in the forest plot with the most data) from only five RCTs. It was not possible to determine the impact of exercise rehabilitation on the secondary outcomes of cardiopulmonary haemodynamics, functional class or B‐type natriuretic peptide due to insufficient data. No studies reported on the effects of rehabilitation, time to clinical worsening or mortality. The quality of evidence was generally low, with no studies reporting allocation concealment, and the potential for selection bias, as there were few details provided regarding screening of potential artificialness. All outcomes were short term, measured immediately following the rehabilitation period, so the longer‐term effects of exercise rehabilitation remain unknown.
Overall completeness and applicability of evidence
Most participants in the studies had a diagnosis of PAH, so our results should be applied primarily in that group. There was a small number of participants with CTEPH, however their results could not be extracted separately, so it is difficult to be confident regarding the effects of exercise rehabilitation in this group. Of the studies completed to date, none have included groups of participants who had PH associated with connective tissue disease or congenital heart disease, PH due to left heart disease, or PH due to lung disease, so our results cannot be applied to these groups. Few participants in functional class IV were included, so the impact of exercise rehabilitation in those with the most severe disease remains unclear. Importantly, all studies only included participants who were stable on medical therapy (including no recent syncope), so it is in this group that exercise rehabilitation can be applied.
Three of the six studies used an inpatient rehabilitation programme of at least three weeks in duration, with exercise training taking place seven days per week (Mereles 2006; Ley 2013; Ehlken 2016). The magnitude of improvement in exercise outcomes appeared to be greater following these programmes compared to those who used an outpatient exercise‐based rehabilitation model, where supervised training took place only two to three times per week (Chan 2013; Ganderton 2013). However we were unable to determine whether the underlying diagnosis of participants also affected the outcomes. The inpatient exercise rehabilitation programmes delivered closer supervision, more sophisticated monitoring and a higher frequency of training than the outpatient programmes, which may contribute to better exercise outcomes. Such inpatient cardiorespiratory rehabilitation programmes are common in some parts of Europe, but are virtually non‐existent in other parts of the world such as the UK, Australia and the USA. Such differences in health system organisation may affect the type of exercise rehabilitation model that can be applied in PH. However it should be noted that improvements following outpatient training, although smaller in magnitude, were clinically important.
The exercise rehabilitation protocols tested included lower limb endurance training (walking or cycling), usually with resistance exercises for the upper and lower limbs. These protocols are similar to those recommended for standard pulmonary (Spruit 2013) and cardiac (Piepoli 2014) rehabilitation programmes. Additional components in some studies included stretching, breathing techniques such as pursed lip breathing, body perception, yoga, and strengthening of respiratory muscles (Mereles 2006). Further data is required to identify the contribution of these additional components to rehabilitation outcomes. The similarity of the core rehabilitation components to those delivered in pulmonary and cardiac rehabilitation programmes (lower limb endurance training, upper and lower limb resistance training) suggests that people with PH could receive their rehabilitation within these existing services, which could improve uptake into practice. However some studies in this review used specialised exercise prescription and monitoring practices that may not occur routinely in existing cardiopulmonary rehabilitation programmes (e.g. low‐intensity interval training, continuous monitoring of oxyhaemoglobin saturation and heart rate, restriction of exercise heart rate to less than 120 beats per minute) (Mereles 2006; Ley 2013; Ehlken 2016). Whilst no significant adverse events were documented during supervised exercise training in the studies included in this review, it is clear that exercise is not entirely without risk in PH (Morris 2015) and international guidelines currently suggest that exercise rehabilitation should be undertaken "...by centres experienced in both PH patient care and rehabilitation of compromised patients" (Galie 2015).
Quality of the evidence
It was encouraging that five out of six included studies reported blinding of outcome assessors, which is important for rehabilitation studies where many of the important outcomes (exercise capacity, HRQoL) could be affected by knowledge of group assignment. Random sequence generation and allocation concealment were generally not well reported. However the major source of potential bias related to reporting of participant selection. For three of the six studies it was not clear how many people had been assessed in order to achieve the required sample size. Pulmonary hypertension comprises a diverse group of patients with wide variation in disease severity. In contrast the participants in the included trials were predominantly from Group 1 and tended to have mild to moderate disease. It remains possible that the participants in these studies were a highly selected group who responded well to exercise training. Future studies should carefully report their screening and selection procedures in accordance with CONSORT requirements (Schulz 2010).
