Interventions for managing taste disturbances
Abstract
Background
The sense of taste is very much essential to the overall health of an individual. It is a necessary component to enjoy one's food, which in turn provides nutrition to an individual. Any disturbance in taste perception can hamper quality of life in such patients by influencing their appetite, body weight and psychological well‐being. Taste disorders have been treated using different modalities of treatment and there is no consensus for the best intervention. Hence this Cochrane Review was undertaken. This is an update of the Cochrane Review first published in November 2014.
Objectives
To assess the effects of interventions for the management of patients with taste disturbances.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 4 July 2017); the Cochrane Central Register of Controlled Trials (CENTRAL; 2017 Issue 6) in the Cochrane Library (searched 4 July 2017); MEDLINE Ovid (1946 to 4 July 2017); Embase Ovid (1980 to 4 July 2017); CINAHL EBSCO (1937 to 4 July 2017); and AMED Ovid (1985 to 4 July 2017). The US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for trials. Abstracts from scientific meetings and conferences were searched on 25 September 2017. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included all randomised controlled trials (RCTs) comparing any pharmacological agent with a control intervention or any non‐pharmacological agent with a control intervention. We also included cross‐over trials in the review.
Data collection and analysis
Two pairs of review authors independently, and in duplicate, assessed the quality of trials and extracted data. Wherever possible, we contacted trial authors for additional information. We collected adverse events information from the trials.
Main results
We included 10 trials (581 participants), nine of which we were able to include in the quantitative analyses (566 participants). We assessed three trials (30%) as having a low risk of bias, four trials (40%) at high risk of bias and three trials (30%) as having an unclear risk of bias. We only included studies on taste disorders in this review that were either idiopathic, or resulting from zinc deficiency or chronic renal failure.
Of these, nine trials with 544 people compared zinc supplements to placebo for patients with taste disorders. The participants in two trials were children and adolescents with respective mean ages of 10 and 11.2 years and the other seven trials had adult participants. Out of these nine, two trials assessed the patient‐reported outcome for improvement in taste acuity using zinc supplements (risk ratio (RR) 1.40, 95% confidence interval (CI) 0.94 to 2.09; 119 participants, very low‐quality evidence). We meta‐analysed for taste acuity improvement using objective outcome (continuous data) in idiopathic and zinc‐deficient taste disorder patients (standardised mean difference (SMD) 0.44, 95% CI 0.23 to 0.65; 366 participants, three trials, very low‐quality evidence). We also analysed one cross‐over trial separately using the first half of the results for taste detection (mean difference (MD) 2.50, 95% CI 0.93 to 4.07; 14 participants, very low‐quality evidence), and taste recognition (MD 3.00, 95% CI 0.66 to 5.34; 14 participants, very low‐quality evidence). We meta‐analysed taste acuity improvement using objective outcome (dichotomous data) in idiopathic and zinc‐deficient taste disorder patients (RR 1.42, 95% 1.09 to 1.84; 292 participants, two trials, very low‐quality evidence). Out of the nine trials using zinc supplementation, four reported adverse events like eczema, nausea, abdominal pain, diarrhoea, constipation, decrease in blood iron, increase in blood alkaline phosphatase, and minor increase in blood triglycerides.
One trial tested taste discrimination using acupuncture (MD 2.80, 95% CI ‐1.18 to 6.78; 37 participants, very low‐quality evidence). No adverse events were reported in the acupuncture trial.
None of the included trials could be included in the meta‐analysis for health‐related quality of life in taste disorder patients.
Authors' conclusions
We found very low‐quality evidence that was insufficient to conclude on the role of zinc supplements to improve taste acuity reported by patients and very low‐quality evidence that zinc supplements improve taste acuity in patients with zinc deficiency/idiopathic taste disorders. We did not find any evidence to conclude the role of zinc supplements for improving taste discrimination, or any evidence addressing health‐related quality of life due to taste disorders.
We found very low‐quality evidence that is not sufficient to conclude on the role of acupuncture for improving taste discrimination in cases of idiopathic dysgeusia (distortion of taste) and hypogeusia (reduced ability to taste). We were unable to draw any conclusions regarding the superiority of zinc supplements or acupuncture as none of the trials compared these interventions.
PICOs
Plain language summary
Interventions for managing taste disturbances
What is the aim of this review?
The aim of this Cochrane Review was to find out what is the best method for the management of zinc‐deficient/idiopathic (of unknown cause) taste disorders and taste disorders secondary to chronic renal failure in children and adults.
Key messages
Giving zinc supplements or acupuncture may have some benefit in treating taste disorders. However, we still need more high‐quality studies to ascertain the role of zinc supplements and acupuncture in treating taste disorders.
What was studied in the review?
The sense of taste is essential to the health and psychological well‐being of an individual. Taste disorders can range from lack of taste, to distortion of taste, to reduced ability to taste. Any disorder in taste perception can lead to conditions like malnutrition and consumption of poisonous food substances. The cause may be due to disease, drugs, radiation treatment, or ageing; or it may result from unknown causes.
Various treatment methods have been used to improve taste sensation. These include the use of zinc compounds, pilocarpine, alpha lipoic acid, transcranial magnetic stimulation, ginkgo biloba and acupuncture.
What are the main results of the review?
We collected and analysed all relevant studies to answer this question and found 10 trials in which a total of 581 subjects received different treatments. Nine trials assessed the benefits of zinc compounds and one trial assessed the effects of acupuncture. We only included studies on taste disorders in this review that were either idiopathic, or resulting from zinc deficiency or chronic renal failure.
Two trials were from Germany, three from Japan, two from the UK, and three from the US. These studies compared zinc with placebo or acupuncture with sham procedure for patients with taste disorders. Two were government funded, three were privately funded, two were funded by a pharmaceutical company and three trials did not mention funding details.
When patients with taste disorders are given zinc, compared to placebo:
‐ we found very low‐quality evidence that was insufficient to conclude on the role of zinc supplements to improve taste acuity reported by patients and very low‐quality evidence that zinc supplements improve taste acuity in patients with zinc deficiency/idiopathic taste disorders;
‐ zinc supplementation showed adverse events like eczema, nausea, abdominal pain, diarrhoea, constipation, decrease in blood iron, increase in blood alkaline phosphatase and minor increase in blood triglycerides;
‐ no studies were found that looked at improvement in taste discrimination or quality of life.
When patients with taste disorders are given acupuncture, compared to sham procedure:
‐ we found very low‐quality evidence that is not sufficient to conclude on the role of acupuncture for improving taste discrimination in cases of idiopathic dysgeusia (distortion of taste) and hypogeusia (reduced ability to taste);
‐ acupuncture trial did not show adverse events;
‐ no studies were found that looked at improvement in taste acuity or quality of life.
We were unable to draw any conclusions regarding the superiority of zinc supplements or acupuncture as none of the trials compared these interventions.
How up‐to‐date is this review?
We searched for studies that had been published up to 4 July 2017.
Authors' conclusions
Summary of findings
| Zinc compared to placebo for the management of taste disturbances | ||||||
| Patient or population: patients with taste disturbances | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with zinc | |||||
| Taste acuity improvement (patient‐reported outcome) assessed with VAS/questionnaire where improvement in dysgeusia is defined as more than 5% improvement in the VAS scores for a mean follow‐up period of 3 months | Study population ‐ zinc‐deficient/idiopathic taste disorder | 1.40 (0.94 to 2.09) | 119 | ⊕⊝⊝⊝ | There is a 40% relative increase in taste acuity improvement (patient‐reported outcome) in patients taking zinc when compared to placebo with a CI of 6% decrease to 109% increase of taste acuity | |
| 407 per 1000 | 569 per 1000 | |||||
| Taste acuity improvement (objective outcome ‐ continuous data) assessed with filter paper strip and filter paper disk methods for a mean follow‐up period of 3 months | ‐ | SMD 0.44 higher | ‐ | 366 | ⊕⊝⊝⊝ | The standardised mean difference for taste acuity improvement in the zinc intervention group is 0.44 higher than the placebo group |
| Taste acuity improvement (objective outcome ‐ dichotomous data) assessed with filter paper disk and Henkin's 3‐drop stimulus method for a mean follow‐up period of 3 months | Study population ‐ zinc‐deficient/idiopathic taste disorder | RR 1.42 | 292 | ⊕⊝⊝⊝ | There is a 42% relative improvement in taste acuity in patients taking zinc when compared to placebo with a CI of 9% to 84% increase of taste acuity | |
| 435 per 1000 | 618 per 1000 | |||||
| Cross‐over trial ‐ taste detection assessed with Henkin's method for a follow‐up period of 6 months | The mean taste detection was 7.5 | MD 2.50 higher | ‐ | 14 | ⊕⊝⊝⊝ | The mean difference for taste detection in the intervention group is 2.50 higher than the placebo group |
| Cross‐over trial ‐ taste recognition assessed with Henkin's method for a follow‐up period of 6 months | The mean taste recognition was 16 | MD 3.00 higher | ‐ | 14 | ⊕⊝⊝⊝ | The mean difference for taste recognition in the intervention group is 3.00 higher than placebo group |
| Adverse events ‐ follow‐up range 12 weeks to 18 weeks | Study population ‐ zinc‐deficient/idiopathic taste disorder and taste disorder secondary to chronic renal failure | 5.20 (0.90 to 30.19) | 335 | ⊕⊝⊝⊝ | Risk of 1 per 1000 assumed in placebo group (as it was 0) There is 420% relative increase in the adverse events in patients taking zinc compared to placebo with 95% CI of 10% decrease to 2919% increase in adverse events | |
| 6 per 1000 | 31 per 1000 | |||||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear randomisation and high risk of bias due to attrition in Sakai 2002. Downgraded by 1 level. | ||||||
| Acupuncture compared to sham control for the management of taste disturbances | ||||||
| Patient or population: idiopathic dysgeusia combined with hypogeusia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with sham (control) | Risk with acupuncture | |||||
| Taste discrimination assessed with 32 taste strips with a follow‐up of 8 weeks | The mean taste discrimination was 14.7 | MD 2.80 higher | ‐ | 37 | ⊕⊕⊝⊝ | The mean difference for taste discrimination in the acupuncture group is 2.80 higher than the sham group |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Brandt 2008 is a single‐blind trial with high risk of performance bias. Downgraded by 2 levels. | ||||||
Background
The sense of taste is important for health and quality of life, yet it is often taken for granted. More than 200,000 people/year visit a physician for chemosensory problems such as taste disorders. Many more taste disorders go unreported (NIDCD 2010). Approximately 240,000 people in Japan have an altered taste sensation and present to health professionals for evaluation (Ikeda 2005). Alterations in taste can lead to loss of appetite, resulting in malnutrition, affecting both physical and psychological well‐being (NIDCD 2009). The US National Health and Nutrition Examination Survey (NHANES) published the results of a survey from 2011 to 2012 where prevalence and risk factors were assessed based on individuals' self‐reported responses to chemosensory alterations. Survey results reflected prevalence of taste alterations were 19% including dysgeusia at 5%, with rates increasing with age (> 80 years of age = 27%) (Rawal 2016).
