降低引发腹主动脉瘤患者死亡率和心血管事件血管危险因素的药物治疗
摘要
研究背景
药物预防已被证明可以降低动脉粥样硬化闭塞性动脉疾病患者心血管事件的风险。然而,预防对于腹主动脉瘤(abdominal aortic aneurysm,AAA)患者的作用尚不清楚。一些研究表明,尽管修复成功,但腹主动脉瘤患者的生存率仍低于健康对照组。腹主动脉瘤患者的冠心病患病率和心血管事件风险增加。尽管存在这种关联,但对于药物预防在降低腹主动脉瘤患者心血管风险方面的有效性我们还是知之甚少。本系统综述是对2014年首次发表的Cochrane系统综述的更新。
研究目的
确定抗血小板、抗高血压或降脂药物在降低腹主动脉瘤(AAA)患者死亡率和心血管事件方面的长期有效性。
检索策略
对于本次更新,Cochrane血管信息专员(Cochrane Vascular Information Specialist, CIS)检索了Cochrane血管专业注册库(Cochrane Vascular Specialised Register )(2016年4月14日)。此外,Cochrane血管信息专员检索了Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials,CENTRAL)(2016年第3期)和试验注册库(2016年4月14日),我们还检索了相关文章的参考文献列表。
纳入排除标准
将腹主动脉瘤患者随机分配接受一种预防性治疗与另一种预防性治疗、相同治疗的不同方案、安慰剂或不治疗的随机对照试验是合格的,符合纳入本系统综述的条件。主要结局包括全因死亡率和心血管死亡率。
资料收集与分析
两位综述作者独立筛选纳入的研究,并完成质量评价和资料提取。我们通过讨论解决了分歧。只有一项研究符合本系统综述的纳入标准,因此我们无法进行meta分析。
主要结果
没有新的研究符合本次更新的纳入标准。本系统综述纳入了一项随机对照试验。一个由227名腹主动脉瘤受试者组成的亚组接受了美托洛尔(N=111)或安慰剂(N=116)。没有明确的证据表明美托洛尔降低了术后30天的全因死亡率(比值比(odds ratio,OR)=0.17,95%置信区间(confidence interval,CI)[0.02, 1.41])、心血管死亡(OR=0.20,95% CI [0.02, 1.76])、腹主动脉瘤相关死亡(OR=1.05,95% CI [0.06, 16.92])或增加的非致死性心血管事件(OR=1.44,95% CI [0.58, 3.57])。此外,在术后6个月时,估计效果与全因死亡率(OR=0.71,95% CI [0.26, 1.95])、心血管死亡(OR=0.73,95% CI [0.23, 2.39])和非致命性心血管事件(OR=1.41,95% CI [0.59, 3.35])的利弊相符。在整个研究人群中均有关于药物不良反应的报告,而腹主动脉瘤受试者亚组则没有药物不良反应的相关报告。我们认为该研究的偏倚风险普遍较低。我们将所有结局的证据质量降级为低。我们因不精确性而降低了证据的质量,因为只有一项受试者人数较少的研究可用,事件数量少,且结果与获益和伤害一致。
作者结论
由于纳入的试验数量有限,所以没有足够的证据得出关于心血管预防在降低腹主动脉瘤患者死亡率和心血管事件方面有效性的任何结论。在得出确切结论之前,需要进一步开展高质量的随机对照试验,以检查多种类型的预防措施并进行长期随访。
PICOs
简语概要
降低引发腹主动脉瘤患者死亡率和心血管事件血管危险因素的药物治疗
研究背景
腹主动脉瘤(Abdominal aortic aneurysm,AAA)是一种可能危及生命的疾病,主动脉会扩大并最终破裂,导致大量内出血。目前的指南建议55毫米或更大的腹主动脉瘤应该通过手术修复,因为在这种尺寸下,破裂的风险超过了手术修复的风险。尺寸在30mm到54mm之间的腹主动脉瘤风险不高,通常可以通过定期扫描进行监测,以检查肿瘤是否进一步增大。最近的研究表明,即使在动脉瘤修复后,腹主动脉瘤患者的存活率也低于未患腹主动脉瘤的人。在大多数情况下,死因是心血管事件,例如心脏病发作或中风。高血压或高胆固醇等疾病会增加心血管死亡的风险。然而,这两种情况都可以通过药物治疗来逆转。鉴于腹主动脉瘤患者的死亡风险增加,确定哪种药物治疗对预防腹主动脉瘤患者的心血管死亡最有效非常重要。
在本系统综述中,来自Cochrane的研究人员检查了药物治疗在治疗血管危险因素、减少腹主动脉瘤患者死亡和心血管死亡和事件的有效性。
研究特点和主要研究结果
在检索所有相关研究后(截至2016年4月14日),我们发现了一项研究,其中227名腹主动脉瘤患者的亚组接受了β受体阻滞剂美托洛尔(降低血压的药物)或安慰剂(模拟治疗)。该研究的结果对于腹主动脉瘤修复后30天或6个月的所有死亡原因和心血管疾病或非致命性心血管事件的死亡都不精确。在整个研究人群中均有关于药物不良反应的报告,而腹主动脉瘤受试者亚组则没有药物不良反应的相关报告。
证据质量
我们认为该研究的偏倚风险普遍较低。我们将证据质量评为低,因为我们在系统综述中只纳入了一项小型研究,报告的事件很少,且结果与获益和伤害一致。
需要更大规模和更长时间的研究来找出哪种治疗最有效。目前,腹主动脉瘤患者接受了广泛的药物治疗,包括抗血小板药物、抗高血压药物和降脂药物。未来的试验应该测试可用的药物以找到最有效的策略,无论是单一药物还是联合治疗。此外,需要评价此类干预措施的可接受性,未来的研究应衡量与这些药物相关的不良副作用及其对生活质量的影响。
Authors' conclusions
Summary of findings
| Metoprolol compared to placebo for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm (AAA) | ||||||
| Patient or population: patients of any age with AAA less than 30 mm in diameter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with metoprolol | |||||
| All‐cause mortality, 30 days1 | Study population | OR 0.17 | 227 | ⊕⊕⊝⊝ | — | |
| 52 per 1000 | 9 per 1000 | |||||
| Cardiovascular death, 30 days3 | Study population | OR 0.20 | 227 | ⊕⊕⊝⊝ | — | |
