Zuclopenthixol versus placebo for schizophrenia
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010598.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 01 December 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Schizophrenia Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
ML ‐ Came up with the concept, devised and drafted the protocol.
MJ ‐ Helped with revising the protocol.
Sources of support
Internal sources
-
Leeds and York Partnerships NHS Foundation Trust, UK.
Both authors were employed by this organisation at the time of writing this review
External sources
-
University of Leeds, UK.
ML is undertaking a masters degree with this organisation and has access to their resources. An adapted version of this review will be submitted as ML's masters dissertation
-
Cochrane Collaboration Programme Grant 2011, Reference number: 10/4001/15, UK.
Declarations of interest
ML ‐ None known.
MJ ‐ None known.
Acknowledgements
The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
We would like to thank Christine Esbensen for help with the development of the protocol (also see Differences between protocol and review)
The search term was developed by the Trials Search Co‐ordinator of the Cochrane Schizophrenia Group, Samantha Roberts and the contact author of this protocol. We would also like to thank Shaimaa Abou Damaa for peer reviewing the protocol. Gabrielle Matta and Rahul Khanna provided peer review comments for the review version and Denise Mitchell copy edited.
Parts of this review were generated using RevMan HAL v 4.2. You can find more information about RevMan HAL here.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 01 | Zuclopenthixol versus placebo for schizophrenia | Review | Michael Lacey, Mahesh B Jayaram | |
| 2013 Jun 18 | Zuclopenthixol versus placebo for schizophrenia | Protocol | Michael Lacey, Mahesh B Jayaram, Christine Esbensen | |
Differences between protocol and review
As only a small number of papers were identified the second author checked all data calculations as opposed to the originally planned 20%.
We added 'in schizophrenia' to objectives make the popluation clearer and match selection criteria.
Although CE was involved in the development of the protocol this author had to stop participation due to personal circumstances. Therefore MJ fulfilled this role. It was agreed that if there were conflicts between ML and MJ a third party would be involved to resolve these. However, there were no conflicts and this was unnecessary.
In the protocol the entries listed for inclusion in the summary of results table were, clinically significant response on global state ‐ as defined by each of the studies, clinically significant response on psychotic symptoms ‐ as defined by each of the studies, significant change in quality of life/satisfaction ‐ as defined by each of the studies and death. These outcomes were considered too narrow and limited so in the final version of the review a broader range of outcomes and ones with clinical usefulness were chosen
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
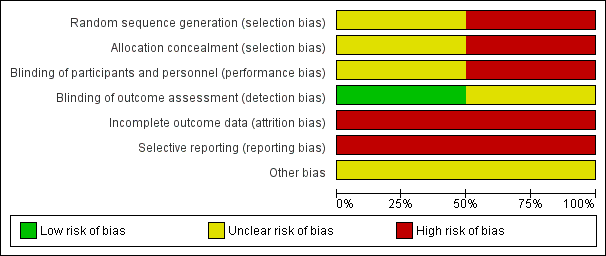
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 1 Clinically significant response: Improvement (CGI) ‐ short term.
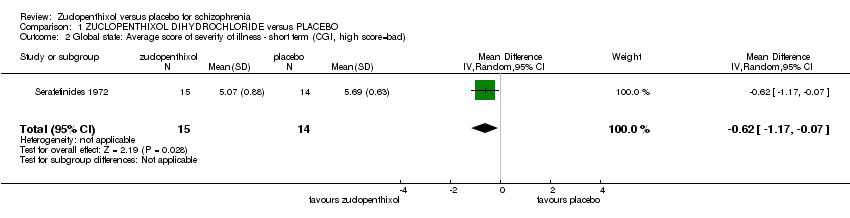
Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad).