The basic tastes are salty, sour, bitter, sweet and umami (taste of meaty/ savoury substances, found in glutamates). It has also been suggested that fatty taste may be recognised as another basic taste quality (Mattes 2009). In humans, there are approximately 5000 taste buds in the oral cavity, situated on the superior surface of the tongue, on the palate, and on the epiglottis (Miller 1995). Taste receptor signalling is not limited to taste buds, but occurs in a variety of tissues like chemosensory cells of the alimentary tract, pancreas, brain and airway epithelium (Kinnamon 2012).
It is important to understand how our taste buds function, both as an organ and in conjunction with other factors, especially our sense of smell. Taste buds are onion‐shaped aggregates of approximately 50 to 100 elongated cells, with a life span of 10 to 11 days (Porter 2010). Due to such a fast turnover rate, the taste cells used for breakfast may be different from those used for lunch (Spielman 1992). They extend from the basal lamina to the surface of the tongue, where their apical microvilli extend through an opening in the epithelium to contact sapid chemicals in the oral cavity. Salts and acids utilise apically located ion channels for transduction, while bitter, sweet and umami stimuli utilise G‐protein‐coupled receptors (GPCRs) and second‐messenger signalling mechanisms (Kinnamon 2012). These taste cells receive tastant from the apical pore and transduce the signal to gustatory nerves that innervate taste buds (Iwatsuki 2012). Taste‐related impulses are then transmitted via the facial, glossopharyngeal and vagus nerves to the nucleus of the solitary tract, and thereafter to the thalamus and upwards to the postcentral gyrus‐facial area and olfactory area of the cortex (Porter 2010). There are super tasters who experience the sense of taste with far greater intensity than average due to an increased number of fungiform papillae, and have extreme sensitivity to n‐propylthiouracil (Bartoshuk 1994).
As important as taste is to food enjoyment, flavour is even more important. It is the distinctive quality of a particular food or drink. Flavour tells us whether we are eating a pear or an apple. In order to perceive flavour, the brain interprets not only taste stimuli, but also olfactory, thermal and tactile sensations. With spicy food, the brain will perceive pain as one aspect of flavour. When one cannot 'taste' food due to the common cold, in reality it is the inability to smell that is affecting the 'flavour' of the food and not the basic tastes of the food. It is important to understand the mechanism by which taste and smell work together. When one chews food, aromas are released that enter the nose through a retronasal passage connecting the roof of the mouth with the nose. Nerve endings in the olfactory bulb in the nose send these smell stimuli to the brain. It is the aroma, when combined with the stimuli of taste, temperature and texture that give the food a 'flavour'. It is the integration of these stimuli by the brain that distinguishes between, for example, eating an apple rather than a pear. Many studies have shown a significant relationship between smell disorders and taste disorders; loss of flavour can increase the salt intake in hyposmia patients (Henkin 2014), as smell loss severity decreased, salivary cAMP and cGMP levels increased consistently with each stepwise change of clinical loss severity (Henkin 2009), and decreased cAMP and cGMP levels in parotid saliva and nasal mucosal secretions of patients diagnosed with taste and smell disorders (Henkin 2007; Henkin 2013).
With the growing population of elderly people globally, and the effects of drugs and other treatment forms of modern medicine at any age, peoples' senses of taste and smell will continue to be adversely affected. The sense of smell is more impaired by aging compared with the sense of taste (Winkler 1999). Both anatomical investigations and human taste threshold studies indicate that age‐related differences in the gustatory system are not as substantial as investigators have suggested in the past (Mistretta 1984). Nutrition surveys have shown that the elderly population with taste loss consume more sweet and salty food (Sergi 2017). A Japanese study has shown that taste hyposensitivity is present even in children (Ohnuki 2014).
Taste and smell protect against malnutrition, depression and their concomitant diseases. The taste or smell of rancid food telling us to avoid it, or perhaps the odour of gas alerting us to danger, are lost or diminished without these senses. Simple pleasures like delicious foods and their aroma enable an individual to enjoy quality of life.
People who suffer from dysgeusia (distortion of taste) are also forced to manage the impact that the disorder has on their quality of life. An altered taste has effects on food choice and intake, and can lead to weight loss, malnutrition, impaired immunity, and a decline in health (Bromley 2000). Patients diagnosed with dysgeusia must use caution when adding sugar and salt to food and must be sure not to over‐compensate for their lack of taste with excess amounts. Since the elderly are often on multiple medications, they are at risk for taste disturbances increasing the chances of developing depression, loss of appetite, and extreme weight loss. This is cause for an evaluation and management of their dysgeusia. In patients undergoing chemotherapy, taste distortions can often be severe and make compliance with cancer treatment difficult. Other problems that may arise include anorexia and behavioural changes that can be misinterpreted as psychiatric delusions regarding food. Symptoms including paranoia, amnesia, cerebellar malfunction and lethargy can also manifest when undergoing histidine treatment. This makes it critical that these patients' dysgeusia is either treated or managed in order to improve their quality of life (Padala 2006).
Description of the condition
The symptoms of taste impairment may vary depending on the cause. Patients may experience a reduced ability to taste (hypogeusia), a distortion of taste (dysgeusia), the total lack of taste (ageusia), or all three. However, the following terms have been used in literature to describe taste abnormalities (Hawkes 2002).
-
Ageusia: absence of taste sense.
-
Hypo or microgeusia: reduction of taste sense.
-
Dysgeusia: distortion of taste sense.
-
Parageusia: distortion due to a specific stimulus.
-
Phantogeusia: distortion when there is no external stimulus.
-
Cacogeusia: unpleasant type of distortion.
-
Torquegeusia: burning type of distortion.
-
Hypergeusia: increased sensitivity to common taste.
-
Gustatophobia: dislike of certain tastes.
-
Heterogeusia: all food and drink taste the same.
-
Presbygeusia: decline of taste sense with age.
-
Type 1 hypogeusia: inability to recognise stimulus with varying degrees of detection.
-
Type 2 hypogeusia: decreased detection or recognition.
-
Type 3 hypogeusia: reduced intensity ability with normal detection and recognition.
The most common causes of taste disorders are drug use (21.7%), zinc deficiency (14.5%), and oral and systemic diseases (7.4% and 6.4%, respectively) (Imoscopi 2012). Anything that negatively affects either the physical make‐up of the taste buds or their cells, saliva production, the nerve pathway, or brain can cause a taste disorder. Therefore, in addition to the normal aging process, a host of other factors such as smoking, infection, nerve diseases, tumours, radiation treatment, drugs, chemicals, head injury, zinc deficiency, dry mouth and poor oral hygiene can also affect the ability to taste.
Taste impairment may be caused not only by an altered threshold of taste and sensory pathway but also by various mental and physical disorders, including depression, taste bud or mucosal lesions, gum disease, dry mouth, gastrointestinal diseases, zinc deficiency and medication. Therefore the symptoms of taste impairment may vary depending on the cause. Subnormal taste often induces appetite loss, which results in malnutrition and impairs quality of life (Kashihara 2011).
Taste disorders are classified based on two principles: type and site of the lesion. Based on the type of lesion, taste disorders are grouped as quantitative dysgeusias (ageusia, hypogeusia and hypergeusia), and qualitative dysgeusias (parageusia, pseudogeusia, phantogeusia, cacogeusia and agnogeusia). Based on the site of the lesion, taste disorders are classified as epithelial, neural and central dysgeusias (Fikentscher 1987). Systemic disorders like renal disorders (Mahajan 1980), alcoholic cirrhosis (Russell 1980), regional enteritis (Solomons 1974), and iatrogenic causes like postradiation therapy (Mossman 1978; Silverman 1983), or chemotherapy (Wickham 1999) can lead to taste disorders. Xerostomia was the strongest risk factor for taste disorder (Rawal 2016).
Description of the intervention
Various treatment modalities have been used to improve taste disorders. These include the use of zinc (Heckmann 2005; Sakai 2002), transcranial magnetic stimulation (Henkin 2011a), alpha lipoic acid (Femiano 2002), ginkgo biloba (Mattes 2004), pilocarpine (Aframian 2007), and acupuncture (Brandt 2008). The ability to manage taste disorders varies with each intervention.
Diminished taste acuity resulting in malnutrition in haemodialysis patients was studied in Mahajan 1980. The subjects were tested for taste acuity related to plasma zinc concentration. This double‐blinded trial was instituted using a zinc supplement (zinc acetate) and a placebo. The same authors studied the effect of zinc supplements on patients undergoing regular haemodialysis (Mahajan 1982). Treatment of taste abnormalities with zinc sulphate was tried in patients receiving external beam radiation therapy (ERT) for head and neck cancers (Halyard 2007; Ripamonti 1998). In a double‐blind, placebo‐controlled trial, the efficacy of zinc picolinate and zinc gluconate were studied in idiopathic zinc deficiency taste disorders (Sakai 2002). Zinc gluconate was tested in patients with drug‐induced taste disorders in Yoshida 1991. Zinc supplements were also tried in taste disorders due to head trauma and malignant tumours of head and neck in Henkin 1976.
Dosage of zinc varied drastically in different trials: capsules containing 22.6 mg of zinc (Barrie 1987); 29 mg of zinc three times a day for three months (Sakai 2002); 45 mg of zinc sulphate three times a day (Ripamonti 1998); and 50 mg of elementary zinc (as zinc acetate) per day (Mahajan 1980).
-
In an open cross‐over trial on idiopathic dysgeusia patients, considering idiopathic dysgeusia as a neuropathy similar to burning mouth syndrome, an alpha lipoic acid intervention was studied (Femiano 2002).
-
Gingko biloba extracts were tried to enhance cognitive, taste and smell functions in dementia patients (Mattes 2004).
-
Repetitive transcranial magnetic stimulation (rTMS) was used in patients with smell and taste disorders (Henkin 2010; Henkin 2011a).
-
Intranasal theophylline was used for the management of patients with smell and taste disorders (Henkin 2012).
Other than these interventional studies, many individual case reports/pilot studies on management of taste disorders are found in the literature. They are:
-
high dose biotin (Greenway 2011);
-
application of glutamate (Sasano 2010);
-
branched‐chain amino acid‐enriched supplementation (Aminofeel) (Nagao 2010);
-
transient cooling of the mouth by using ice cubes (Fujiyama 2010);
-
thioridazine/haloperidol to inhibit phantogeusia (Henkin 2000);
-
miracle fruit (Synsepalum dulcificum) (Wilken 2012).
How the intervention might work
Zinc is an important element in both the maintenance and repair of taste buds. Zinc influences the synthesis of the protein gustin, which is linked to the production of taste buds. A decrease in the salivary gustin/carbonic anhydrase VI is associated with taste and smell disorders and can be effectively treated with zinc supplementation (Henkin 1999). Zinc has also been shown to increase calcium concentration in saliva. Taste buds rely on calcium receptors to work properly (Heckmann 2005). Finally, zinc is an important cofactor for alkaline phosphatase, the most important enzyme in taste bud membranes (Bicknell 1988). Zinc supplementation has shown to be effective in treating taste disorders. It can also be found in natural foods such as meat, cereals, beans and oysters.