| 43 per 1000 | 9 per 1000 | |||||
| AAA‐related death, 30 days4 | Study population | OR 1.05 | 227 | ⊕⊕⊝⊝ | — | |
| 9 per 1000 | 9 per 1000 | |||||
| Nonfatal cardiovascular event, 30 days5 | Study population | OR 1.44 | 227 | ⊕⊕⊝⊝ | — | |
| 78 per 1000 | 108 per 1000 | |||||
| All‐cause mortality, 6 months1 | Study population | OR 0.71 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 63 per 1000 | |||||
| Cardiovascular death, 6 months3 | Study population | OR 0.73 | 227 | ⊕⊕⊝⊝ | — | |
| 60 per 1000 | 45 per 1000 | |||||
| AAA‐related death, 6 months4 | See comments | See comments | See comments | See comments | The incidence of AAA‐related death was not measured at six months. | |
| Nonfatal cardiovascular event, 6 months5 | Study population | OR 1.41 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 117 per 1000 | |||||
| *The risk with placebo was the average risk in the placebo group (i.e. the number of participants with events divided by total number of participants of the placebo group included in the meta‐analysis). The risk in the metoprolol group (and its 95% CI) is based on the assumed risk in the placebo group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Death from all causes. | ||||||
Background
Description of the condition
An abdominal aortic aneurysm (AAA) is an abnormal dilatation of the aorta as it passes below the renal arteries to the point of bifurcation, where it forms the left and right common iliac arteries. The clinical definition of AAA varies, although a maximum infrarenal measurement (a measurement taken below the renal artery branches) of ≥ 30 mm is commonly used (Wanhainen 2008). The prevalence of AAA is six times greater in men than in women (Pleumeekers 1995), with one study demonstrating a prevalence of 1.3% in women and 7.6% in men (Scott 2002). Apart from male gender, other risk factors for AAA include smoking, increased age, and family history of AAA (Blanchard 2000). Conclusive evidence from several studies has shown smoking to be associated with AAA (Badger 2009; Greenhalgh 2008; Wilmink 1999). One study, Wilmink 1999, estimated that the risk of AAA is seven times higher in smokers and three times higher in ex‐smokers compared with age‐matched nonsmokers, and another study reported that 90% of participants with AAA were smokers (Greenhalgh 2008). Increased age has been consistently shown as a significant risk factor (Lloyd 2010; Singh 2001). One population‐based study of 6386 men and women reported no AAA in participants younger than 48 years of age, but from this age onward the prevalence increased linearly in both men and women (Singh 2001). Family history is another known risk factor for AAA. One study reported that 9% to 12% of first‐degree relatives of a participant with an AAA will develop an aneurysm (van Vlijmen‐van Keulen 2002).