Potential biases in the review process
All data were extracted independently by two review authors using Covidence and discrepancies were resolved through discussion (Covidence 2016). Risk of bias ratings were also completed independently by two review authors. We included studies that were published only in abstract form, to ensure that all available trials were included. However, despite attempts to contact the authors of one abstract, additional data were not available (Wilkinson 2007). This may have influenced assessment of trial quality and some estimates of effect. We included an additional subgroup analysis (inpatient versus outpatient rehabilitation setting) that was not included in our protocol. This was because the marked heterogeneity in exercise outcomes prompted us to further explore the differences between studies, but we acknowledge that it is difficult to draw firm conclusions from this analysis due the post hoc nature of the approach.
Agreements and disagreements with other studies or reviews
Currently there are four published systematic reviews on exercise training in PH (Buys 2015; Pandey 2015; Yuan 2015; Babu 2016a), however the included studies, methods of analysis and assessment of study quality differed within these reviews. Like the current review, the systematic review of Buys 2015 examined only controlled trials up to December 2013, not all of which were randomised. The authors extracted five studies, three of which (Mereles 2006; Chan 2013; Ley 2013) were included in our analyses and used an adapted PEDro scale to rate the quality of these studies. This review also included the studies by Fox 2011 and Martinez‐Quintana 2010 both of which were excluded from our analysis as subjects were non‐randomly allocated to exercise or control groups. Overall this review generated similar results as the current review with a large increase in 6MWD (5 studies, MD for exercise group 72.5 m, 95% CI: 46.0 to 99.1) and VO2peak (3 studies, MD for exercise group 2.2 ml/kg‐1/min‐1, 95% CI 46.0 to 99.1). The other three systematic reviews (Pandey 2015; Yuan 2015;Babu 2016a) included both randomised controlled trials and observational studies and hence analysed a larger number of studies. Babu 2016a reported that exercise training resulted in large changes in exercise capacity, health‐related quality of life and very few adverse events in 15 included studies, four of which were classified as randomised controlled trials. These authors did not undertake a meta‐analysis of the studies. Yuan 2015 did undertake a meta‐analysis, reporting large increases in exercise capacity (6MWD and peak exercise capacity), health‐related quality of life (measured using the SF‐36) and few adverse events in the 12 studies they classified as being either randomised (n = 2), observational‐control (n = 4) or observational (n = 6). The authors undertook a subgroup analysis of randomised trials and whilst producing similar results to our study for exercise capacity (MD for exercise group 62 m, 95% CI: 45.6 to 78.8), these authors included data from Weinstein 2013, which we considered to be a duplicate report of one of the studies included in our review (Chan 2013). Moreover Yuan 2015 included the study by Fox 2011 as an RCT, a study excluded from our analysis. Pandey 2015 included 16 studies, with a subgroup of six parallel‐group studies. Similar to Yuan 2015 these authors included the study of Fox 2011 in this analysis. Pandey 2015 also included the study of Martinez‐Quintana 2010, in their parallel‐group analysis, a study again excluded from our analysis. Like other reviews, Pandey reported large increases in exercise capacity measured using the 6MWD and quality of life. Whilst these systematic reviews overall reported treatment effects of a similar magnitude to the current review, there were differences in the rating of the quality of evidence. Using the Downs and Black Quality Index (Downs and Black 1998), Babu 2016a rated the four included RCTs as providing good‐quality evidence (Chan 2013; Weinstein 2013; Ley 2013; Mereles 2006), however issues of possible selection bias were not identified. Pandey 2015 used the Cochrane risk of bias assessment tool to evaluate the quality of the extracted controlled intervention trials. Similar to our findings the authors reported that the majority of studies used random sequence generation and blinded assessment. The authors did not however recognise the potential for selection bias in their analysis. There does not appear to have been any attempt to report on the quality of evidence in the meta‐analysis conducted by Yuan 2015.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Exercise vs control, outcome: 1.1 Exercise capacity: 6MWD
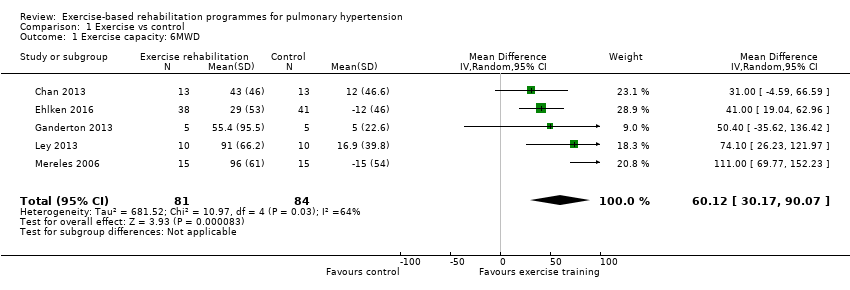
Comparison 1 Exercise vs control, Outcome 1 Exercise capacity: 6MWD.