Alpha lipoic acid (ALA) is an antioxidant that is produced naturally in human cells. Among its functions, it has an important role in the Krebs cycle assisting in the production of nerve growth factor. Research in animals has shown that ALA can improve nerve induction velocity. However, there are contradictory opinions about the efficacy of ALA in treating burning mouth syndrome and dysgeusia (de Moraes 2012; Femiano 2002).
Ginkgo biloba, an herbal extract, may have three effects on the human body: improvement in blood flow to most tissues and organs; protection against oxidative cell damage from free radicals; and blockage of many platelet‐activating factors (aggregation and blood clotting). These anticlotting characteristics may be of help with circulatory problems attributed to aging. It is being used to treat memory loss, and the impact to the brain and circulation may make it helpful in treating taste disorders (Mattes 2004). However, there is no evidence for its clinically significant benefits in dementia patients (Birks 2009).
Transcranial magnetic stimulation (TMS) uses electromagnetic induction to induce weak electric currents stimulating activity in specific parts of the brain with minimal discomfort. A variant of TMS, called repetitive TMS, was used to treat various neurological and psychiatric disorders including migraines, Parkinson's disease, tinnitus, stroke, depression (Henkin 2011a), and phantogeusia (unpleasant taste sensation in the absence of food or drink) (Henkin 2011b).
Research has found that saliva contains specific proteins that are growth factors (nerve growth factor, epidermal growth factor) that make taste buds develop and mature. Without these growth factors, taste buds degenerate (Gardiner 2008). Pilocarpine, by increasing saliva production, gives taste buds greater access to food molecules and may be responsible for maintenance of taste buds. Studies have shown that treatment with pilocarpine enhances taste (Aframian 2007; Leek 2002).
Acupunture is the stimulation of specific acupoints along the skin of the body involving various methods such as the application of heat, pressure or laser or penetration of thin needles. It is a key component of traditional Chinese medicine which aims to treat a range of conditions including dysgeusia. According to traditional Chinese medicine, stimulating specific acupuncture points corrects imbalances in the flow of qi through channels known as meridians. This seeks to re‐establish an equilibrium of forces in the diseased body between the energies of yin and yang (contrary energies such as fire and water, hot and cold), which are distorted in the diseased body (Vent 2010).
Why it is important to do this review
Taste disturbances are not uncommon, have a range of causes and result in significant reduction in quality of life. A systematic review is necessary to summarise the evidence of the effects of the many interventions available to treat taste disturbances and to provide evidence to guide decision‐making. This is an update of the Cochrane Review first published in 2014 (Kumbargere 2014).
Objectives
To assess the effects of interventions for the management of patients with taste disturbances.
Methods
Criteria for considering studies for this review
Types of studies
We included only parallel and cross‐over randomised controlled trials (RCTs) with either a pharmacological or non‐pharmacological intervention in this review.
Types of participants
We included patients with taste disorders diagnosed clinically as dysgeusia, parageusia, ageusia, hypogeusia or phantogeusia regardless of their age, gender, race, profession or residential location. It is a well established fact that many treatment procedures like surgery, radiation therapy and chemotherapy can cause taste perception problems. Once the effect of these treatment procedures diminishes, the taste perception slowly reverts back to normal. Considering these variations, we agreed upon the following exclusion criteria. We excluded the following types of patients in our review.
-
Demolitive surgery of tongue, palate or oropharynx.
-
Presence of oral lesions such as ulcers, stomatitis, candidiasis and necrosis.
-
Cerebral lesions or surgical damage to the nervous system.
-
Endocrinal and neurological disorders known to affect taste, or smell sensitivity, or both.
-
Patients undergoing treatment with drugs known to affect taste perception (e.g. chemotherapy).
-
Patients who underwent treatment affecting salivary function (e.g. radiotherapy).
-
Patients experiencing hyposalivation.
Types of interventions
-
Any intervention versus placebo or no treatment.
-
Any direct comparisons between two active interventions, e.g. drug A versus drug B, or between two doses of the same drug e.g. drug A dose X versus drug A dose Y.
-
We included all routes of drug administration or modes of application.
Types of outcome measures
We considered improvement in taste acuity to at least one quality of taste by subjective/objective assessment scales as the most important outcome. It could be any one of the following.
-
Sip and spit method (traditional method): in this method, a solution of a known concentration of a sweet, salty, bitter, or sour substance is gargled and sloshed in the mouth and then discarded. The patient is asked to identify the taste substance, and the concentration can be varied to determine threshold sensitivity. It is an easy test to administer but assumes severe taste loss. Regional damage (e.g. on the front or tip of the tongue) would be masked by stimulation of the remaining taste cells elsewhere in the mouth.
-
Filter paper disk method (Tomita 1986): in this test, filter paper or a dissolvable strip is impregnated with a known concentration of a sweet, salty, bitter, or sour substance, and the filter paper or strip is placed on a specific part of the tongue or palate. The patient is asked to identify the taste substance. The concentration can be varied to determine threshold sensitivity. This test is also easy to administer. The goal is to activate major regions of taste cells to determine whether the individual has partial taste deficit or damage.
-
Electrogustometry (Tomita 1986): the measurement of taste threshold by passing a controlled anodal current through the tongue. When the current passes through the tongue a unique and distinct metallic taste is perceived.
-
Three stimulus drop technique (Henkin 1963): testing involves a three‐stimuli forced choice drop technique given in a type of staircase technique. The subject is given three drops of liquid, which are placed successively onto the lingual surface. Out of three, two drops are water and one drop is water with a solute, either NaCl (salt), sucrose (sweet), hydrogen chloride (HCl) (sour) or urea (bitter).
-
Edible taste strips (Smutzer 2008): prepared from pullulan hydroxypropyl methylcellulose solutions that are dried to a thin film. The maximal amount of a tastant that could be incorporated in a 2.54 cm × 2.54 cm taste strip is 5% for each class of tastant (sweet, sour, salty, bitter and umami) during strip formation.
-
Filter paper strips method (Mueller 2003): all four of five taste qualities, sweet, sour, salty and bitter are tested using filter paper strips coated with four different concentrations for each taste quality. This is tested randomly on the right and left side of the anterior two‐thirds of the tongue, and the subject is asked to identify the taste from a list of four descriptors.
-
Visual analogue scale (VAS) (traditional method).
-
Self‐reporting questionnaire method (Soter 2008).
-
Spatial taste test (Gondivkar 2009).
-
Clinical bitterness masking test for phantogeusia (Ishimaru 2001).
Primary outcomes
-
Taste acuity improvement ‐ we considered improvement in taste acuity to at least one quality of taste by subjective/objective assessment scales as the most important outcome.
-
Taste discrimination improvement.
Taste acuity includes taste detection and recognition, whereas taste discrimination is the ability to distinguish one taste from the other.
Secondary outcomes
-
Adverse events related to the interventions.
-
Health‐related quality of life.
Search methods for identification of studies
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions.
Electronic searches
The following databases were searched:
-
Cochrane Oral Health's Trials Register (searched 4 July 2017) (Appendix 1);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6) in the Cochrane Library (searched 4 July 2017) (Appendix 2);
-
MEDLINE Ovid (1946 to 4 July 2017) (Appendix 3);
-
Embase Ovid (1980 to 4 July 2017) (Appendix 4);
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 4 July 2017) (Appendix 5);
-
AMED Ovid (Allied and Complementary Medicine; from 1985 to 4 July 2017) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following databases were searched for ongoing trials:
-
the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 4 July 2017) (Appendix 7);
-
the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/; searched 4 July 2017) (Appendix 8).
We searched abstracts from scientific meetings and conferences for appropriate studies through the websites of the following organisations:
-
International Association for Dental Research/American Association for Dental Research Conference Proceedings (to 25 September 2017) (Appendix 9);
-
Association for Research in Otolaryngology Conference Proceedings (to 25 September 2017) (Appendix 10).
The previous version of this review included searches of the metaRegister of Controlled Trials (to 5 March 2014) and the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Clinical Trials Portal (to 5 March 2014). However, these sources are no longer available (see Appendix 11).
We checked reference lists of included studies to identify any further additional studies. We contacted authors of the included studies for relevant unpublished material.
Data collection and analysis
Selection of studies
Two pairs of review authors (Renjith P George (RPG) and Naresh Shetty (NS), and David Levenson (DL) and Debra M Ferraiolo (DMF)) screened the titles and abstracts of all the obtained reports for eligibility, independently and in duplicate. Full papers of relevant RCTs (based on the inclusion and exclusion criteria) were obtained and screened independently and in duplicate by two review authors (Sumanth Kumbargere Nagraj (SKN) and RPG). Any disagreements on eligibility were resolved by discussion. When resolution was not possible, we consulted an arbiter (Adinegara Lutfi Abas). We recorded studies excluded at this point in the Characteristics of excluded studies tables along with reasons for exclusion. We did not mention non‐RCTs or quasi‐RCTs in the Characteristics of excluded studies tables.
Data extraction and management
Two review authors (SKN and RPG) extracted the data independently and in duplicate, using a data extraction form specifically designed for this review. Any disagreements were resolved by discussions. For two studies, we could not resolve the disagreement, and a third review author was asked to do the data extraction independently, and this was deemed final. We entered all of the trial details in the Characteristics of included studies tables in Review Manager 5 (Review Manager 2014).
We recorded the following details for each trial.
-
Publication details like year of publication and language.
-
Demographic details of the report.
-
Inclusion and exclusion criteria.
-
Sample size, method of randomisation, allocation concealment, blinding, type of trial, method of assessing the outcome, and dropouts, if any.
-
Type of intervention.
-
Details of the outcome reported.
-
Duration of follow‐up.
-
Results of the intervention.
-
Funding details.
We contacted the author/s of included/excluded studies via email if clarification of any details or additional data were required.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies using Cochrane's 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We completed a 'Risk of bias' table for each included trial. Within each table, we assessed the following domains of risk of bias: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. For each domain, we described what was reported to have happened, using quotes from the trial, followed by a judgement of 'low risk', 'high risk' or 'unclear risk' of bias. We contacted the trial authors for clarification where necessary, quoting their responses in the risk of bias table. We resolved any disagreements on risk of bias by consulting a third review author (arbiter).
Summarising risk of bias
Studies have been grouped into the following categories.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
We summarised risk of bias graphically using the plots available in Review Manager 5 (Review Manager 2014).
Measures of treatment effect
For dichotomous data, we expressed the estimates of effect of an intervention as risk ratios (RRs) together with 95% confidence intervals (CIs). For continuous data, we used standardised mean difference, as the included studies used different taste scales to measure the same primary outcome (e.g. improvement in taste acuity). For continuous data which were measured using same scales were expressed the estimates of effect as mean difference.
