Needle gauge and tip designs for preventing post‐dural puncture headache (PDPH)
Abstract
Background
Post‐dural puncture headache (PDPH) is one of the most common complications of diagnostic and therapeutic lumbar punctures. PDPH is defined as any headache occurring after a lumbar puncture that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of the patient lying down. Researchers have suggested many types of interventions to help prevent PDPH. It has been suggested that aspects such as needle tip and gauge can be modified to decrease the incidence of PDPH.
Objectives
To assess the effects of needle tip design (traumatic versus atraumatic) and diameter (gauge) on the prevention of PDPH in participants who have undergone dural puncture for diagnostic or therapeutic causes.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL and LILACS, as well as trial registries via the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal in September 2016. We adopted the MEDLINE strategy for searching the other databases. The search terms we used were a combination of thesaurus‐based and free‐text terms for both interventions (lumbar puncture in neurological, anaesthesia or myelography settings) and headache.
Selection criteria
We included randomized controlled trials (RCTs) conducted in any clinical/research setting where dural puncture had been used in participants of all ages and both genders, which compared different tip designs or diameters for prevention of PDPH
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
We included 70 studies in the review; 66 studies with 17,067 participants were included in the quantitative analysis. An additional 18 studies are awaiting classification and 12 are ongoing. Fifteen of the 18 studies awaiting classification mainly correspond to congress summaries published before 2010, in which the available information does not allow the complete evaluation of all their risks of bias and characteristics. Our main outcome was prevention of PDPH, but we also assessed the onset of severe PDPH, headache in general and adverse events. The quality of evidence was moderate for most of the outcomes mainly due to risk of bias issues. For the analysis, we undertook three main comparisons: 1) traumatic needles versus atraumatic needles; 2) larger gauge traumatic needles versus smaller gauge traumatic needles; and 3) larger gauge atraumatic needles versus smaller gauge atraumatic needles. For each main comparison, if data were available, we performed a subgroup analysis evaluating lumbar puncture indication, age and posture.
For the first comparison, the use of traumatic needles showed a higher risk of onset of PDPH compared to atraumatic needles (36 studies, 9378 participants, risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67, I2 = 9%).
In the second comparison of traumatic needles, studies comparing various sizes of large and small gauges showed no significant difference in effects in terms of risk of PDPH, with the exception of one study comparing 26 and 27 gauge needles (one study, 658 participants, RR 6.47, 95% CI 2.55 to 16.43).
In the third comparison of atraumatic needles, studies comparing various sizes of large and small gauges showed no significant difference in effects in terms of risk of PDPH.
We observed no significant difference in the risk of paraesthesia, backache, severe PDPH and any headache between traumatic and atraumatic needles. Sensitivity analyses of PDPH results between traumatic and atraumatic needles omitting high risk of bias studies showed similar results regarding the benefit of atraumatic needles in the prevention of PDPH (three studies, RR 2.78, 95% CI 1.26 to 6.15; I2 = 51%).
Authors' conclusions
There is moderate‐quality evidence that atraumatic needles reduce the risk of post‐dural puncture headache (PDPH) without increasing adverse events such as paraesthesia or backache. The studies did not report very clearly on aspects related to randomization, such as random sequence generation and allocation concealment, making it difficult to interpret the risk of bias in the included studies. The moderate quality of the evidence for traumatic versus atraumatic needles suggests that further research is likely to have an important impact on our confidence in the estimate of effect.
PICOs
Plain language summary
Needle characteristics that reduce the occurrence of post‐dural puncture headache (PDPH)
Background
A lumbar puncture is a needle inserted into the lower part of the spine to draw fluid, to test for conditions affecting the brain and spinal cord. It can also be used for treatment (for instance, for the management of pain in caesarean section).
In general, lumbar punctures are considered safe; however, a number of adverse effects such as backache, tickling sensations (paraesthesia) or even post‐dural puncture headache (PDPH) have been reported. These conditions are not life‐threatening, but can impair the person's physical activity and can be very painful. Several different needle tips (classified as traumatic or atraumatic) and gauges (size/diameter) are used to perform a lumbar puncture. We compared different types of needles to assess the effects of the needle tip and its thickness on the prevention of post‐dural puncture headache.
Study characteristics
We searched the medical literature for studies carried out in any setting comparing needles of different characteristics (i.e. different tip designs and sizes) for the prevention of PDPH. The evidence is current to September 2016. We included 70 studies and were able to include information from 66 of those studies (17,067 participants) in the numerical analysis. An additional 18 studies are awaiting classification and 12 are ongoing.
Key findings
We found that the use of needles with a traumatic tip resulted in a higher risk of PDPH when compared to needles with atraumatic tips. When we compared the different studies comparing various sizes of large and small traumatic gauges, we did not find any difference in effects in terms of the risk of PDPH. Finally, when we compared atraumatic needles with a higher gauge to those with a smaller gauge, we observed no significant differences in terms of the development of PDPH in any of the scenarios analysed. We also found no significant differences in the use of traumatic versus atraumatic needles in the development of adverse effects such as paraesthesia, backache and severe PDPH.
Quality of the evidence
The studies did not report clearly on aspects of their design related to randomization. (This is a method that uses the play of chance to assign participants to comparison groups in a trial). This made it difficult for us to interpret the risk of bias in the included studies. We therefore considered the quality of the evidence for most of the outcomes assessed in this review to be moderate.
Authors' conclusions
Summary of findings
| Traumatic needles compared to atraumatic needles for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needles | Traumatic needles | |||||
| Onset of PDPH | 30 per 1000 | 64 per 1000 | RR 2.14 | 9378 | ⊕⊕⊕⊝ | — |
| Adverse events: paraesthesia | 52 per 1000 | 50 per 1000 | RR 0.96 | 573 | ⊕⊕⊕⊝ | — |
| Adverse events: backache | 155 per 1000 | 147 per 1000 | RR 0.94 | 3027 | ⊕⊕⊕⊝ | — |
| Severe PDPH | 0 per 1000 | 10 per 1000 | RD 0 | 6420 | ⊕⊕⊝⊝ | — |
| Any headache | 221 per 1000 | 290 per 1000 | RR 1.35 | 4104 | ⊕⊕⊕⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Traumatic needle(major gauge) compared to traumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Traumatic needle ‐ smaller gauge | Traumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.86 to 6.47 | 2288 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Adverse events: paraesthesia ‐ not reported | See comment | See comment | Not estimable | — | See comment | We did not identify any studies reporting this outcome. |
| Adverse event: backache | — | — | RR ranged | 948 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Severe PDPH | — | — | RD ranged | 1128 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Any headache | — | — | RR ranged | 771 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Imprecision downgraded by one level due to few events reported in each arm. 3Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit. | ||||||
| Atraumatic needle (major gauge) compared to atraumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needle ‐ smaller gauge | Atraumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.38 to 9.3 | 3134 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: paraesthesia | — | — | RR ranged from 1.03 to 7.61 | 439 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: backache | — | — | RR ranged | 526 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Severe PDPH | — | — | RD ranged | 1983 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Any headache | — | — | RR ranged | 1791 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
Background
Description of the condition
Post‐dural (post‐lumbar or post‐spinal) puncture headache (PDPH) is one of the most common complications of diagnostic, therapeutic or inadvertent lumbar punctures (Bezov 2010; Davignon 2002; Raskin 1990; Sadashivaiah 2009). PDPH is defined as any headache after a lumbar puncture that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of the patient lying down (González‐Martínez 2005; Headache Classification Subcommittee IHS 2004). Most PDPHs occur within three days of the procedure and more than 50% start within the first 48 hours (Turnbull 2003).
The pathophysiology of PDPH has not been fully established. It is well known that puncture of the dura allows cerebrospinal fluid (CSF) to leak from the subarachnoid space, which results in decreased CSF volume and pressure (Grande 2005). This CSF volume loss may cause a downward pull on pain‐sensitive structures, which could explain the occurrence of PDPH (Ahmed 2006; Baumgarten 1987; Davignon 2002; Denny 1987; Harrington 2004). In addition, loss of CSF may cause an increase in blood flow, leading to arterial and venous vasodilatation, which could result in PDPH. A third PDPH mechanism may involve the role of substance P (neurotransmitter/neuromodulator involved in pain perception) and the regulation of neurokinin 1 receptors (NK1Rs) (Clark 1996). Defects in manufactured needles have also been described as a possible source of PDPH (Parker 1997). Laboratory studies have shown significant alteration of the tips of traumatic needles when their introducer needle protrudes through the inner hole of the needle. These altered tips can produce holes in the dura mater of increased diameter, which may require longer healing times and consequently increase the time allowed for leakage of CSF (Bezov 2010; Calthorpe 2004; Parker 1997).
Studies about the incidence of PDPH have reported a wide range of estimates, depending on target populations, types of needles and lumbar puncture techniques (Alstadhaug 2012; Arendt 2009; Lavi 2006; Shaikh 2008; Vallejo 2000). For example, during anaesthetic procedures such as epidural anaesthesia, PDPH is most commonly caused by an unintentional dural puncture (Thew 2008; Turnbull 2003). However, in diagnostic or therapeutic lumbar punctures, the need for adequate CSF flow requires an intentional lesion that may trigger the PDPH phenomenon (Kuczkowski 2006). Estimated frequencies of this event vary from less than 10% after spinal anaesthesia (Vallejo 2000) to 36% after diagnostic lumbar puncture (Lavi 2006; Vallejo 2000), and up to 81% in obstetric patients with inadvertent dural puncture during active labour (Berger 1998; Choi 2003).