The decision to operate on an AAA is made when the risk of rupture is greater than the risk associated with the operation, and burden of co‐morbidity is increasingly important (Ohrlander 2011). The UK Small Aneurysm Trial estimated that the annual rupture rate is 0.3% for AAAs that are less than 4 cm in diameter, 1.5% for 4.0 cm to 4.9 cm AAAs, and 6.5% for 5.0 cm to 5.9 cm AAAs (Brown 1999). In general, the American Heart Association and the UK Aneurysm Screening Programme recommend that patients with infrarenal AAAs measuring ≥ 55 mm should undergo repair to eliminate the risk of rupture (Hirsch 2005). AAAs can be repaired using an open or endovascular approach. Open repair with graft placement is a major procedure and may be preferred when patients are fit because complications are fewer and patients do not routinely require follow‐up. Endograft repair involving stent placement (EVAR) is associated with a lower postoperative risk and is therefore considered when the patient is a high surgical risk or has coexisting medical conditions. The major risks in repairing an AAA are perioperative cardiac events, infection, and death. The 30‐day mortality has been estimated at 5% in elective open surgical AAA repair compared with 1.7% with EVAR (Greenhalgh 2004; Prinssen 2004). However, a recent study showed no significant difference in survival at five years in participants who had undergone open repair compared with EVAR (Brown 2011). Patients with an infrarenal AAA of 30 mm to 54 mm are monitored by ultrasound or computed tomography (CT) scans every three, six, or 12 months for detection of possible expansion and the need for repair. These patients are considered for statin therapy to reduce vascular risk, decrease the risk of rupture and reduce aneurysm growth rates (Davis 2008). Angiotensin‐converting enzyme (ACE) inhibitors and angiotensin receptor blockers have also been proposed to reduce aneurysmal growth (Hackam 2006).
Studies have shown that even after successful surgical repair of an AAA, participants had a poorer survival rate than healthy controls (de Bruin 2014; De Martino 2013; Timmers 2013). A Dutch cohort study measured a survival rate of 59% 10 years after open AAA repair, and patients had a poorer health‐related quality of life than age‐matched controls (Timmers 2013). Another Dutch study compared statin use in patients undergoing AAA repair and found that while statins were associated with fewer cardiovascular deaths, several risk factors remained that were associated with poor survival after AAA repair including age of greater than 70 years, a history of cardiac disease, and moderate to severe tobacco use (de Bruin 2014). A further study of 2637 participants undergoing AAA repair determined that although five‐year survival rates were similar between open and EVAR repair groups, advanced age ≥ 75 years, coronary artery disease, unstable angina or recent myocardial infarction (MI), oxygen‐dependent chronic obstructive pulmonary disease, and an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 were associated with poor survival at five years (De Martino 2013).
A recent study conducted in Australia demonstrated an association between AAA thrombus volume and subsequent cardiovascular events (Parr 2011). AAA thrombus products are released into the circulation where they have the potential to stimulate leukocytes and produce other changes that might promote atherosclerotic plaque activation and acute coronary and cerebrovascular events (Morange 2006; Parry 2009; Smith 2005; Takagi 2009).
AAA size and growth are associated with local generation of inflammation markers such as interleukin‐6, matrix metalliproteinase‐2 (MMP‐2), and MMP‐9 (Schouten 2006). Inflammation also seems to be important in perioperative adverse cardiac events. Larger AAA size is independently associated with an increased incidence of perioperative cardiovascular complications after elective infrarenal AAA repair (Schouten 2006).
Description of the intervention
Pharmacological therapy to reduce cardiovascular risk factors such as hypertension and hypercholesterolaemia. Examples of pharmacological therapy are antiplatelet therapy (e.g. aspirin, clopidogrel, ticlopidine, cilostazol, or any other antiplatelet drugs), antihypertensive drugs (e.g. calcium channel blockers, angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers (β‐blockers), or any other antihypertensive drugs) and lipid‐lowering therapy (e.g. statins).
How the intervention might work
As people with AAA have increased cardiovascular risks, pharmacological therapy may reduce cardiovascular mortality and nonfatal cardiovascular events.