Unit of analysis issues
Cross‐over studies
We had two cross‐over trials in our review. One trial (Eggert 1982) presented the data in graphs and we separately analysed only the data before cross‐over. The other trial (Watson 1983) gave the data in median value and we could not use this in the meta‐analysis of outcomes; however, we used the adverse events data in the meta‐analysis.
Studies with multiple intervention arms
One trial (Sakagami 2009) had three treatment arms. We combined the data using the method described in Section 7.7.3.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We contacted trial authors to obtain missing data whenever possible. If we could not get the missing data, we used per protocol analyses for missing data and assessed the data at a high risk of bias in the 'Risk of bias' tables.
If both mean and standard deviations were reported as graphs, we derived the data from the graphs by magnifying them and approximating the measures of mean and standard deviation.
If the data were described in the form of ordinal outcome, we converted the shorter ordinal scales into dichotomous data by combining relevant adjacent categories.
Assessment of heterogeneity
We assessed heterogeneity of the studies by examining the forest plots, with poor overlap of the confidence intervals indicating the presence of heterogeneity. We used the Chi2 test to assess whether heterogeneity was present and quantified it using the I2 statistic. We used the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions to interpret the I2 statistic: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% indicates considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
According to our protocol we intended to do a test of asymmetry to assess reporting bias, but we did not do this test as we had less than 10 trials included in our meta‐analysis.
Data synthesis
We analysed the data using Review Manager 5 software (Review Manager 2014). We used the data available from the trials with similar comparisons and outcomes in the meta‐analysis. We combined RRs for dichotomous data and standardised mean differences for continuous data (as the trials used different scales), and used a random‐effects model in the meta‐analysis. We used the first‐phase data from one cross‐over trial (Eggert 1982), although we did not pool these data with other studies in the meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses whenever there was heterogeneity.
To identify the reasons for clinical or methodological heterogeneity in meta‐analyses, we carried out subgroup analyses, based on:
-
population: idiopathic dysgeusia/hypogeusia, and dysgeusia and hypogeusia secondary to chronic renal failure; and
-
outcome: patient‐reported outcome (VAS scale, questionnaire), and objective outcome (taste strips or filter paper disks).
Sensitivity analysis
Wherever feasible, we undertook sensitivity analyses to assess the robustness of our findings by excluding studies with high risk of bias.
Summarising and interpreting results
We used the GRADE approach to interpret findings. We used the GRADEpro GDT software (GRADEpro GDT 2015), and imported the data from Review Manager 5 to create 'Summary of findings' tables for each comparison included in this review. We assessed the outcomes with reference to the overall risk of bias of the included studies, the inconsistency of the results, the directness of the evidence, the precision of the estimates, and the risk of publication bias. We categorised the quality of the body of evidence for each assessable outcome as no reason to downgrade the quality of evidence, serious reason (downgraded by one) or very serious reason (downgraded by two). These tables provide the information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined and the sum of available data on the primary outcome and secondary outcomes. We selected the outcomes of improvement in taste acuity, adverse events and taste discrimination, for inclusion in these tables (summary of findings Table for the main comparison; summary of findings Table 2).
Results
Description of studies
Results of the search
The electronic search identified 5728 records from English and other language databases.
We used keywords "taste", "dysgeusia" and "chemosensory" to search for related abstracts, and identified 70 abstracts from the International Association for Dental Research conference proceedings (as of 25 September 2017) and 19 trials from the Association of Research in Otolaryngology conference proceedings (as of 25 September 2017).
The metaRegister of Controlled Trials (mRCT) and the IFPMA trials registry were searched for the original version of this review, but these resources no longer exist. The electronic search of these databases resulted in 21 trials from IFPMA (as on 5 March 2014) and nine trials from mRCT (as on 5 March 2014). Only four trials were related to interventions in patients with taste disorders, and of these, two trials were ongoing (IFPMA, NCT01143285 (excluded) and JapicCTI‐121907 (awaiting classification)) and two trials were completed (clinicaltrials.gov/show/NCT00316563 and JPRN‐C000000401 (awaiting classification)). The completed trial (NCT00316563) is published and we excluded the trial from our review (Brisbois 2011).
At the end of our search, we had 4724 records after removing duplicates, out of which we discarded 4644 and we requested full‐text copies of 80 references. Two pairs of review authors (Renjith P George (RPG) and Naresh Shetty (NS), and David Levenson (DL) and Debra M Ferraiolo (DMF)), independently and in duplicate assessed these papers to determine their eligibility. We identified 10 studies (12 references) which met the inclusion criteria and included them in this review, and 24 were excluded (Figure 1). For details of the studies examined and reasons for inclusion or exclusion, see the Characteristics of included studies and Characteristics of excluded studies tables. Six trials are awaiting classification and one is ongoing. So we will consider them in a future update of the review.

Study flow diagram.
We contacted authors of six included trials and we received clarifications for only four trials. We could not contact authors of three trials due to non‐availability of recent address/email address. One trial did not have any missing data and hence the authors were not contacted (see Characteristics of included studies). We also contacted the authors of two unpublished clinical trials and one of them refused to share the details of the trial (see Characteristics of studies awaiting classification).
Included studies
See Characteristics of included studies table.
Characteristics of the trial settings and investigators
We included 10 randomised controlled trials (RCTs) in the review. Out of these, eight were in the English language, one was in German (Brandt 2008), and one was in Japanese (Ikeda 2013). The countries of origin for the included studies were: two from Germany (Brandt 2008; Heckmann 2005), three from Japan (Ikeda 2013; Sakagami 2009; Sakai 2002), two from the UK (Matson 2003; Watson 1983), and three from the US (Eggert 1982; Mahajan 1980; Mahajan 1982).
Eight trials were parallel‐design trials and two were cross‐over trials (Eggert 1982; Watson 1983).
Out of 10 trials, seven provided grant information and out of these seven, two were government funded (Mahajan 1980; Mahajan 1982), three were privately funded (Brandt 2008; Eggert 1982; Heckmann 2005), one was funded by a pharmaceutical company (Ikeda 2013), one had the intervention drug sponsored by a pharmaceutical company (Sakagami 2009). Three trials did not mention funding details (Matson 2003; Sakai 2002; Watson 1983).
Two of the trials were multicentric (Ikeda 2013; Sakagami 2009) and others were carried out in either one or two centres.
Only one trial (Brandt 2008) studied taste discrimination as the trial outcome; eight trials tested for taste acuity (detection, recognition, or both), and one trial (Matson 2003) studied both taste acuity and taste discrimination as the trial outcome. Two trials (Brandt 2008; Heckmann 2005) have reported the data related to improvement in the mood scale and depression inventory. In addition to these, Brandt 2008 also reported the assessment of 'quality of life' using a visual analogue scale.
Characteristics of the participants
Eight out of 10 trials included only adult patients (Brandt 2008; Heckmann 2005; Ikeda 2013; Mahajan 1980; Mahajan 1982; Matson 2003; Sakagami 2009; Sakai 2002); and two were trials on children (Eggert 1982; Watson 1983). Seven trials included both genders in their trial, one trial included only males (Mahajan 1982), and two trials did not report on the gender distribution (Eggert 1982; Mahajan 1980). The minimum age included in the trials was 0.5 years (Eggert 1982) and the maximum age included in the trials was 83 years (Brandt 2008). The minimum sample size was 15 (Matson 2003) and the maximum sample size was 219 (Ikeda 2013) with an average of 62.4.
Five trials were on renal failure‐induced hypogeusia (Eggert 1982; Mahajan 1980; Mahajan 1982; Matson 2003; Watson 1983), three were on idiopathic dysgeusia/hypogeusia (Brandt 2008; Heckmann 2005; Sakagami 2009), and two were on idiopathic dysgeusia and zinc deficiency‐induced hypogeusia (Ikeda 2013; Sakai 2002).
Characteristics of the interventions
We included one non‐pharmacological intervention (needle acupuncture) in our review (Brandt 2008); the remaining nine trials had zinc (Zn) compounds as the intervention drug. Three trials studied zinc sulphate (Eggert 1982; Matson 2003; Watson 1983) and two used polaprezinc (Ikeda 2013; Sakagami 2009). Zinc acetate was used in two trials (Mahajan 1980; Mahajan 1982), zinc gluconate in one (Heckmann 2005), and zinc picolinate in one trial (Sakai 2002).
Zinc supplement
Zinc sulphate
Two cross‐over trials and one parallel‐group trial studied the effects of zinc sulphate on taste disorders in chronic renal failure patients (Eggert 1982; Matson 2003; Watson 1983). Zinc sulphate was given at the dosage of 0.5 mg/Zn/kg/day to 0.75 mg/Zn/kg/day to all the children included in the trial for six months (Eggert 1982). Zinc sulphate was given at a dose equivalent to 15 mg elemental zinc for children and 50 mg elemental zinc for adults, for a period of six weeks (Watson 1983). In Matson 2003 trial, 220 mg per day (45 mg elemental zinc) was given for a period of six weeks.
Zinc acetate
Two trials studied the effects of zinc acetate on taste disorders in chronic renal failure patients (Mahajan 1980; Mahajan 1982). Both trials tested 25 mg elemental zinc, twice a day in zinc acetate form.
Polaprezinc
Two trials studied the efficacy of polaprezinc on taste disorders (Ikeda 2013; Sakagami 2009). In the trial by Sakagami 2009, polaprezinc was tested in three different dosages, 75 mg, 150 mg, and 300 mg, which was equivalent to 17 mg, 34 mg, and 68 mg of elemental zinc, respectively for 12 weeks. In the trial by Ikeda 2013, polaprezinc was administered as 75 mg (17 mg elemental zinc), twice a day for 12 weeks. Additional to this, the daily intake of dietary zinc was standardised in both the experimental and control groups (Additional Table 1; Additional Table 2; Additional Table 3; Additional Table 4).
| Outcome | Group A | Group B | Time when measured | ||||
| Mean* | SD | n | Mean* | SD | n | ||
| Change of the mean 4 basic taste sensitivity scores from baseline | ‐0.52 | 0.68 | 108 | ‐0.47 | 0.61 | 111 | 4 weeks |
| ‐0.90 | 0.85 | 108 | ‐0.67 | 0.73 | 111 | 8 weeks | |
| ‐1.17 | 0.93 | 108 | ‐0.85 | 0.75 | 111 | 12 weeks | |
| ‐1.28 | 0.94 | 108 | ‐0.97 | 0.76 | 111 | 4 weeks after treatment | |
*Minus change score means better by filter paper disc method by Tomita.