The characteristics of PDPH are often variable. It may be accompanied by neck stiffness, tinnitus, hearing loss, photophobia and nausea, among other symptoms. Other characteristics such as the location and duration of the headache are also unpredictable (Grande 2005). Although PDPH is not a life‐threatening condition, physical activity is often restricted. Patients are usually required to stay in bed for the entire day, and length of hospital stay and use of medical services are increased (Angle 2005). The variability in symptom profiles makes PDPH a diagnosis of exclusion. Alternative diagnoses (e.g. viral meningitis, sinus headache, intracranial haemorrhage) should be ruled out first (Turnbull 2003).
Once PDPH is diagnosed, initial treatment involves conservative measures such as bed rest and analgesics. If PDPH continues for longer than 72 hours, more specific treatment is indicated (Ahmed 2006). Severe PDPH may respond to some therapeutic drugs and to an epidural blood patch (Boonmak 2010; Lavi 2006).
Description of the intervention
Many interventions have been suggested for the prevention of PDPH (e.g. body postures and fluid intake after lumbar puncture). One of the most relevant strategies involves the features of the needles (Arendt 2009). Although the choice of the needle depends mostly on the purpose of the lumbar puncture, several experts have remarked that facets such as the tip and the gauge could be modified to decrease the incidence of PDPH (American Society of Anesthesiologists 2007; Armon 2005).
According to tip design, needles can be divided into traumatic and atraumatic types. Atraumatic needles include Whitacre, Atraucan, Sprotte, Cappe and Deutsch, among others. Traumatic needles include Quincke, Greene, Hingson Ferguson, Lutz, Brace and Rovenstine, among others. Traumatic needles are characterized by a bevelled tip that cuts the dura mater. In contrast, atraumatic needles are characterized by a pencil‐point design. It has been stated that noncutting or atraumatic needles produce a separation of the tissue fibres that heals easily after removal of the needle. Cutting or traumatic needles, on the other hand, favour loss of tissue and trigger a large inflammatory reaction that requires a long time to heal (Calthorpe 2004; Lynch 1992; Wu 1991).
The external diameter of the needle is another factor that may be involved in the mechanisms of PDPH. The external diameter is determined by the cross‐sectional area of the needle; larger diameters are expected to produce larger orifices in the dura mater, thereby allowing increased CSF leakage. Larger gauges are represented by smaller numbers (e.g. 16 gauge, 17 gauge), and smaller gauges are represented by larger numbers (e.g. 29 gauge, 32 gauge) (Calthorpe 2004).
How the intervention might work
Studies that have compared needle internal diameters have found that needles of larger diameter produce larger holes in the dura mater and this could lead to a greater risk of post‐dural puncture headache (Bezov 2010; Lavi 2006; Shaikh 2008; Santanen 2004). However, evidence also suggests that the use of thinner needles increases the difficulty of the procedure and hence the number of bone punctures, causing needle tip deformities (Angle 2003). Some authors advocate the use of needles with cutting/traumatic tips based on the theory that these needles can cause larger lesions than are produced by pencil‐point/atraumatic needles (Calthorpe 2004; Lynch 1992a; Srivastava 2010a). Pencil‐point needles were thought to penetrate and then separate dura mater fibres, resulting in less trauma and subsequently less loss of CSF and a lower incidence of PDPH (Arendt 2009). A large inflammatory reaction caused by larger lesions can lead to faster closing of the injury through rapid migration of the cells involved in scar formation. Microscopic analyses of corpses have revealed that injuries produced by pencil‐point needles are more complex than those produced by cutting needles (Arendt 2009).
Why it is important to do this review
Lumbar puncture is part of everyday clinical practice and is associated with potential adverse effects (Evans 2009; Grande 2005). Prevention strategies should be preferred over treatment of adverse effects (Turnbull 2003). Morbidities associated with CSF loss, besides PDPH, include peripartum seizures, cranial subdural haematomas and subdural fluid collections (Arendt 2009; Janssens 2003). Even though most cases of PDPH are resolved within a few days, a significant number of patients experience at least one week of disability, and others require prolonged or recurrent hospitalizations (van Kooten 2008). Prevention strategies, such as the use of a prophylactic epidural blood patch, caffeine or different postures after lumbar puncture, have not proved effective for the prevention of PDPH in several Cochrane Reviews (Arevalo‐Rodriguez 2013; Basurto 2013; Boonmak 2010).
Objectives
To assess the effects of needle tip design (traumatic versus atraumatic) and diameter (gauge) on the prevention of post‐dural puncture headache (PDPH) in participants who have undergone dural puncture for diagnostic or therapeutic causes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) conducted in any clinical/research setting where dural puncture has been used.
Types of participants
We included participants of all ages and both genders who have undergone lumbar puncture for medical reasons.
Types of interventions
We included studies in participants undergoing lumbar puncture that assessed one of the following interventions.
-
A needle tip design/bevel used for lumbar puncture (i.e. traumatic or atraumatic) versus another needle tip design/bevel.
-
A specified needle gauge (i.e. from 16 gauge to 32 gauge) versus another needle gauge for the same type of tip design (i.e. traumatic or atraumatic).
-
Any combination of the above.
Types of outcome measures
Primary outcomes
-
Onset of PDPH, defined as each headache that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of lying down after a lumbar puncture. We used the valid PDPH diagnostic criteria specified by the International Headache Society (Headache Classification Subcommittee IHS 2004).
-
Adverse events related to lumbar puncture: total adverse events and total serious adverse events. We defined an adverse event as "any untoward medical occurrence that may present during treatment with a pharmaceutical product but that does not necessarily have a causal relationship with this treatment". Due to heterogeneity in the report of adverse events, we choose paraesthesia and backache as the most important adverse events, additional to PDPH, related to needle gauge and tip. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and it is explained in the Differences between protocol and review section.
Secondary outcomes
-
Severe PDPH, according to the definition used in each study, which could be based on specific features (e.g. duration of PDPH), a visual analogue scale (VAS) or other criteria such as the need for specialized treatments to manage the episode of headache (e.g. epidural blood patch).
-
Any headache subsequent to a lumbar puncture, to incorporate any possible data that had not been catalogued as PDPH, according to the definition used in each study.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 9) (see Appendix 2 for details of the search strategy), PubMed, MEDLINE (1966 to September 2016, see Appendix 3), EMBASE via Ovid SP (1982 to September 2016, see Appendix 4), CINAHL (EBSCOhost, 1982 to September 2016, see Appendix 5) and LILACS (1982 to September 2016 see Appendix 6).
We adopted the MEDLINE search strategy in searching the other databases. The search terms are a combination of thesaurus‐based and free‐text terms for both the intervention (lumbar puncture in neurological, anaesthesia or myelography settings) and the headache. We did not impose any language restriction.
Searching other resources
We searched trial registries via the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal up to September 2016. In addition, we searched the reference lists from retrieved studies, information from clinical trial registration websites and conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (JJA and LM) independently selected studies for eligibility using Early Review Organizing Software (EROS) (Ciapponi 2011; Ciapponi 2011a; Glujovsky 2010). We reviewed the titles and abstracts of all identified studies to determine whether they fulfilled the inclusion criteria. We assessed the full texts of selected studies to confirm their relevance for inclusion. We resolved any disagreement by consulting with a third review author (AC). We were not blinded to the authors' names and institutions, the journal of publication or the study results at any stage of the review.
Data extraction and management
Three review authors (NG‐C, SB and LM) independently used pre‐designed data forms to extract information from the original study reports about participants, methods of randomization, blinding, comparisons of interest, numbers of participants originally randomly assigned by arm, follow‐up losses and outcomes (double data entry) (Appendix 7). We recorded the reasons for exclusion of potential studies in the Characteristics of excluded studies table. We resolved any disagreement by discussion with a fourth review author (IA‐R). We entered the extracted data into Review Manager 5 for the analyses (RevMan 5.3).
Assessment of risk of bias in included studies
Two review authors (NG‐C and IA‐R) independently assessed the risk of bias of included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered seven domains (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias). We did not consider blinding of personnel because of the nature of the intervention (lumbar puncture). We resolved any disagreements by discussion with a third review author (MRF).
Measures of treatment effect
We presented results as summary risk ratios (RRs) for incidence of PDPH, adverse events, severe PDPH and any headache along with 95% confidence intervals (CIs). We calculated the number needed to treat for an additional beneficial outcome (NNTB) as the reciprocal of risk differences (RDs) (McQuay 1998).
Unit of analysis issues
We did not expect to encounter any unit of analysis issues, as we did not expect to find cross‐over studies or cluster‐randomized trials. However, we identified four such studies with our search strategies and excluded them from quantitative analysis. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
Dealing with missing data
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis (i.e. we attempted to include in the analyses all randomized patients in the denominator of the assessed groups).
Assessment of heterogeneity
We assessed statistical heterogeneity of effect sizes by means of the I2 statistic. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than to sampling error (Higgins 2003; Higgins 2011). If we identified at least moderate heterogeneity (i.e. I2 > 30%), we explored it by performing prespecified subgroup analyses. If we identified substantial heterogeneity (I2 > 80%), we did not present the pooled result.