Comparison 1 Exercise vs control, Outcome 2 Exercise capacity: VO2peak.

Comparison 1 Exercise vs control, Outcome 3 Exercise capacity: Peak power.

Comparison 1 Exercise vs control, Outcome 4 Exercisecapacity: Anaerobic threshold.
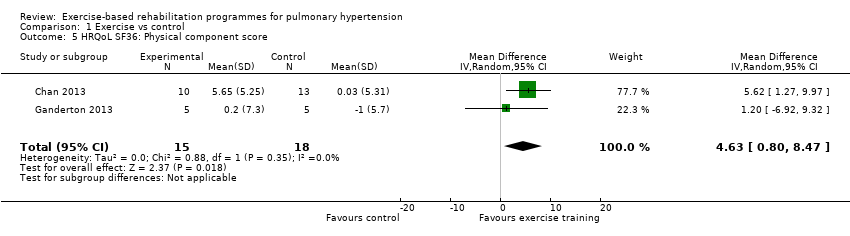
Comparison 1 Exercise vs control, Outcome 5 HRQoL SF36: Physical component score.

Comparison 1 Exercise vs control, Outcome 6 HRQoL SF36: Mental component score.
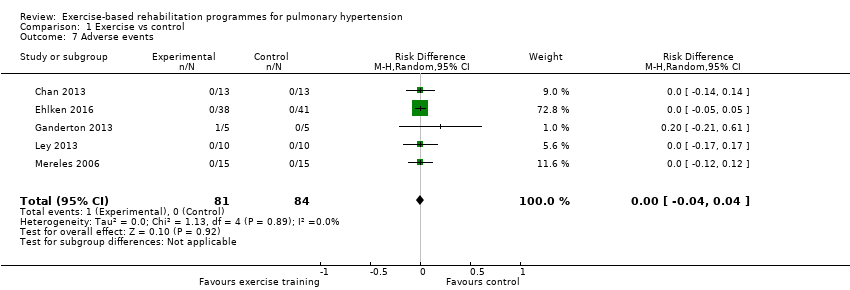
Comparison 1 Exercise vs control, Outcome 7 Adverse events.