SD = standard deviation.
| Outcome | Group A events (Improved) | Group A total | Group B events | Group B total | Time when measured |
| Improved/not improved | 60 | 108 | 48 | 111 | 12 weeks |
| Outcome | Group A (Placebo) n = 27 | Group B (17 mg zinc) n = 27 | Group C (34 mg zinc) n = 25 | Group D (68 mg zinc) n = 28 | Time when measured | ||||
| Secondary outcome | Mean | SD | Mean | SD | Mean | SD | Mean | SD | 12 weeks |
| Mean filter paper disk test scores (filter paper disk) | 4.095 | 1.148 | 4.350 | 1.030 | 3.448 | 0.928 | 3.454 | 1.138 | ─ |
| Mean serum zinc level | 1.8 | 12.7 | 5.7 | 13.5 | 11.4 | 16.6 | 20.6 | 21.3 | ─ |
| ‐ | Group A | Group B | Group C | Group D | ─ | ||||
| Increase in the average score of subjective symptoms | 0.6 | 0.9 | 1.2 | 1.0 | ─ | ||||
SD = standard deviation.
| Primary outcome: quantitative analysis of taste perception using filter paper disk method | Event (success) Cured + improved | No event (fail) Unchanged, neither cured nor improved nor worsened; aggravated | Total |
| Experimental intervention (17 mg zinc) | SE = 14 | FE = 13 | NE = 27 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 0.824; OR = 0.634; RD = 0.447 | |||
| Experimental intervention (34 mg zinc) | SE = 20 | FE = 5 | NE = 25 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 0.318; OR = 2.353; RD = 0.17 | |||
| Experimental intervention (68 mg zinc) | SE = 25 | FE = 3 | NE = 28 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 1.418; OR = 4.902; RD = 0.263 | |||
OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group.
Zinc picolinate
One trial studied the effects of zinc picolinate on taste disorders at the dosage of 28.9 mg, three times a day for three months (Sakai 2002). No dietary instructions to increase dietary zinc were given to either of the groups in this trial (Additional Table 5).
| Filter paper disk method | Event (success) Improved (+ cured) | No event (fail) Unchanged | Total (n = 73) |
| Experimental intervention (zinc picolinate) | SE = 28 | FE = 9 | NE = 37 |
| Control intervention (placebo) | SC = 16 | FC = 20 | NC = 36 |
| RR = 1.703; OR = 3.889; RD = 0.312 | |||
| Experimental intervention (zinc picolinate) | SE = 22 | FE = 12 | NE = 34 |
| Control intervention (placebo) | SC = 18 | FC = 17 | NC = 35 |
| RR = 1.258 ; OR = 1.732; RD = 0.133 | |||
OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group.
Zinc gluconate
One trial studied the effects of zinc gluconate on taste disorders at the dosage of 140 mg/day (equivalent to 20 mg elemental zinc) for three months (Heckmann 2005) (Additional Table 6; Additional Table 7).
| Outcome | Group A (zinc treatment) | Group B (placebo) | Time when measured | ||||
| Mean | SD | n | Mean | SD | n | At the end of 3 months | |
| Primary outcome | ─ | ||||||
| Taste test (32‐filter paper strip method by Mueller 2003) | 25.7 | 6.5 | 26 | 21.2 | 5.7 | 24 | ─ |
| Self‐rated impairment in % (VAS scale of 10 cm length equivalent to 100%; 0 to 10; 0 = no impairment; 10 = extremely impaired) | 45.0 | 4.4 | 26 | 43.8 | 3.6 | 24 | ─ |
| Secondary outcome | ─ | ||||||
| Beck Depression Inventory (BDI) | 7.5 | 7.0 | 26 | 11.3 | 10.9 | 24 | ─ |
| Zerssen Mood Scale (ZMS) | 10.7 | 7.5 | 26 | 18.8 | 14.6 | 24 | ─ |
| Zinc in serum (µg/dL) | 81.53 | 19.61 | 26 | 72.01 | 10.22 | 24 | ─ |
SD = standard deviation; VAS = visual analogue scale.
| Type of intervention | Event (success) Improved | No event (fail) | Total |
| Experimental intervention (zinc) | SE = 13 | FE = 13 | NE = 26 |
| Control intervention | SC = 6 | FC = 18 | NC = 24 |
| RR = 2; OR = 3; RD = 0.25 | |||
OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group.
Acupuncture
We included one trial that studied the effects of 15 acupuncture treatments on taste disorders over a period of eight weeks (Brandt 2008). Two patients did not require further acupuncture treatment after 10 treatments. De‐activated laser acupuncture was used as a sham control (Additional Table 8).
| Outcome | Group A | Group B | Time when measured | ||||||
| Mean | SD* | n = 17 | Mean | SD* | n = 20 | ||||
| Taste discrimination | 11.7 (before)/ 17.5 (after) | 4 (before)/ | ─ | 11.9 (before)/ 14.7(after) | 5 (before)/ | ─ | Before and after treatment | ||
| Quality of life | Not estimable (changes per group only given for each of the 5 individual questions of the questionnaire, but no combined score/analysis stated). Only information given: "both treatments resulted in an increased quality of life, however, no statistically significant difference could be found" | Before and after treatment | |||||||
| Depressive symptoms | 11 (before)/ 6 (after)* | 5 (before) / 4 (after)* | ─ | 10.5 (before)/ 10 (after)* | 7 (before)/ 7 (after)* | ─ | Before and after treatment | ||
| Quote: "The psychological well‐being of the intervention groups increased for 94.1% of all patients in the intervention group, but only for 60% of patients in the control group. This difference was statistically significant" | |||||||||
| Subjective well‐being | 16 (before)/ 12 (after)* | 10 (before)/ 7 (after)* | ─ | 20 (before)/ 18 (after)* | 9 (before)/ 14 (after)* | ─ | Before and after treatment | ||
| Quote: "58.8% of all patients in the intervention group felt better, whereas only 45% of all patients in the control group felt better. This difference was not statistically significant" | |||||||||
*Only given in graph ‐> estimated from graph.
SD = standard deviation.
Excluded studies
See Characteristics of excluded studies tables for further details.
We excluded 37 non‐RCTs and quasi‐RCTs without any explanation. We procured 24 full‐text articles and excluded these with reasons (Characteristics of excluded studies).
Four trials included patients with taste disorders under medications which might affect taste perception (Brisbois 2011; Green 2013; Lyckholm 2012; UMIN000027177).
Ten trials reported the intervention effects on either normal volunteers or subjects without taste disorders (Atkin‐Thor 1978; Dahl 1984; Deniz 2016; Hartman‐Petrycka 2016; Kamphuis 2003; Mahajan 1992; Ohno 2003; Stewart‐Knox 2008; Tupe 2009; Treldal 2016).
Six trials were aimed at prevention of taste disorders in patients undergoing chemotherapy or radiotherapy (Halyard 2007; Jham 2009; Najafizade 2013; NCT01143285; Ripamonti 1998; Strasser 2008).
Three trials included patients with taste disorders due to trauma, cranial injuries, oral lesions, and neurological problems, etc. (Henkin 1976; Sprenger 1983; Yoshida 1991).
One trial included patients with taste disorders secondary to radiotherapy, including parotid carcinoma cases, where the patients could have experienced xerostomia (Velargo 2012).
Studies awaiting classification
See Characteristics of studies awaiting classification tables for further details.
We grouped six trials as studies awaiting classification. One clinical trial was completed but unpublished; hence the trial group refused to share the results (JPRN‐C000000401). The full‐text was not available for three trials, and hence we could not decide on the inclusion/exclusion of the studies (Mahajan 1979; Sanchez 1993; Sturniolo 1985). We need additional information for two trials (JapicCTI‐121907; Sakai 2017).
Ongoing studies
See Characteristics of ongoing studies table for further details.
We identified one ongoing clinical trial. NCT02475928 was supposed to be completed by December 2016. We will consider the results of this trial in our future update of the review if it is published or the authors agree to share the results.
Risk of bias in included studies
See the 'Risk of bias' tables within Characteristics of included studies for further details. For a graphical summary, see Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We documented the risk of bias for included studies based on the full‐text articles. Wherever there was a need for clarification, we contacted the authors. Based on the available data, the 'Risk of bias' assessment was either 'low risk', 'high risk' or 'unclear risk'. If the trial report mentioned it as 'double‐blind' we considered it as at low risk of bias for blinding and if the trial was 'single‐blind' (Brandt 2008), we assessed it as at high risk of bias for this domain.
We assessed three trials (30%) as at low overall risk of bias (Heckmann 2005; Mahajan 1980; Mahajan 1982), four trials (40%) as at high overall risk of bias (Brandt 2008; Eggert 1982; Sakai 2002; Watson 1983), and three trials (30%) as at unclear risk of bias (Ikeda 2013; Matson 2003; Sakagami 2009).
Allocation
Seven of the included trials (Brandt 2008; Eggert 1982; Heckmann 2005; Mahajan 1980; Mahajan 1982; Sakagami 2009; Watson 1983) reported the method of sequence generation and five of the included studies (Eggert 1982; Heckmann 2005; Mahajan 1980; Mahajan 1982; Watson 1983) reported concealment of allocation (Figure 2).
Blinding
Six trials (Eggert 1982; Ikeda 2013; Matson 2003; Sakagami 2009; Sakai 2002; Watson 1983) described their studies as "double‐blind", but no other details were given.
Out of 10 included trials, blinding of participants and personnel was not done in one trial (Brandt 2008).
Blinding of outcome assessors was unclear in one trial (Brandt 2008), whereas three trials described that assessors were blinded (Heckmann 2005; Mahajan 1980; Mahajan 1982).
Incomplete outcome data
Attrition bias was reported in three of the included studies (Eggert 1982; Sakai 2002; Watson 1983).
Selective reporting
None of the included trials had reporting bias.
Other potential sources of bias
We have assessed biases in this section according to the Cochrane Handbook for Systematic Reviews of Interventions, Section 8.15 (Higgins 2011).
One of the trials had a high risk of other bias as this cross‐over trial did not have any washout period (Eggert 1982). We could not rule out the influence of the pharmaceutical company in the Sakagami 2009 trial. Routine medications (no details of those medications described) were continued during the trial which could have caused dysgeusia in the Watson 1983 trial. Matson 2003 used the sip and spit method for taste assessment and this method cannot detect region damage of taste cells which might give wrong results.
Effects of interventions
See: Summary of findings for the main comparison Zinc compared to placebo for the management of taste disturbances; Summary of findings 2 Acupuncture compared to sham control for the management of taste disturbances
Zinc supplements versus placebo
See Summary of findings table 1.
Out of 10 included trials, nine compared zinc supplements with placebo for taste disorder patients. One of these nine trials reported the results using median values (Watson 1983) and three trials reported the results in graphs (Eggert 1982; Matson 2003; Watson 1983). Two trials assessed patient‐reported outcomes (Heckmann 2005; Sakai 2002), and eight trials reported objective improvement based on different taste detection tests (Eggert 1982; Heckmann 2005; Ikeda 2013; Mahajan 1980; Mahajan 1982; Matson 2003; Sakagami 2009; Sakai 2002).
Taste acuity improvement
Taste acuity improvement ‐ Patient‐reported outcome
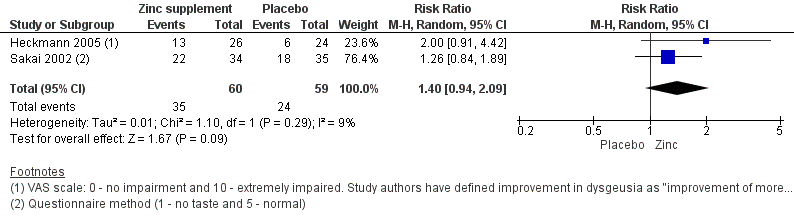
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Taste acuity improvement ‐ Patient‐reported outcome.