Assessment of reporting biases
We assessed reporting bias through careful attention to assessment of quality, particularly the quality of study methodology. We also used funnel plot analysis to assess publication bias.
Data synthesis
We summarized the findings using random‐effects models with the DerSimonian‐Laird method. We carried out statistical analyses using Review Manager 5 (RevMan 5.3).
Subgroup analysis and investigation of heterogeneity
For the primary outcomes, we considered subgroup analyses for the following factors, as appropriate.
-
Participants undergoing dural puncture for anaesthesia only, diagnosis only or myelography only.
-
Pregnant women only.
-
Gender: it has been reported that women are at twice the risk of men (Alstadhaug 2012; Bezov 2010; Evans 2009).
-
Age (younger than 18 years of age, older than 65 years of age and 18 to 65 years of age). Due to heterogeneity in the reporting of age, we classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section
-
Posture during the lumbar puncture (e.g. lateral, sitting).
-
Type of surgery: in participants receiving anaesthesia, we analysed the primary outcome by type of surgical procedure if data were available. As we mentioned in the Background, some patients such as obstetric women have an increased risk of PDPH. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
Sensitivity analysis
We performed a sensitivity analysis to compare the results from using only those RCTs classified as having a 'low risk of bias' in three core domains: allocation concealment, incomplete outcome data and blinding of outcome assessment (Higgins 2011). In addition, we performed a sensitivity analysis to measure the risk difference (RD) in those analysis that presented zero events in both treatment arms. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
'Summary of findings' tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with all outcomes (onset of PDPH and adverse events), and we constructed a 'Summary of findings' table using the GRADE profiler software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the quality of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Balshem 2011; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g). For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations. We included the following outcomes in the 'Summary of findings' tables: onset of PDPH, adverse events (i.e. paraesthesia, backache), severe PDPH and any headache.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We searched the databases in February 2015, identifying a total of 1201 references. We found four additional references using other research strategies. After reviewing the references by title and abstract, we selected 138 of them to review as full texts (see Figure 1). After reading the articles, we included 70 studies (distributed in 75 references). We excluded 35 studies. We classified 12 as ongoing studies and 15 as studies awaiting assessment. We reran the search in September 2016, identifying a total of 96 new references. We selected a further three studies for in‐depth review (Castrillo 2015; Fama 2015; Hong 2015). We added these three potential new studies of interest to a list of ‘Characteristics of studies awaiting classification' and we will incorporate them into the formal review findings during the review update.
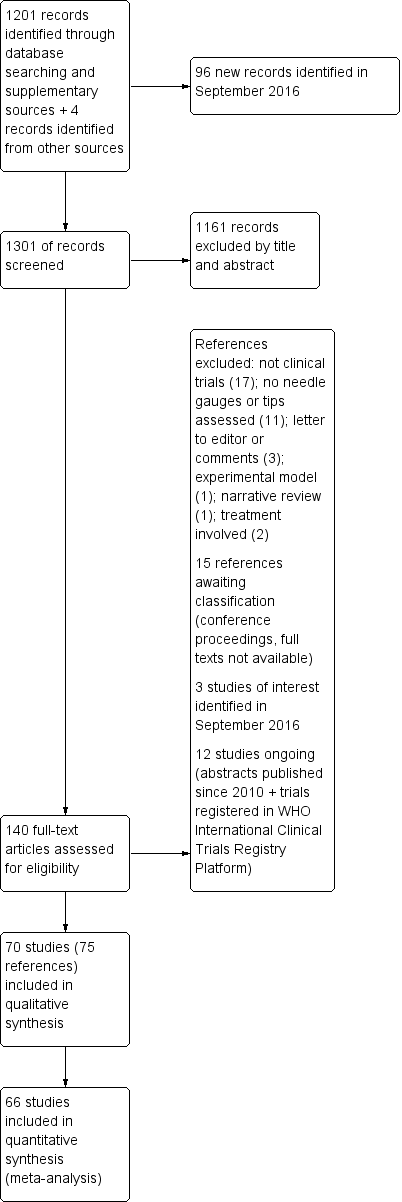
Study flow diagram
Included studies
We included 70 studies in the qualitative synthesis of the review, accounting for 75 references (see Figure 1 and Characteristics of included studies). However, we excluded four of these from the quantitative data analysis as their results were obtained using a different unit of analysis to the one planned for this review (procedures instead of participants: four studies). One of these studies was a study with a cross‐over design (Crock 2014), and it included children receiving treatment for leukaemia. The remaining three were parallel‐group trials that included all the lumbar punctures undertaken on the participants during the lifespan of the study (Hafer 1997; Kokki 1999; Lavi 2006); however, in some cases it was not clear if the procedures or the participants were randomized (Kokki 1999; Lavi 2006).
The quantitative analysis included 66 studies with a total of 17,067 participants (mean 258.6 participants; standard deviation (SD) 236.7; interquartile range (IQR) 100 to 311), published between 1972 and 2013. The sample sizes of the studies included ranged from 40 to 1522 participants (see Characteristics of included studies).
We classified the studies according to the needle tip design used, as follows: traumatic needles = Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon; atraumatic needles = Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor. Thirty‐nine studies (10,715 participants) compared traumatic needles versus atraumatic needles. Eleven studies compared traumatic needles of different gauges (2896 participants) and 15 studies compared atraumatic needles of different gauges (4095 participants). Four studies provided information for two different comparisons (Kokki 1998; Shah 2010; Shaikh 2008; Shutt 1992). The type of needle tip used could not be determined in seven of the studies (Geurts 1990; Harrison 1993; McGann 1992; Rasmussen 1989a; Rasmussen 1989b; Tourtellotte 1972; Wilkinson 1991). In one case, a hybrid point needle (a combination of diamond and pencil points) was compared to an atraumatic needle (Standl 2004). Two references provided information on two studies in the same publication and we analysed these as two independent groups of data (Rasmussen 1989a; Rasmussen 1989b; Srivastava 2010a; Srivastava 2010b).
Most of the studies included both genders, however 20 only included women and one only included men (Saenghirunvattana 2008). Similarly, most of the studies included patients in all age ranges; three only included under 18 year‐olds (Kokki 1996; Kokki 1998; Kokki 2000), and one study only included over 60 year‐olds (Kim 2011). The 25 gauge needle was the most frequently assessed (414 groups), followed by the 22 gauge (20 groups). In one study, it was not possible to identify the gauge of the needle used or its brand (Kokki 2000). A Quincke needle was used in 57 groups, followed by Whitacre needles (31 groups) and Sprotte (21 groups).
Among the indications for lumbar puncture, 57 studies undertook this procedure to administer anaesthesia. The most common reasons for the administration of anaesthesia were caesarean section (15 studies), followed by orthopaedic interventions (eight studies). The remaining studies combined different types of subumbilical surgery such as urologic surgery, outpatient surgery and tubal ligation among others. Five studies used lumbar puncture as a diagnosis method, including for the detection of infections, while a further seven studies used lumbar puncture for myelography. The most common site for puncture was between lumbar vertebrae (L) 2‐3 and 3‐4 (12 studies), followed by L3 to 4 (nine studies). Nineteen studies did not report puncture site and 25 studies reported that the puncture was undertaken by trained and experienced professionals, whereas 35 studies did not provide such information. The most common body position during the procedure was a lateral position (23 studies) and a seated position (21 studies).
Excluded studies
We excluded a total of 35 studies from the review as most of them were not clinical trials. In 11 cases, the studies were not designed to evaluate needles, their gauge or tip for the prevention of PDPH. Readers can find more information in the Characteristics of excluded studies table.
Studies awaiting classification
In total we classified 18 studies as awaiting classification. We found 15 of these during the February 2015 search (Bano 2004; Buttner 1990; De Andres 1994; Fyneface‐Ogan 2006; Harrison 1994; Jager 1995; Jensen 1999; Kaul 1996; Knudsen 1998; Lim 1992; Maclean 1994; Mignonsin 1991; Palmieri 1993; Puolakka 1997; Vandana 2004). These 15 studies mainly correspond to congress summaries published before 2010, in which the available information does not allow the complete evaluation of all their risks of bias and other characteristics. Also, the fact that they were written so long ago makes the likelihood of them being published as complete articles very low. We also classified articles that could not be obtained as full texts from the authors, the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group and the Iberoamerican Cochrane Centre as awaiting assessment.
We reran the search in September 2016 and selected a further three studies for in‐depth review (Castrillo 2015; Fama 2015; Hong 2015).
Ongoing studies
We classified 12 studies as ongoing (Ahmed 2012; Akdemir 2011; Bertolotto 2014; Bertolotto 2014a; Bham 2010; IRCT201009292080N4; Lorthe 2014; NCT00370604; NCT01821807; NCT02384031; Shah 2011; Shaikh 2013), given that we were only able to find summaries of their results. However, we considered that they could be subject to publication in a short time given the year of reference (after 2010). See Characteristics of ongoing studies.
Risk of bias in included studies
We assessed the risk of bias of the studies in seven categories. We provide a summary of our assessment of the risk of bias of the included studies in Figure 2 and Figure 3.