Comparison 1 Exercise vs control, Outcome 8 HRQoL SF36: Physical function.
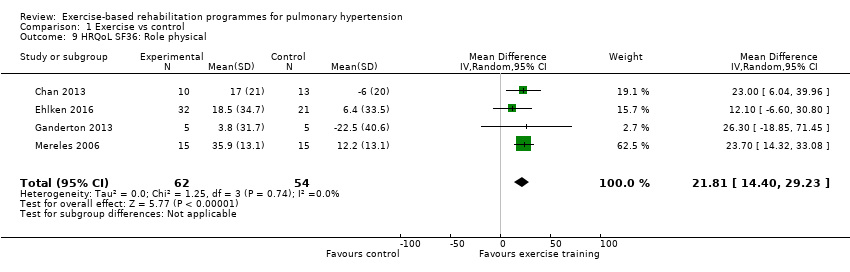
Comparison 1 Exercise vs control, Outcome 9 HRQoL SF36: Role physical.

Comparison 1 Exercise vs control, Outcome 10 HRQoL SF36: Bodily pain.
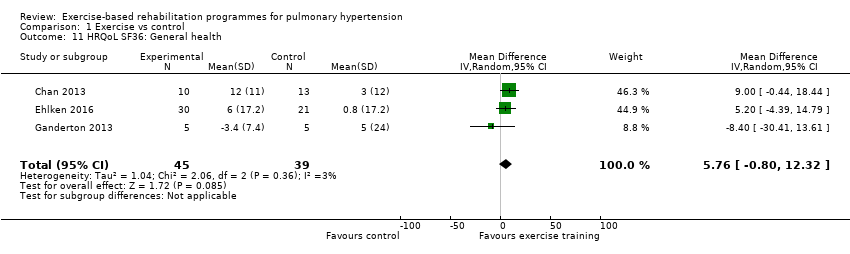
Comparison 1 Exercise vs control, Outcome 11 HRQoL SF36: General health.
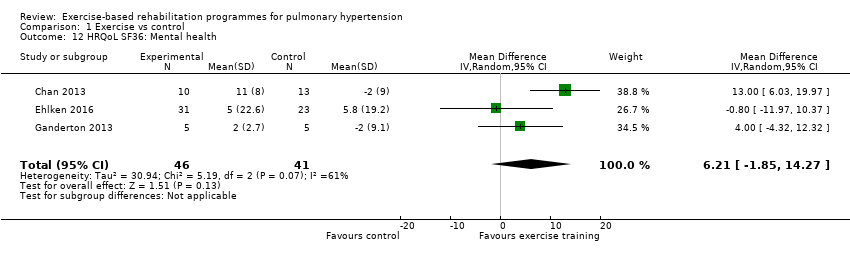
Comparison 1 Exercise vs control, Outcome 12 HRQoL SF36: Mental health.

Comparison 1 Exercise vs control, Outcome 13 HRQoL SF36: Role emotional.

Comparison 1 Exercise vs control, Outcome 14 HRQol SF36: Vitality.
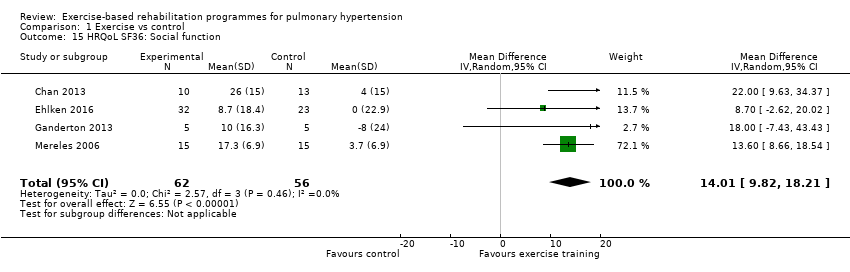
Comparison 1 Exercise vs control, Outcome 15 HRQoL SF36: Social function.

Comparison 1 Exercise vs control, Outcome 16 HRQoL: CAMPHOR activities.

Comparison 1 Exercise vs control, Outcome 17 HRQoL: CAMPHOR symptoms.

Comparison 1 Exercise vs control, Outcome 18 HRQoL: CAMPHOR QoL.
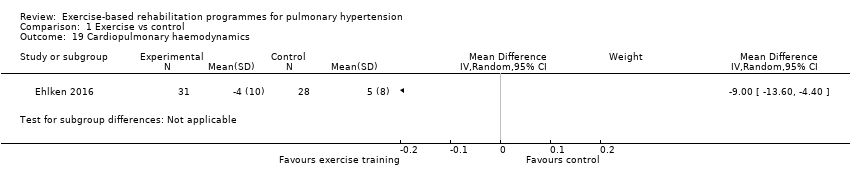
Comparison 1 Exercise vs control, Outcome 19 Cardiopulmonary haemodynamics.

Comparison 1 Exercise vs control, Outcome 20 Functional class.
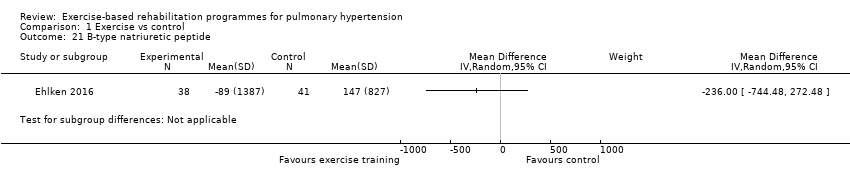
Comparison 1 Exercise vs control, Outcome 21 B‐type natriuretic peptide.
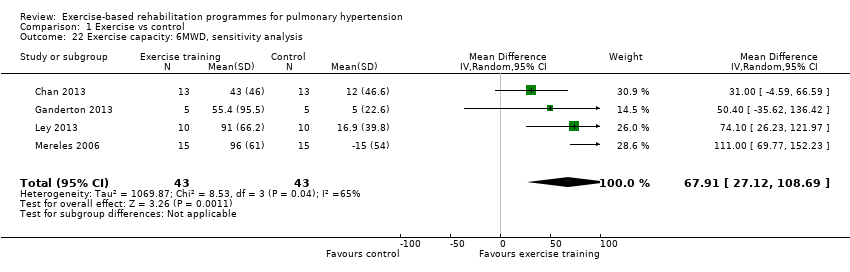
Comparison 1 Exercise vs control, Outcome 22 Exercise capacity: 6MWD, sensitivity analysis.

Comparison 1 Exercise vs control, Outcome 23 Exercise capacity: VO2peak, sensitivity analysis.
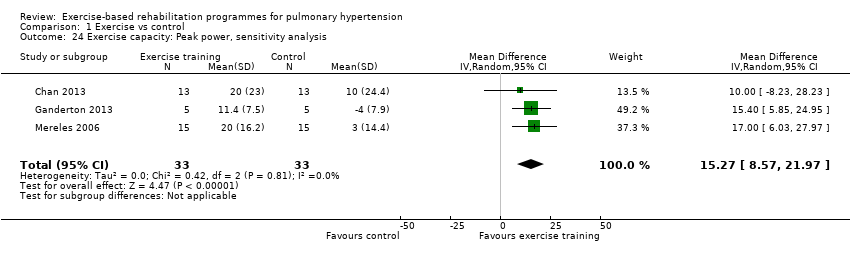
Comparison 1 Exercise vs control, Outcome 24 Exercise capacity: Peak power, sensitivity analysis.