We first evaluated our primary outcome, taste acuity as reported by the patient. Two trials (Heckmann 2005; Sakai 2002) assessed the patient‐reported outcome for taste acuity improvement. The Heckmann 2005 trial was at low risk of bias and the Sakai 2002 trial at high risk of bias.
Heckmann 2005 used zinc gluconate in idiopathic dysgeusia patients and assessed the improvement using a visual analogue scale (VAS) (0 to 10 where 0 was no impairment and 10 was extremely impaired). However, the trial authors presented the dichotomized results using the criteria as described in the Characteristics of included studies table. Sakai 2002 used zinc picolinate in patients either with idiopathic dysgeusia or zinc‐deficient dysgeusia, and assessed the improvement using a questionnaire method (1 to 5 where 1 was no taste and 5 was normal). The forest plot shows an I2 of 9%, depicting low heterogeneity, and the confidence interval (CI) ranges from 0.94 to 2.09, indicating that the effect estimate ranges between no benefit to appreciable benefit. The overall effect favours zinc supplements over placebo for this patient‐reported outcome (risk ratio (RR) 1.40, 95% CI 0.94 to 2.09; 2 trials, 119 participants; Analysis 1.1).
Taste acuity improvement ‐ Objective outcome
Taste acuity can be tested objectively using methods like the filter paper disk method, and the three‐drop technique, etc.. We included eight trials (Eggert 1982; Heckmann 2005; Ikeda 2013; Mahajan 1980; Mahajan 1982; Matson 2003; Sakagami 2009; Sakai 2002) that used different objective taste testing methods to see the improvement in taste acuity. Heckmann 2005 used the filter paper strip method whereas Ikeda 2013; Sakagami 2009; and Sakai 2002 used the filter paper disk method. Eggert 1982; Mahajan 1980; and Mahajan 1982 used the three‐drop stimulus method in their trials. Matson 2003 used the sip and spit method.
Ikeda 2013 described the results in both continuous data and dichotomous data. Sakagami 2009 described the results as continuous data for taste acuity whereas Mahajan 1982 and Sakai 2002 described the results as dichotomous data. Heckmann 2005 reported continuous data (in addition to dichotomous data for this patient‐reported outcome). Hence, we analysed the data from Heckmann 2005; Ikeda 2013; and Sakagami 2009 under continuous data (Analysis 1.2) and data from Ikeda 2013; Mahajan 1982; and Sakai 2002 under dichotomous data (Analysis 1.5).
Mahajan 1980 described the results as continuous data for each taste sensation and was analysed separately (Analysis 1.3).
We used the data from the first half of the cross‐over trial by Eggert 1982 and analysed the data for taste detection and taste recognition (Analysis 1.4).
We could not include Matson 2003 and Watson 1983 in the meta‐analysis related to taste improvement due to missing data.
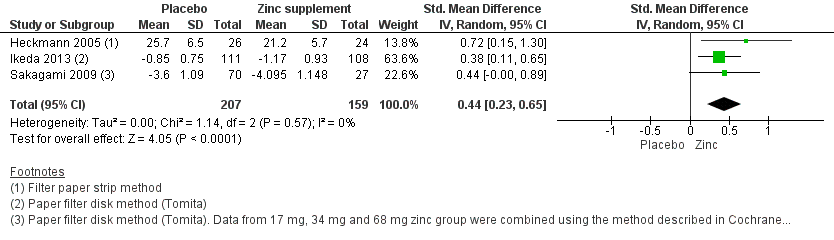
Taste acuity improvement ‐ Objective outcome (continuous data)
Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data.
Three trials were included in the meta‐analysis for this outcome (Heckmann 2005; Ikeda 2013; Sakagami 2009) as they studied taste disorders in idiopathic dysgeusia. One of these trials (Heckmann 2005) was at low risk of bias and two trials (Ikeda 2013; Sakagami 2009) were at unclear risk of bias.
In the Heckmann 2005 trial, 32 filter paper strips impregnated with various tastants were used, and an average was taken for each group. An improvement by six points in the taste test was regarded as substantial. Ikeda 2013 and Sakagami 2009 used the filter paper disk method. Ikeda 2013 explained the results in mean improvement of the taste grades whereas Sakagami 2009 explained the results in mean improvement for three different dosages of zinc (17 mg, 34 mg, and 68 mg). Hence, we combined these data according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 7.7.3.8 (Higgins 2011). The grade was expressed as negative value in the Sakagami 2009 trial because the values were regarded as improvement only when it was less than the baseline data.
In the Ikeda 2013 trial, the average zinc intake from the diet in both the intervention and control groups were the same (obtained from food frequency questionnaire method and was 7.9 mg/day). But there were no data available for the zinc intake from the diet in the Sakagami 2009 trial.
Three trials included for meta‐analysis (Heckmann 2005; Ikeda 2013; Sakagami 2009) used different taste detection tests, and hence we calculated the standardised mean difference (SMD). The overall effect favoured zinc supplements (SMD 0.44, 95% CI 0.23 to 0.65; 3 trials, 366 participants; Analysis 1.2).
Taste acuity improvement for different taste sensations
The results of the Mahajan 1980 trial were described as an improvement for each individual taste quality. The salt, sugar and bitter taste acuity (detection and recognition) significantly improved in the interventional group compared to the control group. Bitter taste quality improvement was similar in both groups. The data were derived from the graphs. The mean difference (MD) for the Mahajan 1980 trial was calculated for four types of taste sensations: salt (MD 285, 95% CI 238.75 to 331.25; 22 participants; Analysis 1.3); sweet (MD 190, 95% CI 142.36 to 237.64; 22 participants; Analysis 1.3); sour (MD 10, 95% CI ‐16.43 to 36.43; 22 participants; Analysis 1.3); and bitter (MD 2.40, 95% CI 2.14 to 2.66; 22 participants; Analysis 1.3).
In a similar way, Matson 2003 reported three taste perceptions (sweet, sour, and salt) before and after the intervention. In this trial, sour was often confused with salt, and sour solutions of different concentrations were not distinguishable. There was no difference in the taste scores after six weeks in either of the groups. We could not include this trial in the meta‐analysis as the data related to the placebo group were missing.
Watson 1983 reported taste acuity for four taste sensations (sweet, sour, salt, and bitter) before and after the intervention as median values using graphs. We could not extract the data as the percentage of change between pre‐ and post‐treatment was minimal and was not detectable in the graph.
Taste acuity improvement ‐ Cross‐over trial
Eggert 1982 described the results as taste detection and taste recognition in children with chronic renal failure in a cross‐over trial. We took the results of taste recognition before the cross‐over to prevent the carry‐over effect (the trial did not have any washout period). The data were derived from the graphs.
Eggert 1982 showed improvement in taste detection (MD 2.50, 95% CI 0.93 to 4.07; 14 participants; Analysis 1.4) and taste recognition for the zinc group (MD 3, 95% CI 0.66 to 5.34; 14 participants; Analysis 1.4).
Taste acuity improvement ‐ Objective outcome (dichotomous data)

Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous.
Only three trials (Ikeda 2013; Mahajan 1982; Sakai 2002) described the objective outcome as dichotomous data. Meta‐analysis of these three studies showed high heterogeneity because of the different populations (idiopathic and zinc‐deficient taste disorder in the Ikeda 2013 and Sakai 2002 trials and taste disorder secondary to chronic renal failure in the Mahajan 1982 trial). Hence, we did subgroup analyses.
Meta‐analysis of idiopathic and zinc‐deficient taste disorder showed improvement in the taste acuity for the zinc supplement group (RR 1.42, 95% CI 1.09 to 1.84; 292 participants; Analysis 1.5).
A lack of events in the placebo group in the Mahajan 1982 trial resulted in a high upper limit in the CI (RR 25, 95% CI 1.65 to 379.57; 24 participants; Analysis 1.5). Effect estimates of both the subgroups favoured zinc supplements.
Taste discrimination improvement
Matson 2003 used zinc sulphate for testing taste discrimination and was at unclear risk of bias. Taste discrimination was tested using the sip and spit method. However, the trial did not report the details of the placebo group and thus we could not meta‐analyse the results.
Adverse events
See Additional Table 9; Additional Table 10; Analysis 1.6; and Summary of findings table 1 for adverse events in trials comparing zinc with placebo for the management of taste disturbances.
| Outcome | Group A ‐ Zinc picolinate events | Group A total | Group B ‐ Placebo events | Group B total | Time when measured |
| Adverse events | 6 | 37 | 0 | 36 | 3 months |
| Outcome | Group A ‐ 17 mg zinc events | Group A total | Group B ‐ 34 mg zinc events | Group B total | Group C ‐ 68 mg zinc events | Group C total | Group D ‐ Placebo events | Group D total | Time when measured |
| Adverse events | 5 | 27 | 6 | 25 | 7 | 28 | 3 | 27 | 12 weeks |
Out of nine trials using zinc supplementation, four reported adverse events (Ikeda 2013; Sakagami 2009; Sakai 2002; Watson 1983). Ikeda 2013 reported one case of eczema. Sakai 2002 reported adverse events like nausea, abdominal pain and diarrhoea in 16% of patients (Additional Table 9). Watson 1983 reported nausea and vomiting in one patient after the zinc intervention only. Sakagami 2009 reported adverse events like stomach discomfort, abdominal distension, constipation, decrease in blood iron, increase in blood alkaline phosphatase and minor increase in blood triglycerides in all four groups of the trial (Additional Table 10). We did not include this trial in the analysis since it did not report any further details on the number of events. Zinc intervention groups reported adverse events compared to placebo (RR 5.20, 95% CI 0.90 to 30.19; 3 trials, 335 participants; Analysis 1.6).
The other five trials (Eggert 1982; Heckmann 2005; Mahajan 1980; Mahajan 1982; Matson 2003) did not give any data on adverse events.
Health‐related quality of life
None of the studies reported data on health‐related quality of life. Heckmann 2005 reported that the signs of depression in the zinc group were less severe (Beck Depression Inventory, P < 0.05; mood scale, P < 0.05) and these findings were independent of the actual levels of zinc in serum/saliva (Additional Table 6).
Acupuncture versus sham control
See summary of findings Table 2.
Taste acuity improvement
There were no data on taste acuity improvement in the included acupuncture trial (Brandt 2008).
Taste discrimination improvement
See Analysis 2.1 and Summary of findings table 2 for the main comparison of acupuncture to sham for the management of taste disturbances.
Brandt 2008 tested taste discrimination using acupuncture and was at high risk of bias. Taste discrimination was tested using the filter paper strip method and results were described in means of filter paper strips. The acupuncture group showed improvement in taste discrimination compared to the sham group (MD 2.80, 95% CI ‐1.18 to 6.78; 1 trial, 37 participants; Analysis 2.1). As we had only one trial using acupuncture, we considered that the evidence is insufficient to conclude if there is any difference between acupuncture and sham acupuncture with laser to improve taste discrimination.