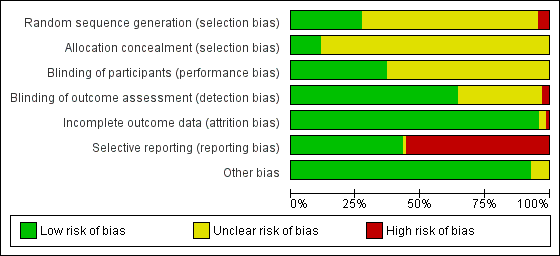
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
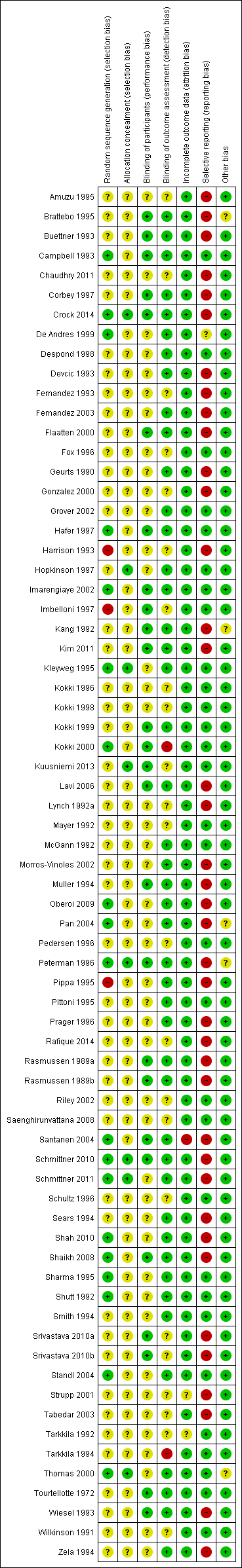
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 19 studies, the authors reported a valid method of randomization (Campbell 1993; Crock 2014; De Andres 1999; Hafer 1997; Imarengiaye 2002; Kleyweg 1995; Kokki 2000; Oberoi 2009; Pan 2004; Peterman 1996; Santanen 2004; Schmittner 2010; Schmittner 2011; Shah 2010; Shaikh 2008; Sharma 1995; Shutt 1992; Standl 2004; Thomas 2000), whereas this information was not clearly reported in the remaining 48 studies. As mentioned above, in three studies the authors reported an invalid method of randomization (Harrison 1993; Imbelloni 1997; Pippa 1995), and we rated them as at high risk of selection bias.
Eight studies undertook and reported adequate random allocation concealment (Crock 2014; Hopkinson 1997; Kleyweg 1995; Kuusniemi 2013; Peterman 1996; Schmittner 2010; Schmittner 2011; Thomas 2000), whereas this information was absent in the rest of the included studies.
Blinding
Twenty‐six studies reported blinding of participants (Brattebo 1995; Buettner 1993; Campbell 1993; Corbey 1997; Crock 2014; Flaatten 2000; Hafer 1997; Imarengiaye 2002; Imbelloni 1997; Kang 1992; Kim 2011; Kokki 1999; Kokki 2000; Kuusniemi 2013; Lavi 2006; Muller 1994; Peterman 1996; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Tourtellotte 1972; Wiesel 1993), and we assessed them as at low risk of bias. However, the remaining 44 studies did not report this information clearly. Two studies reported an open assessment process to the researchers and assessors, and we considered them to have a high risk of bias for blinding of outcome assessment (Kokki 2000; Tarkkila 1994). Twenty‐one studies did not provide enough information to assess the blinding of outcome assessment, and in the remaining 47 studies we classified the risk of bias as low. In 21 studies we classified the risk of bias as low for both blinding of participants and blinding of outcome assessment (Brattebo 1995; Buettner 1993; Campbell 1993; Corbey 1997; Crock 2014; Flaatten 2000; Hafer 1997; Imarengiaye 2002; Kang 1992; Kim 2011; Kokki 1999; Lavi 2006; Muller 1994; Peterman 1996; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Shaikh 2008; Tourtellotte 1972; Wiesel 1993).
Incomplete outcome data
Significant numbers of patients were lost or excluded from the final analysis of one study (Santanen 2004), and two further studies presented unclear data (Strupp 2001; Tarkkila 1992). In the studies with minimal attrition bias, we often found that the data analyses were undertaken by protocol and we took this into account for data gathering, including all the randomized patients in the denominators of the assessed groups.
Selective reporting
A full report of adverse events associated with the different types of needle is fundamental for the complete assessment of their usefulness in the assessed clinical scenarios. We found that 39 studies did not report other adverse events associated with the needles (such as paraesthesia and backache) (Amuzu 1995; Brattebo 1995; Buettner 1993; Chaudhry 2011; Corbey 1997; Crock 2014; Devcic 1993; Fernandez 1993; Fernandez 2003; Flaatten 2000; Geurts 1990; Gonzalez 2000; Harrison 1993; Kang 1992; Kim 2011; Lavi 2006; Lynch 1992a; Morros‐Vinoles 2002; Muller 1994; Oberoi 2009; Pan 2004; Peterman 1996; Pippa 1995; Prager 1996; Rafique 2014; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Schmittner 2011; Sears 1994; Shah 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003; Wiesel 1993; Zela 1994), whereas the remaining studies reported at least one additional adverse event to PDPH.
Other potential sources of bias
We found other sources of bias in five studies, mainly related to the unclear role of the sponsors in the development of the research (Brattebo 1995; Kang 1992; Pan 2004; Schmittner 2010; Thomas 2000). We identified no additional sources of bias in the remaining studies.
Effects of interventions
See: Summary of findings for the main comparison Traumatic needles compared to atraumatic needles for prevention of post‐dural puncture headache (PDPH); Summary of findings 2 Larger traumatic needles compared to smaller traumatic needles for prevention of post‐dural puncture headache (PDPH); Summary of findings 3 Larger atraumatic needles compared to smaller atraumatic needles for prevention of post‐dural puncture headache (PDPH)
See: summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3.
Comparison between traumatic and atraumatic needles
Primary outcome: Onset of post‐dural puncture headache (PDPH)
This comparison included information from 36 studies with a total of 9378 participants and 448 events (incidence of PDPH = 4.77%). The traumatic needles showed a greater risk of PDPH compared with the atraumatic ones (risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67), with low heterogeneity among the studies (I2 = 9%) (Analysis 1.1; Figure 4). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table for the main comparison).
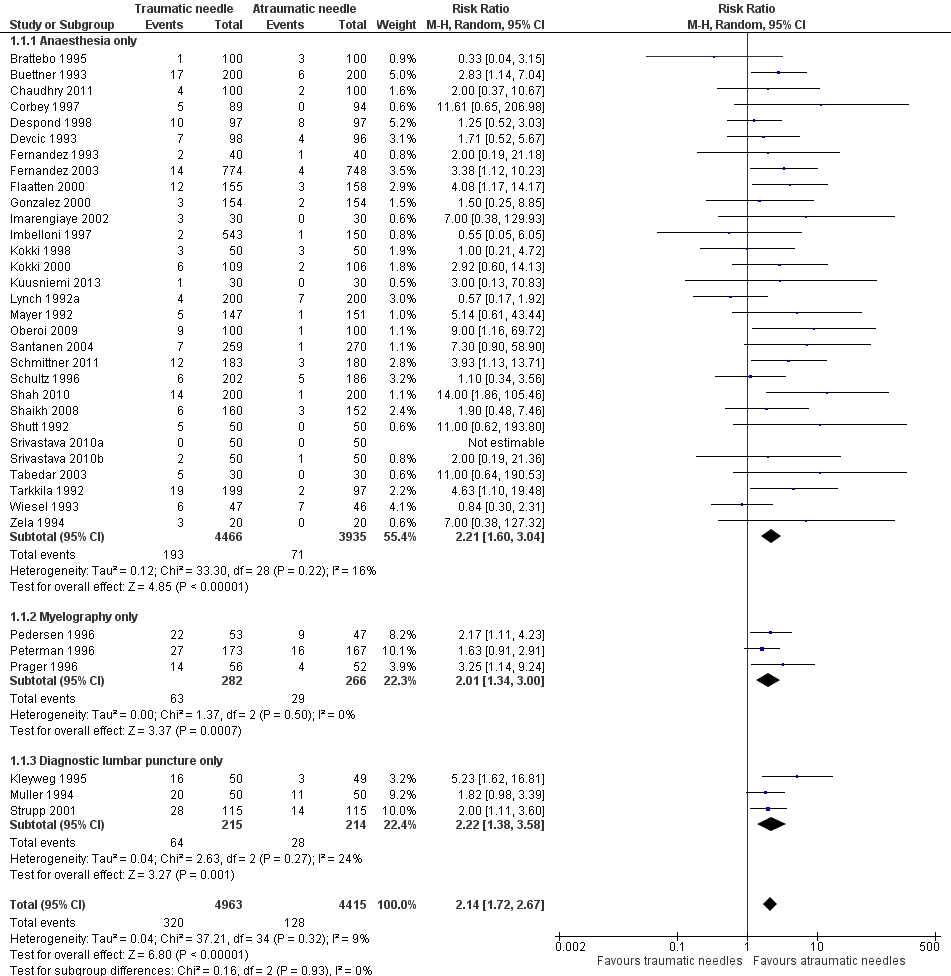
Forest plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
In the subgroup analysis of needle gauge size, 20 studies (6213 participants) compared 22, 25 or 27 gauge traumatic and atraumatic needles (Buettner 1993; Chaudhry 2011; Corbey 1997; Despond 1998; Fernandez 1993; Flaatten 2000; Kleyweg 1995; Kuusniemi 2013; Oberoi 2009; Pedersen 1996; Peterman 1996; Prager 1996; Santanen 2004; Schmittner 2010; Shah 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003). We observed no significant heterogeneity between the three subgroups (I2 subgroup test = 0%). The estimated RR for each of these subgroups is similar to the overall estimate reported above (22 gauge RR 2.15, 95% CI 1.56 to 2.97; 25 gauge RR 2.48, 95% CI 1.56 to 3.95; 27 gauge RR 2.87, 95% CI 1.81 to 4.53) (Analysis 1.2), with no evidence of significant heterogeneity in any of the subgroups (I2 = 0%).