Why it is important to do this review
Three Cochrane systematic reviews on the effectiveness of surgical treatment of AAA have been conducted. Badger 2014 and Paravastu 2014 both compared endovascular versus open surgical repair for AAA, while Filardo 2015 examined immediate repair versus routine ultrasound surveillance. Another published Cochrane review, Rughani 2012, examined the effectiveness of medical treatments in terms of the expansion rate of small abdominal aortic aneurysms. However, these reviews have focused on treatment of AAA and ruptured AAA rather than on treatment of vascular risk factors associated with cardiovascular mortality in participants with AAA.
Acquired risk factors such as hypertension and hypercholesterolaemia are often reversible through pharmacological therapy. Given the increased risk of mortality with AAA, it is important to determine which prophylaxis is most effective in preventing cardiovascular death in people with AAA. To date, no systematic review has been conducted to study the effectiveness of medical treatments in reducing cardiovascular mortality in people with AAA. This review sought to provide evidence on the most effective medical treatment for this important problem.
This is an update of a Cochrane review first published in 2014.
Objectives
To determine the long‐term effectiveness of antiplatelet, antihypertensive or lipid‐lowering medication in reducing mortality and cardiovascular events in people with abdominal aortic aneurysm (AAA).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in which participants with abdominal aortic aneurysm (AAA) were randomly allocated to one prophylactic treatment versus another, a different regimen of the same treatment, a placebo, or no treatment. We planned to include published studies and studies in progress, if preliminary results were available. Non‐English studies were eligible and we sought translations, where appropriate, for inclusion in the review.
Types of participants
Men and women of any age with AAA of less than 30 mm in diameter as measured by standardised techniques such as ultrasound examination or CT. We also included participants who had undergone endovascular or open surgical repair for AAA. In participants who had an AAA repair, the time period included in this review was the postoperative rather than the surveillance phase. We only included mixed population studies where data on the subset of participants with AAA were available.
Types of interventions
-
Antiplatelet therapy (e.g. aspirin, clopidogrel, ticlopidine, cilostazol, or any other antiplatelet drugs).
-
Antihypertensive drugs (e.g. calcium channel blockers, angiotensin‐converting enzyme (ACE) inhibitors, beta‐blockers (β‐blockers), or any other antihypertensive drugs).
-
Lipid‐lowering therapy (e.g. statins).
-
Combination treatment (e.g. antiplatelet drug plus antihypertensive or statin) versus single treatment.
-
Combination treatment versus no treatment.
Where possible, we planned to compare one intervention with another treatment, a different regimen of the same treatment, placebo, or no treatment. We included any type, method, duration, timing, mode of delivery, and dose of medical treatment. We excluded studies in which participants were not treated with a specific regimen but were given numerous medications as it would not be possible to attribute outcomes or side effects to one particular regimen.
This review concerns medical interventions in which the principal actions are to modify cardiovascular risk factors. Therefore, we did not include any alternative treatments for which the primary purpose was to treat the aneurysm itself, for example to reduce growth rates or prevent rupture, or both.
Types of outcome measures
Primary outcomes
-
All‐cause mortality.
-
Cardiovascular mortality (fatal myocardial infarction (MI), fatal stroke, other vascular deaths).
Secondary outcomes
-
AAA‐related death.
-
Nonfatal cardiovascular events (nonfatal MI, nonfatal stroke, or transient ischaemic attack).
-
Major amputation.
-
Quality of life.
-
Drug‐related morbidity.
-
Drug‐related mortality.
We excluded outcomes that were specific to the aneurysm itself (for example, change in size or rupture rates).
Search methods for identification of studies
We sought translations of any trials that were not in the English language.
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
-
The Cochrane Vascular Specialised Register (14 April 2016).
-
The Cochrane Central Register of Controlled Trials (CENTRAL (2016, Issue 3)) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy the CIS used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial databases for details of ongoing and unpublished studies using the terms abdominal aneurysm (14 April 2016):
-
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
-
ClinicalTrials.gov (http://clinicaltrials.gov/).
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the search strategies.
Searching other resources
We reviewed the reference lists of relevant studies.
Data collection and analysis
Selection of studies
One review author (LR) used the selection criteria to identify trials for inclusion and assessed the titles and abstracts of all identified studies for relevance and design. The second review author (EA) independently confirmed this selection, and we resolved any disagreements through discussion. We obtained the full‐text articles of any potentially relevant studies. Two review authors independently assessed the full‐text articles. We resolved any disagreements by discussion. We listed all studies excluded after full‐text assessment in a 'Characteristics of excluded studies' table. We planned to include any studies that were published in duplicate only once in the review. We constructed a PRISMA diagram to illustrate the study selection process.