Adverse events
The acupuncture trial (Brandt 2008) did not give any data on adverse events in either group.
Health‐related quality of life
Brandt 2008 assessed health‐related quality of life in dysgeusia patients. The trial reported significant improvement in psychological symptoms in the interventional group (acupuncture) and increased quality of life in both groups (no statistically significant difference between the two groups) (Additional Table 8). We could not meta‐analyse the health‐related quality of life outcome due to missing data.
Discussion
Summary of main results
The main objective of this review was to evaluate the efficacy of various interventions to improve taste acuity and taste discrimination. We included 10 randomised controlled trials (RCTs) in our review. We assessed three trials (30%) as at low risk of bias, four trials (40%) as at high risk of bias, and three trials (30%) as at unclear risk of bias.
Nine trials compared the efficacy of different zinc supplements for improvement of taste acuity, and one trial compared the efficacy of acupuncture for improvement of taste discrimination in dysgeusia patients. However, the Mahajan 1980 and Matson 2003 trials were not included in the 'Summary of findings' tables because each taste sensation was analysed, rather than overall taste acuity and Matson 2003 did not report data for the placebo group. We did not pool the first half of the cross‐over trial by Eggert 1982 because of the different trial designs and we did not include Mahajan 1982 in the 'Summary of findings' tables due to the large confidence interval (CI). Based on the available data, we conducted a meta‐analysis for taste acuity and taste discrimination separately.
Out of seven trials that reported taste acuity as their outcome, two trials (Heckmann 2005; Sakai 2002) documented this as a patient‐reported outcome. We assessed the body of evidence for this comparison using GRADE which incorporates risk of bias, the directness of the evidence, inconsistency of the results, the precision of the estimates, and the risk of publication bias (summary of findings Table for the main comparison). We assessed the evidence from these two trials, which included 119 participants, with a mean trial period of three months. The quality of the evidence assessed was very low and was insufficient to conclude if there is any benefit of zinc supplementation for improvement in taste acuity.
Three trials (Heckmann 2005; Ikeda 2013; Sakagami 2009) described taste improvement (summary of findings Table for the main comparison). We used GRADE to assess the evidence from these three trials, which included 366 participants with a mean trial period of three months. The quality of the evidence assessed was very low. We assessed one trial (Eggert 1982) as having very low‐quality evidence for taste detection and taste recognition improvement.
We assessed two trials (Ikeda 2013; Sakai 2002) in zinc‐deficient/idiopathic taste disorder patients which described taste acuity improvement. The evidence assessed from these trials included 292 participants with a trial period of three months. We assessed the quality of the evidence as very low and further research may change the estimate.
Two trials reported taste discrimination as the outcome (Brandt 2008; Matson 2003). We assessed the evidence from Brandt 2008, which included 37 participants with a trial period of eight weeks, using acupuncture, as very low (summary of findings Table 2).
Overall completeness and applicability of evidence
We systematically searched for trials according to the methodology written in our protocol. We included all RCTs that fit the inclusion criteria in our review. All the included trials had patients with dysgeusia or hypogeusia. None of them reported phantogeusia, ageusia, parageusia or cacogeusia in their participants.
For taste acuity improvement, we included nine trials using zinc supplements in different forms. We included two trials for taste discrimination improvement using acupuncture and zinc sulphate. Zinc supplement is the most commonly used intervention for taste disorders and our review highlights the usage of zinc in the majority of the included trials. Taste acuity and taste discrimination were our two primary outcomes. Health‐related quality of life due to taste impairment is an important issue to be addressed. We only found two trials addressing health‐related quality of life due to taste disorders. We included in the analysis all trials that reported adverse events appropriately.
Trials which were not included in the meta‐analysis were explained in the results. We did not exclude any trial due to missing data. In a cross‐over trial (Eggert 1982) without a sufficient washout period and suspected carry‐over effect, we included data before the cross‐over.
This review has limited evidence on the primary outcomes of taste acuity and taste discrimination, and is not conclusive in demonstrating improvement in taste perception with zinc supplements or acupuncture. However, the review encourages further high quality RCTs with primary outcomes of taste acuity and taste discrimination using zinc supplements or acupuncture for taste disorder patients to derive definitive conclusions and recommendations.
Quality of the evidence
We included seven parallel and two cross‐over RCTs with 566 participants in the meta‐analyses. The quality of the evidence is discussed for each outcome. One parallel‐group RCT with 15 participants is discussed in the review but not included in the meta‐analyses.
Zinc supplements versus placebo
Taste acuity ‐ patient‐reported outcome
Of the two trials comparing zinc supplements and placebo that were suitable for pooling in this meta‐analysis for the outcome of patient‐reported taste acuity, we considered one to be at low risk of bias (Heckmann 2005) and one to be at high risk of bias (Sakai 2002). We downgraded the quality of the evidence by one due to high attrition bias, imprecision, and publication bias (summary of findings Table for the main comparison). The results, therefore, do not allow us to draw a robust conclusion regarding the patient‐reported outcome in taste acuity. We assessed the results of this outcome to be of very low quality.
Both trials excluded dysgeusia/hypogeusia subjects secondary to systemic illnesses. However, Sakai 2002 included idiopathic dysgeusia and dysgeusia due to zinc deficiency. Neither of the trials mentioned dietary zinc which could have been a major confounder here. Future studies on zinc interventions in dysgeusia cases should standardise dietary zinc to clearly ascertain the role of zinc in the improvement of dysgeusia. Gender differences between these two trials (Heckmann 2005; Sakai 2002) could have been another confounder that should be considered.
Taste acuity ‐ objective outcome
We included seven trials in the meta‐analysis for this outcome. Three trials (Heckmann 2005; Ikeda 2013; Sakagami 2009) reported taste acuity improvement as objective, continuous data, and we found the quality of the evidence to be very low. One trial (Mahajan 1980) presented the data in the form of a graph for each taste sensation (salt, sweet, bitter, sour) and could not be grouped with the other three trials. One cross‐over trial (Eggert 1982) showed better taste detection and taste recognition improvement in the zinc group among chronic renal failure patients and presented the data in the form of a graph. Due to clinical heterogeneity, we did not include this trial in the meta‐analysis. We assessed the evidence for the outcomes of these two comparisons from Mahajan 1980 and Eggert 1982 studies separately to be of very low quality.
Three trials (Ikeda 2013; Mahajan 1982; Sakai 2002) reported the objective outcome for taste acuity in the form of dichotomous data. We did subgroup analyses based on the diagnosis and graded results of idiopathic/zinc‐deficient taste disorders subgroup separately. The results showed very low‐quality evidence for taste acuity improvement (summary of findings Table for the main comparison). As there were no events in the placebo group and all participants in the intervention group showed improvement, we could not grade the results of the other subgroup, taste disorders secondary to chronic renal failure.
Watson 1983 reported taste recognition as median values in the form of a graph for each taste sensation and did not mention interquartile range for post‐treatment changes. Matson 2003 did not report data for the placebo group. Hence we could not include these two trials in the meta‐analysis.
Adverse events
We included only three trials in the meta‐analysis which reported adverse events in the zinc supplements group. The reported adverse events were either gastrointestinal or dermatological. The results were of very low quality and therefore do not allow us to draw a strong conclusion regarding the prediction of adverse events related to zinc supplementation (summary of findings Table for the main comparison).
We could not assess the quality of the evidence for the health‐related quality of life outcome as this outcome was not meta‐analysed in this comparison.
Acupuncture versus sham control
Taste discrimination
We included only one trial (Brandt 2008) for the outcome of taste discrimination, and we assessed the quality of the evidence as very low (summary of findings Table 2). The results therefore do not allow us to draw a robust conclusion regarding taste discrimination using acupuncture.
We could not assess the quality of the evidence for the outcomes taste recognition, health‐related quality of life, and adverse events as these outcomes were not meta‐analysed in this comparison.
Potential biases in the review process
We have taken steps to minimise bias in every stage of the review. We searched all the above mentioned databases, conference proceedings, and trial registries to include all relevant reports. We included foreign language reports and alternative medicine reports in our review. We tried to contact trial authors for missing data through emails. If the reports were very old, we tried to get the contact details of the authors through peer contacts, Google search and university/hospital websites where they were previously affiliated. Nevertheless, there could be unpublished data which we could not trace with the above methods. Trials with missing data were included qualitatively.
Two review authors independently reviewed data extraction forms obtained from translators and cross‐checked doubtful areas using Google translator. We tried our best to follow the methodology stated in the protocol. However, post hoc changes in the inclusion criteria could have introduced some bias in our review (Differences between protocol and review).
Agreements and disagreements with other studies or reviews
We found two systematic reviews on dysgeusia associated with cancer therapies (Hovan 2010; McLaughlin 2014).
The purpose of the review by Hovan 2010 was to select relevant scientific papers written since 1989, which focused on the prevalence and management of dysgeusia as an oral side effect of cancer treatment. The literature search in the review was limited to English language papers published between 1990 and 2008. A total of 30 papers were reviewed. The trial authors concluded that from the current literature, there does not appear to be a predictable way of preventing or treating dysgeusia.
In the review by McLaughlin 2014, a meta‐analysis was done to assess the relationship between the impaired taste sensation and the type of treatment and tumour site in head and neck cancer treatment survivors.
In our review, we excluded prevention studies and trials including taste disorders secondary to cancer treatment. We included idiopathic dysgeusia/hypogeusia and dysgeusia/hypogeusia secondary to renal failure. The difference in opinion could be due to these reasons.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.1 Taste acuity improvement ‐ Patient‐reported outcome.

Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data.

Forest plot of comparison: 1 Zinc versus placebo, outcome: 1.5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous.

Comparison 1 Zinc versus placebo, Outcome 1 Taste acuity improvement ‐ Patient‐reported outcome.

Comparison 1 Zinc versus placebo, Outcome 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data.

Comparison 1 Zinc versus placebo, Outcome 3 Taste acuity improvement for different taste sensations.

Comparison 1 Zinc versus placebo, Outcome 4 Taste acuity improvement ‐ Cross‐over study.

Comparison 1 Zinc versus placebo, Outcome 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous.

Comparison 1 Zinc versus placebo, Outcome 6 Adverse events.