In the subgroup analysis performed for indication of lumbar puncture, we observed no differences in the results (I2 subgroup test = 0%). Most of the studies involved anaesthesia procedures (30 studies, 8401 participants, incidence of PDPH = 3.14%). In this subgroup, the atraumatic needles presented significantly less risk of PDPH in comparison with the use of traumatic needles, similar to the analysis using the whole sample (RR 2.21, 95% CI 1.60 to 3.04; I2 = 16%) (Analysis 1.1). The results were similar for the myelography by lumbar puncture subgroup (three studies: Pedersen 1996; Peterman 1996; Prager 1996) (RR 2.01, 95% CI 1.34 to 3; I2 = 0%), and the diagnostic lumbar puncture subgroup (three studies: Kleyweg 1995; Muller 1994; Strupp 2001) (RR 2.22, 95% CI 1.38 to 3.58; I2 = 24%). The funnel plot figure indicates slight asymmetry related to the studies with small sample sizes and null or favourable outcomes when using traumatic needles (Figure 5).
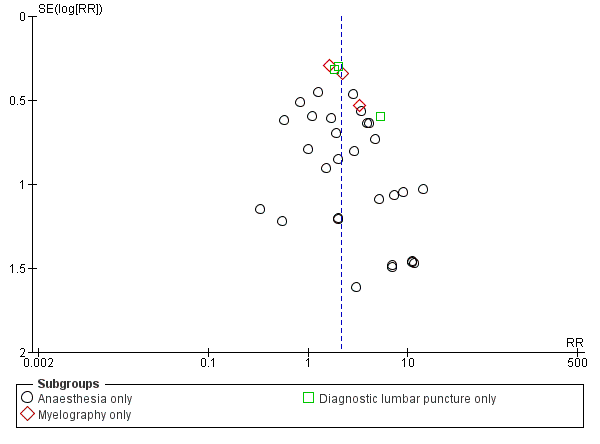
Funnel plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
In addition, we identified nine studies that only included women (mostly in labour) (Devcic 1993; Imarengiaye 2002; Mayer 1992; Oberoi 2009; Pedersen 1996; Shaikh 2008; Shutt 1992; Srivastava 2010b; Tabedar 2003) and we found no studies that only included men (Analysis 1.3).
In the subgroup analysis for the type of surgery used in the anaesthesia studies, there were no significant subgroup differences between caesarean section, orthopaedic interventions and subumbilical or lower limb surgeries (test of subgroup differences: I2 = 12%). Orthopaedic surgical studies presented moderate heterogeneity (I2 = 55%), but there was no significant difference in risk between traumatic and atraumatic needles (RR 1.35, 95% CI 0.58 to 3.19). In contrast, the risk of PDPH for caesarian and other surgeries was lower in the atraumatic needle group, with no or minimal heterogeneity (I2 = 0% and 18%, respectively) (Analysis 1.4).
In addition, in the subgroup analysis performed for body position during the lumbar puncture there was heterogeneity (I2 subgroup test = 76.9%) (Analysis 1.5). These differences may be due to the results observed in the subgroup of punctures administered to patients in a lateral position, in which the risk associated with traumatic needles increased significantly when compared to the global result (nine studies, RR 4.70, 95% CI 2.39 to 9.24; I2 = 0%). In the subgroup of punctures administered to sitting participants, with traumatic needles the risk ratio was similar to the analysis including the whole sample (11 studies, RR 2.11, 95% CI 1.52 to 2.94; I2 = 0%).
Finally, in the subgroup analysis performed for age range, we observed no differences (I2 subgroup test = 0%). In this comparison, only two studies focused on children under 18 (Kokki 1998; Kokki 2000), and the estimate in this subgroup was not precise (RR 1.69, 95% CI 0.56 to 5.12; I2 = 0%), due to the low number of events (14 in total) (Analysis 1.6).
Primary outcome: adverse events/paraesthesia
Paraesthesia was reported in three studies, which included a total of 573 participants and 29 paraesthesias (incidence of paraesthesia = 5.06%) (Imarengiaye 2002; Kuusniemi 2013; Mayer 1992). We found no differences between the use of traumatic needles versus atraumatic needles for this adverse event (RR 0.96, 95% CI 0.47 to 1.96; I2 = 0%) (Analysis 1.7). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table for the main comparison).
Primary outcome: adverse events/backache
Backache was reported in 12 studies (Brattebo 1995; Chaudhry 2011; Flaatten 2000; Imarengiaye 2002; Imbelloni 1997; Kokki 1998; Kokki 2000; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Schultz 1996; Thomas 2000), including a total of 3027 participants and 454 backache events (incidence of backache = 14.9%). We found no differences between the use of traumatic needles versus atraumatic needles for this adverse event (RR 0.94, 95% CI 0.78 to 1.13; I2 = 14%) (Analysis 1.8). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table for the main comparison).
Secondary outcome: severe PDPH
For this comparison, we analysed the information taken from 24 studies with a total of 6420 participants and 87 events (incidence of severe PDPH = 1.35%) (Brattebo 1995; Chaudhry 2011; Corbey 1997; Despond 1998; Devcic 1993; Fernandez 1993; Fernandez 2003; Imbelloni 1997; Kokki 1998; Lynch 1992a; Mayer 1992; Muller 1994; Pedersen 1996; Peterman 1996; Prager 1996; Shah 2010; Shaikh 2008; Shutt 1992; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003; Tarkkila 1992; Wiesel 1993). Nine studies presented zero events in both arms and they do not count for the RR analysis (Brattebo 1995; Fernandez 1993; Imbelloni 1997; Kokki 1998; Lynch 1992a; Mayer 1992; Shah 2010; Srivastava 2010a; Srivastava 2010b). A sensitivity analysis measuring the risk difference (RD) allowed us to include all the studies and presents a similar risk between traumatic and atraumatic needles, with considerable heterogeneity (RD 0.00, 95% CI 0.00 to 0.01; I2 = 42%). The heterogeneity observed in this analysis is due to the study focused on diagnostic lumbar punctures (Muller 1994). Excluding this study eliminates the heterogeneity completely and maintains the non‐significant results (Analysis 1.9). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well the presence of the aforementioned considerable heterogeneity. (See summary of findings Table for the main comparison).
Secondary outcome: any headache
For this comparison, we analysed the information taken from 18 studies with a total of 4104 participants and 636 events (general incidence of any headache = 15.4%) (Brattebo 1995; Buettner 1993; Chaudhry 2011; Corbey 1997; Despond 1998; Flaatten 2000; Fox 1996; Imarengiaye 2002; Kokki 1998; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Peterman 1996; Prager 1996; Saenghirunvattana 2008; Santanen 2004; Shutt 1992; Thomas 2000). The estimated RR for this outcome was 1.35 (95% CI 1.17 to 1.57) (Analysis 1.10), with minimal heterogeneity (I2 = 5%). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table for the main comparison).
Comparison between larger gauge traumatic needles versus smaller gauge traumatic needles
Primary outcome: Onset of PDPH
For this comparison, we analysed the information taken from 10 studies with a total of 2288 participants and 185 events (incidence of PDPH = 8.09%) (Grover 2002; Kang 1992; Kim 2011; Kokki 1996; Pippa 1995; Rafique 2014; Schmittner 2010; Shah 2010; Shaikh 2008; Tarkkila 1994). We decided against overall pooling of results because a needle gauge could be considered small in one comparison but large in another (for example, a 25 gauge needle could be considered as smaller in a 23 versus 25 gauge comparison, but larger in a 25 versus 27 gauge comparison). Instead, we grouped and analysed studies according to the gauges evaluated (23 versus 25 gauge, 25 versus 27 gauge, 25 versus 29 gauge, 26 versus 27 gauge and 21 versus 25 gauge). The RRs for these comparisons ranged from 0.86 to 6.47 and they were not were not statistically significant except for a single study in the 26 versus 27 gauge subgroup (23 versus 25 gauge RR 2.08, 95% CI 0.20 to 21.55; 25 versus 27 gauge RR 1.82, 95% CI 0.98 to 3.39; 25 versus 29 gauge RR 2.13, 95% CI 0.46 to 9.78; 26 versus 27 gauge RR 6.47, 95% CI 2.55 to 16.43; 21 versus 25 gauge RR 0.86, 95% CI 0.30 to 2.44) (Analysis 2.1).