Comparison 1 Exercise vs control, Outcome 25 Exercise capacity 6MWD, PAH subgroup only.
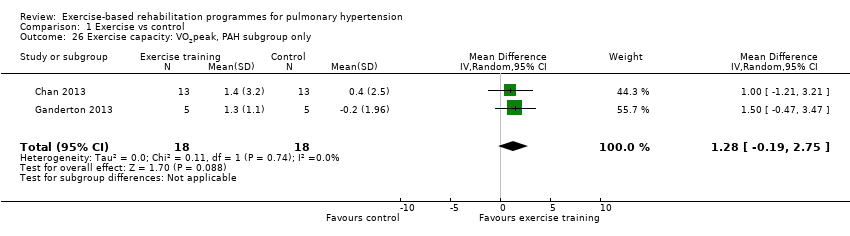
Comparison 1 Exercise vs control, Outcome 26 Exercise capacity: VO2peak, PAH subgroup only.
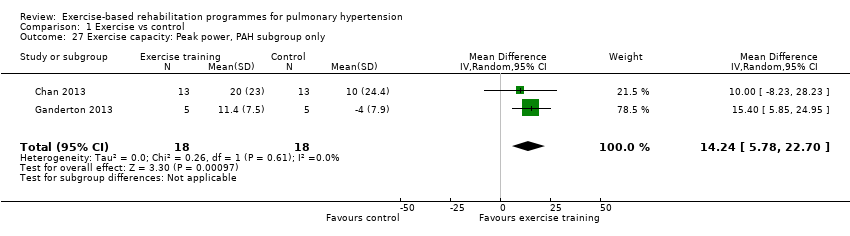
Comparison 1 Exercise vs control, Outcome 27 Exercise capacity: Peak power, PAH subgroup only.

Comparison 1 Exercise vs control, Outcome 28 Exercise capacity: Anaerobic threshold, PAH subgroup only.