Comparison 2 Acupuncture versus sham control, Outcome 1 Taste discrimination.
| Zinc compared to placebo for the management of taste disturbances | ||||||
| Patient or population: patients with taste disturbances | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with zinc | |||||
| Taste acuity improvement (patient‐reported outcome) assessed with VAS/questionnaire where improvement in dysgeusia is defined as more than 5% improvement in the VAS scores for a mean follow‐up period of 3 months | Study population ‐ zinc‐deficient/idiopathic taste disorder | 1.40 (0.94 to 2.09) | 119 | ⊕⊝⊝⊝ | There is a 40% relative increase in taste acuity improvement (patient‐reported outcome) in patients taking zinc when compared to placebo with a CI of 6% decrease to 109% increase of taste acuity | |
| 407 per 1000 | 569 per 1000 | |||||
| Taste acuity improvement (objective outcome ‐ continuous data) assessed with filter paper strip and filter paper disk methods for a mean follow‐up period of 3 months | ‐ | SMD 0.44 higher | ‐ | 366 | ⊕⊝⊝⊝ | The standardised mean difference for taste acuity improvement in the zinc intervention group is 0.44 higher than the placebo group |
| Taste acuity improvement (objective outcome ‐ dichotomous data) assessed with filter paper disk and Henkin's 3‐drop stimulus method for a mean follow‐up period of 3 months | Study population ‐ zinc‐deficient/idiopathic taste disorder | RR 1.42 | 292 | ⊕⊝⊝⊝ | There is a 42% relative improvement in taste acuity in patients taking zinc when compared to placebo with a CI of 9% to 84% increase of taste acuity | |
| 435 per 1000 | 618 per 1000 | |||||
| Cross‐over trial ‐ taste detection assessed with Henkin's method for a follow‐up period of 6 months | The mean taste detection was 7.5 | MD 2.50 higher | ‐ | 14 | ⊕⊝⊝⊝ | The mean difference for taste detection in the intervention group is 2.50 higher than the placebo group |
| Cross‐over trial ‐ taste recognition assessed with Henkin's method for a follow‐up period of 6 months | The mean taste recognition was 16 | MD 3.00 higher | ‐ | 14 | ⊕⊝⊝⊝ | The mean difference for taste recognition in the intervention group is 3.00 higher than placebo group |
| Adverse events ‐ follow‐up range 12 weeks to 18 weeks | Study population ‐ zinc‐deficient/idiopathic taste disorder and taste disorder secondary to chronic renal failure | 5.20 (0.90 to 30.19) | 335 | ⊕⊝⊝⊝ | Risk of 1 per 1000 assumed in placebo group (as it was 0) There is 420% relative increase in the adverse events in patients taking zinc compared to placebo with 95% CI of 10% decrease to 2919% increase in adverse events | |
| 6 per 1000 | 31 per 1000 | |||||
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Unclear randomisation and high risk of bias due to attrition in Sakai 2002. Downgraded by 1 level. | ||||||
| Acupuncture compared to sham control for the management of taste disturbances | ||||||
| Patient or population: idiopathic dysgeusia combined with hypogeusia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Risk with sham (control) | Risk with acupuncture | |||||
| Taste discrimination assessed with 32 taste strips with a follow‐up of 8 weeks | The mean taste discrimination was 14.7 | MD 2.80 higher | ‐ | 37 | ⊕⊕⊝⊝ | The mean difference for taste discrimination in the acupuncture group is 2.80 higher than the sham group |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Brandt 2008 is a single‐blind trial with high risk of performance bias. Downgraded by 2 levels. | ||||||
| Outcome | Group A | Group B | Time when measured | ||||
| Mean* | SD | n | Mean* | SD | n | ||
| Change of the mean 4 basic taste sensitivity scores from baseline | ‐0.52 | 0.68 | 108 | ‐0.47 | 0.61 | 111 | 4 weeks |
| ‐0.90 | 0.85 | 108 | ‐0.67 | 0.73 | 111 | 8 weeks | |
| ‐1.17 | 0.93 | 108 | ‐0.85 | 0.75 | 111 | 12 weeks | |
| ‐1.28 | 0.94 | 108 | ‐0.97 | 0.76 | 111 | 4 weeks after treatment | |
| *Minus change score means better by filter paper disc method by Tomita. | |||||||
| Outcome | Group A events (Improved) | Group A total | Group B events | Group B total | Time when measured |
| Improved/not improved | 60 | 108 | 48 | 111 | 12 weeks |
| Outcome | Group A (Placebo) n = 27 | Group B (17 mg zinc) n = 27 | Group C (34 mg zinc) n = 25 | Group D (68 mg zinc) n = 28 | Time when measured | ||||
| Secondary outcome | Mean | SD | Mean | SD | Mean | SD | Mean | SD | 12 weeks |
| Mean filter paper disk test scores (filter paper disk) | 4.095 | 1.148 | 4.350 | 1.030 | 3.448 | 0.928 | 3.454 | 1.138 | ─ |
| Mean serum zinc level | 1.8 | 12.7 | 5.7 | 13.5 | 11.4 | 16.6 | 20.6 | 21.3 | ─ |
| ‐ | Group A | Group B | Group C | Group D | ─ | ||||
| Increase in the average score of subjective symptoms | 0.6 | 0.9 | 1.2 | 1.0 | ─ | ||||
| SD = standard deviation. | |||||||||
| Primary outcome: quantitative analysis of taste perception using filter paper disk method | Event (success) Cured + improved | No event (fail) Unchanged, neither cured nor improved nor worsened; aggravated | Total |
| Experimental intervention (17 mg zinc) | SE = 14 | FE = 13 | NE = 27 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 0.824; OR = 0.634; RD = 0.447 | |||
| Experimental intervention (34 mg zinc) | SE = 20 | FE = 5 | NE = 25 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 0.318; OR = 2.353; RD = 0.17 | |||
| Experimental intervention (68 mg zinc) | SE = 25 | FE = 3 | NE = 28 |
| Control intervention (placebo) | SC = 17 | FC = 10 | NC = 27 |
| RR = 1.418; OR = 4.902; RD = 0.263 | |||
| OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group. | |||
| Filter paper disk method | Event (success) Improved (+ cured) | No event (fail) Unchanged | Total (n = 73) |
| Experimental intervention (zinc picolinate) | SE = 28 | FE = 9 | NE = 37 |
| Control intervention (placebo) | SC = 16 | FC = 20 | NC = 36 |
| RR = 1.703; OR = 3.889; RD = 0.312 | |||
| Experimental intervention (zinc picolinate) | SE = 22 | FE = 12 | NE = 34 |
| Control intervention (placebo) | SC = 18 | FC = 17 | NC = 35 |
| RR = 1.258 ; OR = 1.732; RD = 0.133 | |||
| OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group. | |||
| Outcome | Group A (zinc treatment) | Group B (placebo) | Time when measured | ||||
| Mean | SD | n | Mean | SD | n | At the end of 3 months | |
| Primary outcome | ─ | ||||||
| Taste test (32‐filter paper strip method by Mueller 2003) | 25.7 | 6.5 | 26 | 21.2 | 5.7 | 24 | ─ |
| Self‐rated impairment in % (VAS scale of 10 cm length equivalent to 100%; 0 to 10; 0 = no impairment; 10 = extremely impaired) | 45.0 | 4.4 | 26 | 43.8 | 3.6 | 24 | ─ |
| Secondary outcome | ─ | ||||||
| Beck Depression Inventory (BDI) | 7.5 | 7.0 | 26 | 11.3 | 10.9 | 24 | ─ |
| Zerssen Mood Scale (ZMS) | 10.7 | 7.5 | 26 | 18.8 | 14.6 | 24 | ─ |
| Zinc in serum (µg/dL) | 81.53 | 19.61 | 26 | 72.01 | 10.22 | 24 | ─ |
| SD = standard deviation; VAS = visual analogue scale. | |||||||
| Type of intervention | Event (success) Improved | No event (fail) | Total |
| Experimental intervention (zinc) | SE = 13 | FE = 13 | NE = 26 |
| Control intervention | SC = 6 | FC = 18 | NC = 24 |
| RR = 2; OR = 3; RD = 0.25 | |||
| OR = odds ratio: odds of event in experimental group/odds of event in control group; RD = risk difference: risk of event in experimental group/risk of event in control group; RR = risk ratio: risk of event in experimental group/risk of event in control group. | |||
| Outcome | Group A | Group B | Time when measured | ||||||
| Mean | SD* | n = 17 | Mean | SD* | n = 20 | ||||
| Taste discrimination | 11.7 (before)/ 17.5 (after) | 4 (before)/ | ─ | 11.9 (before)/ 14.7(after) | 5 (before)/ | ─ | Before and after treatment | ||
| Quality of life | Not estimable (changes per group only given for each of the 5 individual questions of the questionnaire, but no combined score/analysis stated). Only information given: "both treatments resulted in an increased quality of life, however, no statistically significant difference could be found" | Before and after treatment | |||||||
| Depressive symptoms | 11 (before)/ 6 (after)* | 5 (before) / 4 (after)* | ─ | 10.5 (before)/ 10 (after)* | 7 (before)/ 7 (after)* | ─ | Before and after treatment | ||
| Quote: "The psychological well‐being of the intervention groups increased for 94.1% of all patients in the intervention group, but only for 60% of patients in the control group. This difference was statistically significant" | |||||||||
| Subjective well‐being | 16 (before)/ 12 (after)* | 10 (before)/ 7 (after)* | ─ | 20 (before)/ 18 (after)* | 9 (before)/ 14 (after)* | ─ | Before and after treatment | ||
| Quote: "58.8% of all patients in the intervention group felt better, whereas only 45% of all patients in the control group felt better. This difference was not statistically significant" | |||||||||
| *Only given in graph ‐> estimated from graph. | |||||||||
| Outcome | Group A ‐ Zinc picolinate events | Group A total | Group B ‐ Placebo events | Group B total | Time when measured |
| Adverse events | 6 | 37 | 0 | 36 | 3 months |
| Outcome | Group A ‐ 17 mg zinc events | Group A total | Group B ‐ 34 mg zinc events | Group B total | Group C ‐ 68 mg zinc events | Group C total | Group D ‐ Placebo events | Group D total | Time when measured |
| Adverse events | 5 | 27 | 6 | 25 | 7 | 28 | 3 | 27 | 12 weeks |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Taste acuity improvement ‐ Patient‐reported outcome Show forest plot | 2 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.94, 2.09] |
| 2 Taste acuity improvement ‐ Objective outcome ‐ Continuous data Show forest plot | 3 | 366 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.23, 0.65] |
| 3 Taste acuity improvement for different taste sensations Show forest plot | 1 | 88 | Mean Difference (IV, Random, 95% CI) | 119.43 [14.48, 224.39] |
| 3.1 Salt | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 285.0 [238.75, 331.25] |
| 3.2 Sweet | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 190.0 [142.36, 237.64] |
| 3.3 Sour | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐16.43, 36.43] |
| 3.4 Bitter | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 2.4 [2.14, 2.66] |
| 4 Taste acuity improvement ‐ Cross‐over study Show forest plot | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 2.66 [1.35, 3.96] |
| 4.1 Taste detection | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 2.5 [0.93, 4.07] |
| 4.2 Taste recognition | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 3.00 [0.66, 5.34] |
| 5 Taste acuity improvement ‐ Objective outcome ‐ Dichotomous Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Idiopathic and zinc‐deficient taste disorders | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.09, 1.84] |
| 5.2 Taste disorder secondary to chronic renal failure | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 25.00 [1.65, 379.57] |
| 6 Adverse events Show forest plot | 3 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 5.20 [0.90, 30.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Taste discrimination Show forest plot | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐1.18, 6.78] |