The results obtained when comparing 29 with 25 gauge needles present the greatest heterogeneity (I2 = 69%; Analysis 2.1). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See summary of findings Table 2).
All the studies included in this comparison were undertaken using anaesthesia and included a mixed population, which is the reason why we did not carry out a subgroup analysis for indication for lumbar puncture or gender. Analysis by type of surgery showed no subgroup differences. The estimates presented in the caesarean section subgroup and the orthopaedic surgeries subgroup showed no differences in the risk of PDPH with the use of traumatic needles of any gauge (Analysis 2.2). In the analyses performed for age subgroups, we found no differences by subgroup. In the studies in children and the over 60 years age group there were no significant differences in the risk of PDPH between the use of larger or smaller gauges; however, this information was derived from only one study for each of the subgroups mentioned (Analysis 2.3). In studies in the no age distinction group, we found a significantly higher risk of PDPH for larger gauge needles (RR 2.09, 95% CI 1.11 to 3.95), but with significant heterogeneity (I2 = 69%, P = 0.002). There were no significant differences in the risk of PDPH between the use of larger or smaller gauges in the subgroup analyses by body position (Analysis 2.4).
Primary outcome: adverse events/paraesthesia
No studies in this comparison reported this outcome.
Primary outcome: adverse events/backache
Backache was reported in three studies that included a total of 948 participants and 188 events (backaches) (incidence of backache = 19.8%) (Grover 2002; Kang 1992; Tarkkila 1994). The RRs for these comparisons ranged from 0.81 to 2.00 and were not statistically significant (25 versus 29 gauge RR 2.00, 95% CI 1.00 to 4.02; 26 versus 27 gauge RR 0.91, 95% CI 0.66 to 1.24; 25 versus 27 gauge RR 0.81, 95% CI 0.44 to 1.49) (Analysis 2.5). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table 2).
Secondary outcome: severe PDPH
For this outcome, we analysed the information from six studies with a total of 1128 participants and three events (incidence of severe PDPH = 0.2%) (Grover 2002; Kim 2011; Pippa 1995; Rafique 2014; Shah 2010; Shaikh 2008). We grouped and analysed studies according to the gauges evaluated (23 versus 25 gauge, 25 versus 27 gauge, 25 versus 29 gauge and 21 versus 25 gauge). We conducted analyses with risk differences, which allowed us to incorporate all studies in the estimate. The RDs for these comparisons were 0.00 in all cases and were not statistically significant (23 versus 25 gauge RD 0.00, 95% CI ‐0.07 to 0.07; 25 versus 27 gauge RD 0.00, 95% CI ‐0.01 to 0.01; 25 versus 29 gauge RD 0.00, 95% CI ‐0.04 to 0.04; 21 versus 25 gauge RD 0.00, 95% CI ‐0.02 to 0.02) (Analysis 2.6). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as the few events reported. (See summary of findings Table 2).
Secondary outcome: any headache
For this comparison, we analysed the information taken from three studies with a total of 771 participants and 195 events (incidence of any headache = 25.2%) (Kang 1992; Kim 2011; Kokki 1996). The RRs for these comparisons ranged from 0.75 to 1.56 and were not statistically significant (23 versus 25 gauge RR 1.29, 95% CI 0.98 to 1.68; 25 versus 29 gauge RR 1.56, 95% CI 0.86 to 2.82; 26 versus 27 gauge RR 0.75, 95% CI 0.18 to 3.07) (Analysis 2.7). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table 2).
Comparison between larger gauge atraumatic needles versus smaller gauge atraumatic needles
This comparison involved all studies that compared different gauges of atraumatic needles. From each study we selected only comparisons between larger gauge versus smaller gauge needles for this analysis.
Primary outcome: Onset of PDPH
For this comparison, we analysed the information taken from 13 studies with a total of 3134 participants and 75 events (incidence PDPH = 2.33%) (Amuzu 1995; Campbell 1993; De Andres 1999; Hopkinson 1997; Kokki 1998; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sears 1994; Shah 2010; Sharma 1995; Shutt 1992; Smith 1994). As we mentioned above, we decided against overall pooling of results because a needle gauge could be considered small in one comparison but large in other (for example, a 25 gauge needle could be considered as smaller in a 23 versus 25 gauge comparison, but larger in a 25 versus 27 gauge comparison). We found no significant differences in the analyses comparing gauges (22 versus 24 gauge RR 0.98, 95% CI 0.20 to 4.81; 22 versus 25 gauge RR 3.00, 95% CI 0.32 to 28.50; 24 versus 25 gauge RR 5.62, 95% CI 1.00 to 31.67; 25 versus 26 gauge RR 0.76, 95% CI 0.30 to 1.90; 25 versus 27 gauge RR 3.72, 95% CI 0.59 to 23.64; 26 versus 27 gauge RR 1.79, 95% CI 0.30 to 10.73; 27 versus 29 gauge RR 1.59, 95% CI 0.58 to 4.37) (Analysis 3.1). We found few incidence data for each of the gauge subgroups mentioned and we did not find benefits derived from the use of smaller atraumatic needles compared to larger ones. The funnel plot figure does not show any asymmetry in relation to the data classified by gauge (Figure 6). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See summary of findings Table 3).
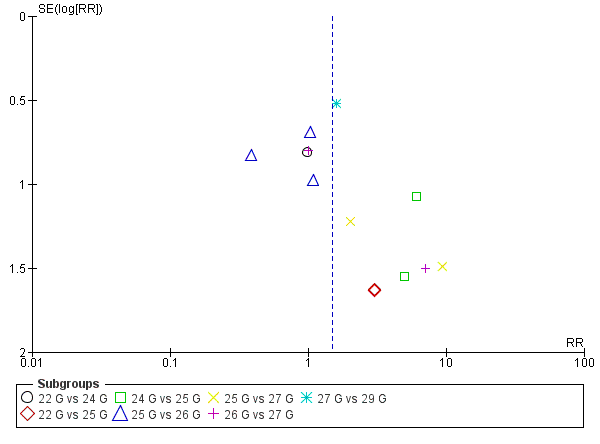
Funnel plot of comparison: 3 Atraumatic needles: different gauges, outcome: 3.1 PDPH major gauge versus minor gauge by number.
The studies included in this comparison had participants with indication for anaesthesia. Analyses by type of surgery showed no effect derived from the type of needles with respect to the presentation of PDPH (Analysis 3.2). The subgroup analyses performed for gender and body position also showed no differences in the results for PDPH.
Primary outcome: adverse events/paraesthesia
Two studies that included a total of 439 participants reported 51 paraesthesias (incidence of paraesthesia = 11.6%) (Hopkinson 1997; Sharma 1995). We found no statistically significant difference in paraesthesia related to the size of gauge used; the pooled estimate presented considerable heterogeneity (RR 2.19, 95% CI 0.31 to 15.30; I2 = 72%) (Analysis 3.5). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table 3).
Primary outcome: adverse events/backache
Four studies including a total of 526 participants reported 105 incidences of backache (incidence = 19.9%) (De Andres 1999; Kokki 1998; Sharma 1995; Smith 1994). The RRs for these comparisons ranged from 0.95 to 5.00 and they were not statistically significant (25 versus 29 gauge RR 5.00, 95% CI 0.62 to 40.28; 26 versus 27 gauge RR 1.29, 95% CI 0.69 to 2.40; 25 versus 27 gauge RR 0.95, 95% CI 0.56 to 1.61; 25 versus 26 gauge RR 1.19, 95% CI 0.58 to 2.42) (Analysis 3.6). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table 3).
Secondary outcome: severe PDPH
For this outcome, we analysed the information taken from eight studies with a total of 1983 participants and five events (incidence of severe PDPH = 0.25%) (Campbell 1993; De Andres 1999; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sears 1994; Sharma 1995; Smith 1994). We grouped and analysed studies according to the gauges evaluated (22 versus 24 gauge, 22 versus 25 gauge, 24 versus 25 gauge, 25 versus 26 gauge, 25 versus 27 gauge, 26 versus 27 gauge and 27 versus 29 gauge). We conducted analyses with RDs, which allowed us to incorporate all studies in the estimate. The RDs for these comparisons ranged from 0.00 to 0.01 and they were not statistically significant (22 versus 24 gauge RD 0.00, 95% CI ‐0.01 to 0.01; 22 versus 25 gauge RD 0.00, 95% CI ‐0.02 to 0.02; 24 versus 25 gauge RD 0.01, 95% CI ‐0.02 to 0.03; 25 versus 26 gauge RD 0.01, 95% CI ‐0.01 to 0.03; 25 versus 27 gauge RD 0.01, 95% CI ‐0.02 to 0.04; 26 versus 27 gauge RD 0.00, 95% CI ‐0.02 to 0.02; 27 versus 29 gauge RD 0.00, 95% CI ‐0.01 to 0.01) (Analysis 3.7). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See summary of findings Table 3).