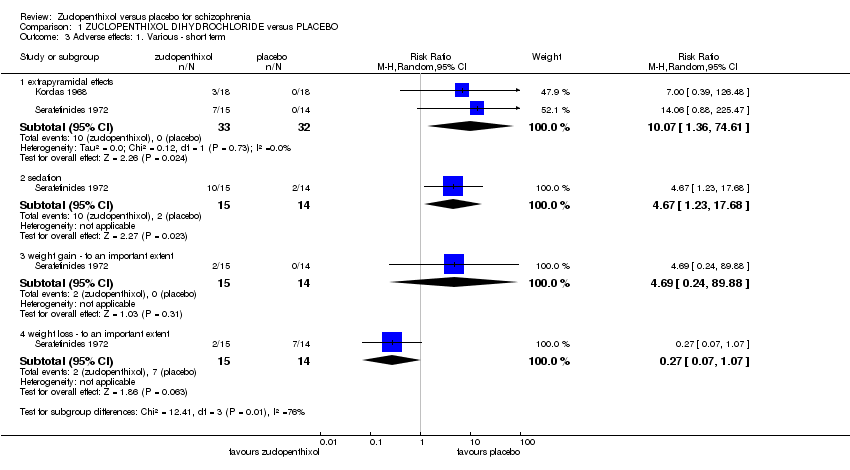
Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 3 Adverse effects: 1. Various ‐ short term.
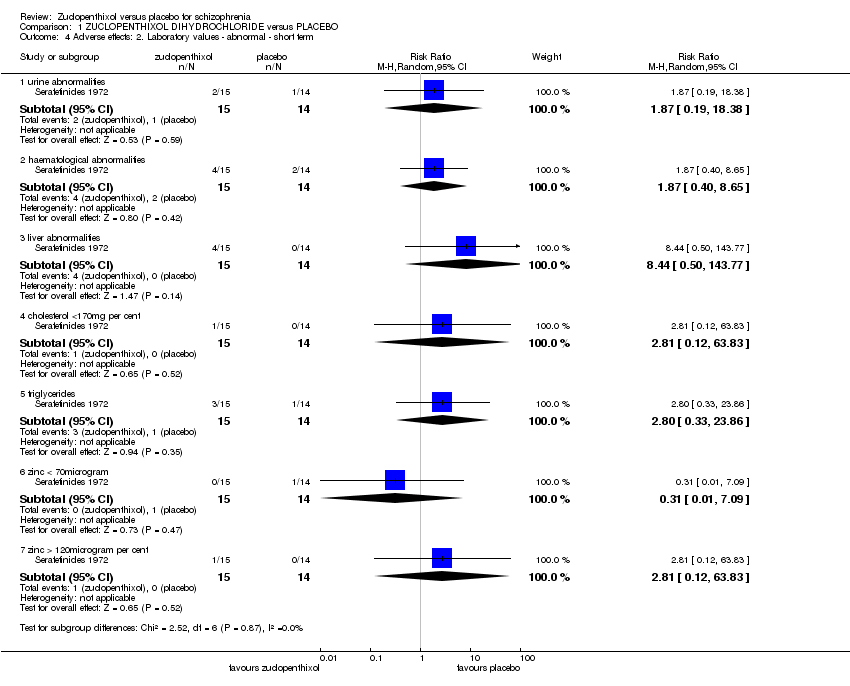
Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term.

Comparison 1 ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO, Outcome 5 Leaving the study early.
| Excluded Study | Comparison | Existing review |
| Chlorpromazine versus molindone | ||
| Chlorpromazine versus placebo | ||
| Chlorpromazine versus placebo | ||
| Clopenthixol versus risperidone | ||
| D‐fenfluramine versus placebo |
| Methods | Allocation: randomised, full description of methods of randomisation and allocation concealment. |
| Participants | Diagnosis: people with schizophrenia (according to a diagnostic criteria). |
| Interventions | 1. Zuclopenthixol dihydrochloride. N = 150. 2. Placebo. N = 150. |
| Outcomes | Global state: clinically important response to treatment, average score/change of the global state. Mental state: general measurement and specific domains (depressive symptoms, positive symptoms, negative symptoms). Leaving the study early, due to any reason, due to inefficacy of treatment, and due to adverse events. Adverse events: any serious adverse event recorded. Service use: number of hospitalisations, days in hospital. |
| Methods | Allocation: randomised, fully explicit description of methods of randomisation and allocation concealment. |
| Participants | Diagnosis: people with schizophrenia (according to a diagnostic criteria). Acutely disturbed behaviour as described by the study |
| Interventions | 1. Zuclopenthixol acetate either alone or in combination with other medications. n = 150 2. Placebo. n = 150 |
| Outcomes | Global state: clinically important response to treatment, average score/change of the global state. Mental state: general measurement and specific domains (such as depressive symptoms, positive symptoms, negative symptoms) Leaving the study early, due to any reason, due to inefficacy of treatment, and due to adverse events. Adverse events: any serious adverse event recorded. Pharmacological interactions. |
| ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO for schizophrenia | ||||||
| Patient or population: people with with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ZUCLOPENTHIXOL DIHYDROCHLORIDE versus PLACEBO | |||||
| Clinically significant response on global state ‐ as defined by each of the studies, Improvement (CGI) ‐ short term ‐ as rated by nurse | Study population | RR 2.57 | 29 | ⊕⊝⊝⊝ | For this SOF table outcome CGI data as rated by a nurse and a psychiatrist were both available. The nurse data were chosen as it includes both control event and | |
| 286 per 1000 | 734 per 1000 | |||||
| Relapse as defined by the studies | No studies reported these important outcomes | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies. | ||||||
| Adverse effects: Sedation | Low | RR 4.67 | 29 | ⊕⊝⊝⊝ | No use of formal rating scales.Several other adverse events were recorded but sedation considered important. | |
| 50 per 1000 | 234 per 1000 | |||||
| Moderate | ||||||
| 150 per 1000 | 701 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 1000 per 1000 | |||||
| Leaving the study early | Study population | RR 0.93 | 29 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 93 per 1000 | |||||
| Average change in quality of life/satisfaction | No studies reported these important outcomes | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated 'very serious' ‐ randomisation method unclear as describes "randomly assigned". Incomplete outcome data and does not accurately describe losses. Patients blinded but unclear if raters and clinicians are blinded | ||||||