Data extraction and management
Two review authors (LR, EA) independently extracted the data. We recorded information about the trial design; AAA definition and measurement methods; baseline characteristics of participants; treatment type, method, duration, timing, mode of delivery, and dose. We reported all‐cause mortality and cardiovascular mortality data as the primary outcome measures. Also, we collected information on non‐cardiovascular events and adverse events in accordance with the secondary outcome measures. We planned to contact the study authors for further information if we required clarification. We resolved any disagreements in data extraction and management by discussion.
Assessment of risk of bias in included studies
Two review authors (LR, EA) independently used the Cochrane 'Risk of bias' assessment tool, Higgins 2011, to assess the risk of bias in the included study). This tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting, and any other relevant biases. We resolved any disagreements by discussion.
Measures of treatment effect
We planned to base the analysis on intention‐to‐treat data from the individual clinical trials. As the primary and secondary outcomes are all binary measures, we computed odds ratios (ORs) using a fixed‐effect model. We calculated the 95% confidence intervals (CIs) of the effect sizes.
Unit of analysis issues
The unit of analysis was the individual participant. However, as the trial involved repeat measurements on participants at different points in time, it was prone to unit of analysis errors (Deeks 2011). Therefore, for the purpose of this review, we chose cardiovascular mortality at five years as the primary endpoint. We planned to include outcomes at longer follow‐up periods as secondary outcomes if reported.
Dealing with missing data
We sought information about dropouts, withdrawals, and other missing data. If not reported, we attempted to contact the study authors.
Assessment of heterogeneity
The inclusion of studies on a wide range of medical treatments was likely to result in a high degree of heterogeneity. We therefore planned to assess the heterogeneity between pooled studies by using the Chi2 test regarding the characteristics and quality of included studies (Deeks 2011).
We planned to perform the Chi2 test to assess heterogeneity in identified subgroups, and we planned to use the I2 statistic to measure the degree of inconsistency between studies. An I2 statistic result of greater than 50% may represent moderate to substantial heterogeneity (Deeks 2011). Only one study met the inclusion criteria for the review and therefore it was not necessary to measure the heterogeneity between studies.
Assessment of reporting biases
We planned to assess reporting biases such as publication bias using funnel plots (Sterne 2011). As only one study met the inclusion criteria of this review, which was at a low risk of reporting bias, we did not perform this.
Data synthesis
Two review authors (LR, EA) independently extracted the data. One review author (LR) entered the data into Review Manager 5 (RevMan 5) (RevMan 2014). The second review author (EA) cross‐checked data entry, and we resolved any discrepancies by consulting the source publication.
We used a fixed‐effect model to meta‐analyse the data.
Subgroup analysis and investigation of heterogeneity
Where possible, we planned to analyse clinically relevant subgroups based on drug and participant groupings including the following.
-
Diameter of aneurysm.
-
Type of repair (e.g. endovascular versus surgical).
-
Type of repair (e.g. endovascular or surgical) versus no repair.
-
Diabetes.
-
Year of publication.
However, as only one study with 227 participants met the inclusion criteria, it was not possible to perform subgroup analyses.
Sensitivity analysis
We planned to conduct a sensitivity analysis by excluding studies at a high risk of bias to measure the effect on the results. However, as there was only one included study we were unable to conduct a sensitivity analysis.
'Summary of findings' table
We presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data for all‐cause mortality, cardiovascular mortality, AAA‐related death, and nonfatal cardiovascular events in a 'Summary of findings' table, according to the GRADE principles as described by Higgins 2011 and Atkins 2004. We used the GRADEprofiler Guideline Development Tool (GRADEpro GDT) software to assist in the preparation of the 'Summary of findings' table (GRADEpro GDT 2014).
Results
Description of studies
Results of the search
See Figure 1.