Secondary outcome: any headache
For this outcome, we analysed the information taken from seven studies with a total of 1791 participants and 206 events (incidence of any headache = 11.5%) (Campbell 1993; Hopkinson 1997; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sharma 1995; Smith 1994). We grouped and analysed studies according to the gauges evaluated (22 versus 25 gauge, 24 versus 25 gauge, 25 versus 26 gauge, 25 versus 27 gauge and 27 versus 29 gauge). The RRs for these comparisons ranged from 1.13 to 2.17 and they were not statistically significant (22 versus 25 gauge RR 2.17, 95% CI 0.85 to 5.51; 24 versus 25 gauge RR 1.17, 95% CI 0.49 to 2.77; 25 versus 26 gauge 1.13, 95% CI 0.65 to 1.99; 25 versus 27 gauge RR 1.87, 95% CI 0.65 to 5.39; 27 versus 29 gauge RR 1.80, 95% CI 0.85 to 3.83) (Analysis 3.8). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See summary of findings Table 3).
Sensitivity analysis
In accordance with our protocol, we selected studies with a low risk of bias for allocation concealment, blinding of outcome assessment and presence of incomplete data (attrition bias). Six studies fulfilled these requirements for the main outcome of onset of PDPH (Hopkinson 1997; Kleyweg 1995; Peterman 1996; Schmittner 2010; Schmittner 2011; Thomas 2000). Only three of them could be analysed together as they made similar comparisons (traumatic needles versus atraumatic needles) and possessed data regarding the main outcome (PDPH) (Kleyweg 1995; Peterman 1996; Schmittner 2011). The analysis of these three studies showed significant risk of PDPH when using traumatic needles (RR 2.78, 95% CI 1.26 to 6.15), but with moderate heterogeneity (I2 = 51%) (Analysis 1.11).
Discussion
Summary of main results
We assessed the evidence from 66 studies in 17,067 participants, which showed several important aspects for each comparison analysed.
Firstly, in the comparison between traumatic versus atraumatic needles, after analysing information from 9378 participants, we found that the risk of post‐dural puncture headache (PDPH) is almost doubled when a traumatic needle is used (risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67). The number of participants required to be treated with atraumatic needles to prevent an additional new episode of PDPH (NNTB) is 24 (95% CI 20 to 30 participants undergoing lumbar punctures). We observed these results regardless of lumbar puncture indication, gender, age or risk of bias issues. Likewise, we found that only three of the studies included in this review reported paraesthesia as a possible primary outcome after lumbar puncture, with an incidence of 5.06% (Imarengiaye 2002; Kuusniemi 2013; Mayer 1992). We identified no difference in the occurrence of paraesthesia between traumatic and atraumatic needles. This may be due to the low number of events. Twelve studies reported backache, with an incidence of 14.9% (Brattebo 1995; Chaudhry 2011; Flaatten 2000; Imarengiaye 2002; Imbelloni 1997; Kokki 1998; Kokki 2000; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Schultz 1996; Thomas 2000). Despite the higher number of events, we found no important differences between the two needle types. Finally, we found significant differences in the risk of any headache in this comparison (RR 1.35, 95% CI 1.17 to 1.57), but not in the risk of severe PDPH or backache.
Secondly, with respect to the comparison of different gauges of traumatic needles, after analysing the information from 2288 participants of both genders, we found heterogeneous results about the risk associated with larger gauges versus smaller gauges. Overall, studies comparing various sizes of large and small gauges showed no significant differences in the effects on risk of PDPH. We analysed this information by factors such as type of surgery, age and body position, but these factors did not explain the heterogeneity. In addition, we found a scarcity of data related to adverse events: only three studies reported backache and found no differences in risk according to gauge (Grover 2002; Kang 1992; Tarkkila 1994).
Finally, in the comparison of gauges for atraumatic needles, after analysing the information from 3134 participants, we found a large number of gauge comparisons, all with few data. Studies comparing various gauge sizes (large and small) showed no significant differences in the effects on risk of PDPH. Similarly, we did not find significant differences in adverse events, severe PDPH or any headache.
Overall completeness and applicability of evidence
We carried out a thorough search and identified a reasonable number of studies evaluating the effectiveness and safety of different gauges and needle types for the prevention of PDPH. The 66 studies included in the numerical analysis enrolled 17,067 participants. Needle tips, gauges, indications for lumbar puncture and operators all varied and participants were from different age groups and genders. The studies were also conducted over a long period of time. The included studies represent the characteristics of the population usually undergoing lumbar puncture procedures either for diagnostic or therapeutic reasons, which is important for the external validity of this review and should increase the applicability of the results.
The systematic search for study selection and data extraction that we undertook should have minimized the likelihood of missing relevant studies. Also, the funnel plots we produced were highly symmetric, suggesting that a minimal chance of having missed relevant studies and that there is no evidence of publication bias.
The evidence presented consistently showed benefits derived from the use of atraumatic needles and is sufficient to address the main objectives of this review. However, new studies (including those that are ongoing) could help to increase the precision of the different measures of effect, as well as to clarify the actual risk in some selected subgroups (for instance, the comparison between traumatic needles by gauge). Similarly, we think that new studies could also help to provide additional data on adverse events related to the use of needles, or even information about technical difficulties related to the use of smaller gauge needles.
Finally, we did not find any information related to gauge differences in diagnostic and myelography settings. New studies might help to identify any benefits related to greater gauge versus finer gauge needles in these specific scenarios.
Quality of the evidence
We considered the quality of the evidence for the first comparison (traumatic versus atraumatic needles) to be moderate for most of the outcomes assessed. We downgraded the quality of the evidence in these cases due to lack of reporting of aspects related to randomization, such as random sequence generation and allocation concealment, which made it difficult for us to interpret the risk of bias for the included studies. Given that it is not possible to blind the personnel to the needle used, we only assessed the blinding of participants. However, we found that participant blinding was only reported in 50% of included studies. Likewise, we found that a considerable number of studies did not report other adverse events associated with the use of needles, for example paraesthesia and backache. The quality of the evidence for the secondary outcome of severe PDPH was also downgraded from high to low due to both the presence of risk of bias and inconsistency (42%), which was caused by one study focusing on diagnostic lumbar punctures. The secondary outcome 'any headache' was affected by similar reporting problems to those previously mentioned for the primary outcomes and we therefore reduced the quality of this evidence to moderate from high.
The primary outcomes for the second comparison (larger gauge versus smaller gauge traumatic needles) were also affected by concerns about risk of bias and we downgraded the quality of the evidence from high to moderate. The secondary outcomes were not affected by heterogeneity but we considered the quality of the evidence to be moderate due to concerns about risk of bias, similar to those related to the primary outcomes.
Finally, we considered the quality of evidence for the outcomes in the third comparison (larger gauge versus smaller gauge atraumatic needles) to be moderate for most of the outcomes, due to imprecision and risk of bias issues.
Potential biases in the review process
We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
This review was comprehensive in identifying clinical trials addressing the issue of the effectiveness and safety of needle gauges and tips in the prevention of post‐dural puncture headache. However, 18 studies did not provide enough information to be able to classify them as included or excluded, because they were published only as conference proceedings, or because we did not have access to the full texts when we were completing this review. Also, we considered 12 of the studies to be 'ongoing' due to their date of publication as abstracts. We may be able to decide whether or not to include these studies once they have been published as full texts.
A potential source of bias in the review process was that we made some decisions about the analysis after seeing the data from the included studies. First, in order to assess adverse events, we had to define those events (except PDPH) related to the use of needles in anaesthesia, myelography and diagnostic lumbar punctures after the publication of the protocol. Most of the events usually reported in studies, such as nausea and vomiting, were already included in the definition of PDPH; we therefore selected paraesthesia and backache as the two most important adverse events related to the intervention assessed. In this review, we did not consider other events related to technical difficulties with the use of smaller needles; for example, the number of attempts before a successful puncture or the anaesthesiologist’s satisfaction regarding the use of these needles. Secondly, we did not expect to encounter any unit of analysis issues, as we do not expect to find cross‐over studies or cluster‐randomized trials. However, we did identify one cross‐over study with our search strategies. In order to avoid bias in the development of our review, we did not include numerical results related to this study in our analyses because we consider that the patients' history of PDPH could be an important factor to take into account when analysing the possibility of a new episode of PDPH. In a future update we can examine other analysis options in order to try to deal with this information. Finally, we modified the subgroup analysis for age due to heterogeneity in the reporting of this outcome. We classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more, and we analysed the numerical information in these three new categories. Although we planned to present risk ratios, in cases where there were no events in one of the arms we presented risk differences. However, we also presented risk ratios in these cases as a sensitivity analysis.
It is also important to mention as a potential source of bias in the review process the fact that we reran the search strategy in September 2016 and found three studies of interest. We added these studies to the list of Studies awaiting classification and we will incorporate them into the review during a review update.
Agreements and disagreements with other studies or reviews
The literature includes a number of examples of reviews that have evaluated several issues related to the use of needles for different purposes. One of our identified studies included a meta‐analysis of other trials using 27 gauge atraumatic versus 27 gauge traumatic needles , which found a RR of developing PDPH of 0.38 (95% CI 0.19 to 0.75) in the atraumatic group compared to the traumatic group (Flaatten 2000). In our review, we found an effect in all the gauges assessed (22, 25 and 27 gauge), confirming the conclusions presented by these authors. Likewise, Halpern 1994 compared noncutting spinal needles (Sprotte or Whitacre) with cutting needles and larger spinal needles with smaller needles. They found a reduction in the incidence of severe PDPH when noncutting spinal needles were used rather than cutting needles (odds ratio (OR) 0.26, 95% CI 0.11 to 0.62) and no important difference in back pain. They also found a reduction in severe PDPH when a small spinal needle was used compared with a large needle of the same type (OR 0.18, 95% CI 0.09 to 0.36). There was no important difference in the incidence of back pain. The direction of the effect is consistent with our findings.