| ZUCLOPENTHIXOL ACETATE versus PLACEBO for schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | ZUCLOPENTHIXOL ACETATE versus PLACEBO | ||||
| Clinically significant response on global state | No studies reported any of these important outcomes | ||||
| Relapse as defined by the studies | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies. | |||||
| Other adverse effects, general and specific | |||||
| Leaving the study early | |||||
| Average change in quality of life/satisfaction | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | |||||
| ZUCLOPENTHIXOL DECANOATE versus PLACEBO for schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | ZUCLOPENTHIXOL DECANOATE versus PLACEBO | ||||
| Clinically significant response on global state | No studies reported any of these important outcomes | ||||
| Relapse as defined by the studies | |||||
| Clinically significant response on psychotic symptoms ‐ as defined by each of the studies | |||||
| Other adverse effects, general and specific | |||||
| Leaving the study early | |||||
| Average change in quality of life/satisfaction | |||||
| Significant change in quality of life/satisfaction ‐ as defined by each of the studies | |||||
| Focus of the review | Participants | Reference |
| Zuclopenthixol acetate | acutely ill people with schizophrenia | |
| Zuclopenthixol decanoate | people with schizophrenia | |
| Zuclopenthixol dihydrochloride | people with schizophrenia |
| Individual data | Mean | Sum of mean squares | SD | |||||||||||||||
| CGI severity score clopenthixol | 3 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 5.07 | 396 | 0.88 |
| Individual data | Mean | Sum of mean squares | SD | |||||||||||||
| CGI Scores Placebo | 4 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5.69 | 426 | 0.63 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinically significant response: Improvement (CGI) ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 as rated by psychiatrist | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 1.2 as rated by nurse | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.06, 6.20] |
| 2 Global state: Average score of severity of illness ‐ short term (CGI, high score=bad) Show forest plot | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.07] |
| 3 Adverse effects: 1. Various ‐ short term Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 extrapyramidal effects | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 10.07 [1.36, 74.61] |
| 3.2 sedation | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.67 [1.23, 17.68] |
| 3.3 weight gain ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [0.24, 89.88] |
| 3.4 weight loss ‐ to an important extent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.07, 1.07] |
| 4 Adverse effects: 2. Laboratory values ‐ abnormal ‐ short term Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 urine abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.19, 18.38] |
| 4.2 haematological abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.40, 8.65] |
| 4.3 liver abnormalities | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [0.50, 143.77] |
| 4.4 cholesterol <170mg per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 4.5 triglycerides | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.8 [0.33, 23.86] |
| 4.6 zinc < 70microgram | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.09] |
| 4.7 zinc > 120microgram per cent | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 63.83] |
| 5 Leaving the study early Show forest plot | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.06, 13.54] |