Comparison 1 Exercise vs control, Outcome 29 Exercise capacity: 6MWD, subgroup analysis for setting of rehabilitation.
| Exercise compared to control for pulmonary hypertension | |||||
| Patient or population: people with pulmonary hypertension | |||||
| Outcomes | Illustrative comparative effects* (95% CI) | No of participants | Quality of the evidence | Comments | |
| Response on control | Treatment effect | ||||
| Control | Exercise | ||||
| Change in functional exercise capacity (6MWD) | Median change = 5 m | The mean exercise capacity 6MWD in the intervention groups was 60.12 higher | 165 | ⊕⊕⊝⊝ | Subgroup PAH: (2 studies, n = 36), mean 6MWD for intervention group was 33.84 m higher (0.95 to 66.73 higher); these studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component Minimal important difference was 30 metres |
| Exercise capacity: VO2peak Oxygen uptake, ml/kg/min | Median change = ‐0.25 ml/kg/min | The mean VO2peak in the intervention groups was 2.41 ml/kg/min higher | 145 | ⊕⊕⊝⊝ | Subgroup PAH (2 studies, n = 36), the mean VO2peak in the intervention groups was 1.28 ml/kg/min higher (‐0.19 to 2.75 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
| Exercise capacity: peak power watts | Median change = 1 watt | The mean exercise capacity: peak power in the intervention groups was 16.44 W higher | 145 | ⊕⊕⊝⊝ | Subgroup PAH (2 studies, n = 36), the mean peak power in the intervention groups was 14.24 watts higher (5.78 to 22.70 higher); these two studies used outpatient exercise rehabilitation whilst other studies contributing to meta‐analysis had an inpatient training component |
| HRQoL SF‐36: PCS units Follow‐up median 11 weeks | Median change = ‐0.49 units | The mean HRQoL SF‐36: PCS in the intervention groups was 4.63 higher (0.80 to 8.47 higher) | 33 | ⊕⊕⊝⊝ | Both studies were only PAH |
| HRQoL SF‐36: MCS units Follow‐up median 11 weeks | Median change = ‐0.31 units | The mean HRQoL SF‐36: MCS in the intervention groups was 4.17 higher (0.01 to 8.34 higher) | 33 | ⊕⊕⊝⊝ | Both studies were only PAH |
| *The basis for the response on control is the median control group response across studies | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Two studies did not report random sequence generation, no studies reported allocation concealment 3 Imprecision (2 small studies of 33 participants) and neither reported allocation concealment | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exercise capacity: 6MWD Show forest plot | 5 | 165 | Mean Difference (IV, Random, 95% CI) | 60.12 [30.17, 90.07] |
| 2 Exercise capacity: VO2peak Show forest plot | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 2.41 [1.38, 3.44] |
| 3 Exercise capacity: Peak power Show forest plot | 4 | 145 | Mean Difference (IV, Random, 95% CI) | 16.44 [10.90, 21.99] |
| 4 Exercisecapacity: Anaerobic threshold Show forest plot | 3 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.53, 1.58] |
| 5 HRQoL SF36: Physical component score Show forest plot | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 4.63 [0.80, 8.47] |
| 6 HRQoL SF36: Mental component score Show forest plot | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 4.17 [0.01, 8.34] |
| 7 Adverse events Show forest plot | 5 | 165 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 8 HRQoL SF36: Physical function Show forest plot | 4 | 118 | Mean Difference (IV, Random, 95% CI) | 6.13 [‐3.73, 16.00] |
| 9 HRQoL SF36: Role physical Show forest plot | 4 | 116 | Mean Difference (IV, Random, 95% CI) | 21.81 [14.40, 29.23] |
| 10 HRQoL SF36: Bodily pain Show forest plot | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 5.64 [‐3.09, 14.36] |
| 11 HRQoL SF36: General health Show forest plot | 3 | 84 | Mean Difference (IV, Random, 95% CI) | 5.76 [‐0.80, 12.32] |
| 12 HRQoL SF36: Mental health Show forest plot | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 6.21 [‐1.85, 14.27] |
| 13 HRQoL SF36: Role emotional Show forest plot | 3 | 87 | Mean Difference (IV, Random, 95% CI) | 2.79 [‐7.43, 13.01] |
| 14 HRQol SF36: Vitality Show forest plot | 4 | 115 | Mean Difference (IV, Random, 95% CI) | 13.47 [7.55, 19.40] |
| 15 HRQoL SF36: Social function Show forest plot | 4 | 118 | Mean Difference (IV, Random, 95% CI) | 14.01 [9.82, 18.21] |
| 16 HRQoL: CAMPHOR activities Show forest plot | 2 | 33 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐3.56, 0.90] |
| 17 HRQoL: CAMPHOR symptoms Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | ‐3.08 [‐7.78, 1.62] |
| 18 HRQoL: CAMPHOR QoL Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐8.03, ‐2.81] |
| 19 Cardiopulmonary haemodynamics Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20 Functional class Show forest plot | 2 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.85, ‐0.35] |
| 21 B‐type natriuretic peptide Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22 Exercise capacity: 6MWD, sensitivity analysis Show forest plot | 4 | 86 | Mean Difference (IV, Random, 95% CI) | 67.91 [27.12, 108.69] |
| 23 Exercise capacity: VO2peak, sensitivity analysis Show forest plot | 3 | 66 | Mean Difference (IV, Random, 95% CI) | 1.94 [0.86, 3.01] |
| 24 Exercise capacity: Peak power, sensitivity analysis Show forest plot | 3 | 66 | Mean Difference (IV, Random, 95% CI) | 15.27 [8.57, 21.97] |
| 25 Exercise capacity 6MWD, PAH subgroup only Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 33.84 [0.95, 66.73] |
| 26 Exercise capacity: VO2peak, PAH subgroup only Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 1.28 [‐0.19, 2.75] |
| 27 Exercise capacity: Peak power, PAH subgroup only Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 14.24 [5.78, 22.70] |
| 28 Exercise capacity: Anaerobic threshold, PAH subgroup only Show forest plot | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 41.31 [‐52.05, 134.67] |
| 29 Exercise capacity: 6MWD, subgroup analysis for setting of rehabilitation Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 29.1 Inpatient exercise training | 3 | 129 | Mean Difference (IV, Random, 95% CI) | 72.79 [28.09, 117.49] |
| 29.2 Outpatient exercise training | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 33.84 [0.95, 66.73] |