Bradbury et al assessed different methods to decrease accidental dural punctures and interventions to reduce PDPH following these punctures in parturients (Bradbury 2013). They identified 14 randomized controlled trials with 11,536 epidural insertions, finding that prophylactic epidural blood patch, lateral positioning of the epidural needle bevel upon insertion, use of Sprotte needles, epidural morphine and administration of cosyntropin reduce PDPH. In the same subgroup of participants, Choi et al found that the use of atraumatic spinal needles with a smaller gauge decreased the risk of PDPH in the obstetric population (Choi 2003). However, the authors remarked that the incidence of this complication in labour is considerable, with an estimate of 52.1% accidental dural punctures (95% CI 51.4% to 52.8%). We found similar benefits in the subgroup of obstetric participants when atraumatic needles were used.
Other reviews included other factors related to needles but these are not assessed in the present review. In 2006, Richman assessed the effect of lumbar puncture needle bevel direction on the incidence of post‐dural puncture headache in adult participants when cutting needles were used (Richman 2006). The authors also evaluated the use of a parallel versus a perpendicular orientation during needle insertion. The results derived from five trials suggested that a parallel/longitudinal insertion resulted in a lower incidence of PDPH (OR 0.29, 95% CI 0.17 to 0.50). Our review did not include information about bevel orientation but we noticed that the needles used in spinal anaesthesia are larger than those usually used. Likewise, Tung et al in 2012 developed a decision‐analytic model to determine the cost of diagnostic lumbar punctures using atraumatic versus traumatic needles (Tung 2012). The authors assumed a healthcare system perspective and determined that the difference in estimated costs between the two needles was the economic outcome measure selected. They found that lumbar punctures performed with an atraumatic needle are associated with an average cost saving of USD 26.07 per patient. Average total healthcare costs with traumatic needles are USD 192.15 versus 166.08 using atraumatic needles in diagnostic lumbar punctures.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.

Funnel plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.

Funnel plot of comparison: 3 Atraumatic needles: different gauges, outcome: 3.1 PDPH major gauge versus minor gauge by number.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 1 PDPH by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 2 PDPH by gauge.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 3 PDPH by gender.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 4 PDPH/anaesthesia: type of surgery.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 5 PDPH by position.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 6 PDPH by age.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 7 AE: paraesthesia.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 8 AE: backache.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 9 Severe PDPH by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 10 Any headache by indication.

Comparison 1 Traumatic needle versus atraumatic needle, Outcome 11 PDPH sensitivity analysis.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 3 PDPH by age.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 4 PDPH by position.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 5 AE: backache.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 6 Severe PDPH by gauge.

Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 7 Any headache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 2 PDPH by type of surgery.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 3 PDPH by gender.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 4 PDPH by position.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 5 AE: paraesthesia.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 6 AE: backache.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 7 Severe PDPH by gauge.

Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 8 Any headache by gauge.
| Traumatic needles compared to atraumatic needles for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needles | Traumatic needles | |||||
| Onset of PDPH | 30 per 1000 | 64 per 1000 | RR 2.14 | 9378 | ⊕⊕⊕⊝ | — |
| Adverse events: paraesthesia | 52 per 1000 | 50 per 1000 | RR 0.96 | 573 | ⊕⊕⊕⊝ | — |
| Adverse events: backache | 155 per 1000 | 147 per 1000 | RR 0.94 | 3027 | ⊕⊕⊕⊝ | — |
| Severe PDPH | 0 per 1000 | 10 per 1000 | RD 0 | 6420 | ⊕⊕⊝⊝ | — |
| Any headache | 221 per 1000 | 290 per 1000 | RR 1.35 | 4104 | ⊕⊕⊕⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Traumatic needle(major gauge) compared to traumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Traumatic needle ‐ smaller gauge | Traumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.86 to 6.47 | 2288 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Adverse events: paraesthesia ‐ not reported | See comment | See comment | Not estimable | — | See comment | We did not identify any studies reporting this outcome. |
| Adverse event: backache | — | — | RR ranged | 948 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Severe PDPH | — | — | RD ranged | 1128 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Any headache | — | — | RR ranged | 771 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Imprecision downgraded by one level due to few events reported in each arm. 3Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit. | ||||||
| Atraumatic needle (major gauge) compared to atraumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needle ‐ smaller gauge | Atraumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.38 to 9.3 | 3134 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: paraesthesia | — | — | RR ranged from 1.03 to 7.61 | 439 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: backache | — | — | RR ranged | 526 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Severe PDPH | — | — | RD ranged | 1983 | ⊕⊕⊝⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Any headache | — | — | RR ranged | 1791 | ⊕⊕⊕⊝ | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH by indication Show forest plot | 36 | 9378 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.72, 2.67] |
| 1.1 Anaesthesia only | 30 | 8401 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.60, 3.04] |
| 1.2 Myelography only | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.34, 3.00] |
| 1.3 Diagnostic lumbar puncture only | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.38, 3.58] |
| 2 PDPH by gauge Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 22 gauge | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.56, 2.97] |
| 2.2 25 gauge | 5 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.56, 3.95] |
| 2.3 27 gauge | 11 | 4076 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.81, 4.53] |
| 3 PDPH by gender Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 9 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.62, 4.17] |
| 4 PDPH/anaesthesia: type of surgery Show forest plot | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Caesarean section | 8 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.60, 6.10] |
| 4.2 Orthopaedic procedures | 3 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.58, 3.19] |
| 4.3 Other surgeries | 19 | 6083 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.50, 3.51] |
| 5 PDPH by position Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Lateral position | 9 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.39, 9.24] |
| 5.2 Sitting position | 11 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.52, 2.94] |
| 6 PDPH by age Show forest plot | 36 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 No distinctions by age | 34 | 9063 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.73, 2.73] |
| 6.2 Only < 18 years | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.56, 5.12] |
| 7 AE: paraesthesia Show forest plot | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.96] |
| 8 AE: backache Show forest plot | 12 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 9 Severe PDPH by indication Show forest plot | 24 | 6420 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.20, 2.94] |
| 9.1 Anesthesia | 19 | 5542 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.88, 3.53] |
| 9.2 Myelography | 4 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.68, 4.28] |
| 9.3 Diagnostic lumbar puncture | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.18, 7.63] |
| 10 Any headache by indication Show forest plot | 18 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.17, 1.57] |
| 10.1 Anaesthesia | 16 | 3656 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 10.2 Myelography | 2 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.21] |
| 11 PDPH sensitivity analysis Show forest plot | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.26, 6.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 23 G vs 25 G | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 1.2 25 G vs 27 G | 4 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.39] |
| 1.3 25 G vs 29 G | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.46, 9.78] |
| 1.4 26 G vs 27 G | 1 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.55, 16.43] |
| 1.5 21 G vs 25 G | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 2 PDPH by type of surgery Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.64, 2.57] |
| 2.2 Orthopaedic surgeries | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.58] |
| 2.3 Other surgeries | 6 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.23, 7.03] |
| 3 PDPH by age Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 No distinctions about age | 8 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.11, 3.95] |
| 3.2 Only children | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 3.3 Only > 60 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 4 PDPH by position Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Lateral position | 5 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.98, 3.16] |
| 4.2 Sitting position | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.56] |
| 5 AE: backache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Severe PDPH by gauge Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 23 G vs 25 G | 1 | 53 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.2 25 G vs 27 G | 3 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 6.3 25 G vs 29 G | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.4 21 G vs 25 G | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7 Any headache Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PDPH larger gauge vs smaller gauge Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 22 G vs 24 G | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.81] |
| 1.2 22 G vs 25 G | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.50] |
| 1.3 24 G vs 25 G | 2 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [1.00, 31.67] |
| 1.4 25 G vs 26 G | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.90] |
| 1.5 25 G vs 27 G | 2 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.59, 23.64] |
| 1.6 26 G vs 27 G | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.30, 10.73] |
| 1.7 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.58, 4.37] |
| 2 PDPH by type of surgery Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 6 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.64, 5.79] |
| 2.2 Orthopaedic procedures | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.30, 5.07] |
| 2.3 Other surgeries | 5 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.83] |
| 3 PDPH by gender Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 8 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.51, 2.20] |
| 4 PDPH by position Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Sitting position | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.06] |
| 4.2 Lateral position | 5 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.65, 5.41] |
| 5 AE: paraesthesia Show forest plot | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.31, 15.30] |
| 6 AE: backache Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Severe PDPH by gauge Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 22 G vs 24 G | 1 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7.2 22 G vs 25 G | 1 | 234 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 24 G vs 25 G | 1 | 304 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.4 25 G vs 26 G | 2 | 311 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.5 25 G vs 27 G | 1 | 212 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.6 26 G vs 27 G | 1 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.7 27 G vs 29 G | 1 | 389 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Any headache by gauge Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 22 G vs 25 G | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.85, 5.51] |
| 8.2 24 G vs 25 G | 2 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.49, 2.77] |
| 8.3 25 G vs 26 G | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.99] |
| 8.4 25 G vs 27 G | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.65, 5.39] |
| 8.5 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |