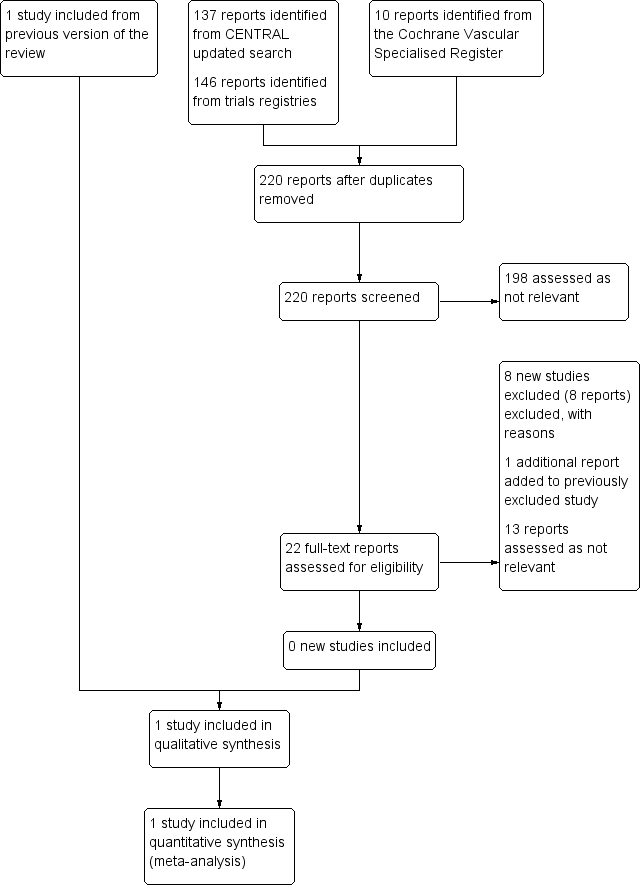
Study flow diagram.
Included studies
See the 'Characteristics of included studies' table.
No new studies met the inclusion criteria for this update.
The review includes one study (Yang 2006). Yang 2006 is a double‐blind, randomised, placebo‐controlled trial that measured the effects of metoprolol on the incidence of cardiac complications at 30 days and six months after vascular surgery. The study included 496 participants who underwent procedures including abdominal aortic repair and infrainguinal or axillofemoral revascularisation. A subgroup of 227 participant had an abdominal aortic repair. Although the trial authors did not present outcome data for the abdominal aortic aneurysm (AAA) subgroup in the full report, we obtained these data through personal communication with the study author and statistician. Of the 227 AAA participants, 111 were randomised to metoprolol and 116 were randomised to a placebo. The doses of metoprolol were as follows: 100 mg in participants weighing ≥ 75 kg, 50 mg for participants weighing between 40 mg and 75 kg, and 25 mg for those weighing ≤ 40 kg. Beta‐blocker therapy was commenced preoperatively on the day of surgery and continued for the duration of the hospital stay. Within two hours postsurgery, the study drug was administered orally or intravenously for 15 minutes (metoprolol 1 mg/mL or saline at 0.2 mL/kg, diluted with 20 mL of saline). Study medication was continued intravenously every six hours or orally twice a day for five days or until hospital discharge, whichever occurred sooner. Intravenous study drug was converted to oral as soon as the participant tolerated oral intake. The trial performed 30‐day and six‐month follow‐ups by telephone for discharged participants. Yang 2006 defined the primary outcome as a composite of cardiac complications at 30 days postoperation including: cardiac death, nonfatal myocardial infarction (MI), congestive heart failure (CHF), unstable angina, and dysrhythmia requiring treatment, defined as atrial fibrillation or ventricular dysrhythmias. In the presence of more than one outcome, the first outcome was recorded. Secondary study outcomes included study drug discontinuation (due to bronchospasm, hypotension, or bradycardia), amputation, and intraoperative hypotension or bradycardia.
Excluded studies
See the 'Characteristics of excluded studies' table.
For this update we excluded seven completed studies (Ashes 2013; Berwanger 2015; Kouvelos 2011; Qu 2014; Schouten 2011; Xia 2014; Xia 2015), and one ongoing study (NCT01225094).
In total, we excluded 17 studies from the review (Ashes 2013; Berwanger 2015; Cesanek 2008; DECREASE Study; Durazzo 2004; Kouvelos 2011; Kouvelos 2013; Mackey 2006; Mangano 1996; NCT01225094; Neilipovitz 2012; POBBLE Trial; POISE Study; Qu 2014; Schouten 2011; Xia 2014; Xia 2015). Two studies, Durazzo 2004 and POBBLE Trial, had AAA subgroups but did not present specific outcome data for these participant. The author of one study, Durazzo 2004, confirmed through personal communication that these data were not available. We were unable to contact the authors of the POBBLE Trial. Ten studies did not report AAA subgroups (Ashes 2013; Berwanger 2015; Cesanek 2008; Kouvelos 2011; Mangano 1996; POISE Study; Qu 2014; Schouten 2011; Xia 2014; Xia 2015). Authors of the POISE Study confirmed that outcome data for AAA participants were not available, but the other nine study authors did not respond (Ashes 2013; Berwanger 2015; Cesanek 2008; Kouvelos 2011; Mangano 1996; Qu 2014; Schouten 2011; Xia 2014; Xia 2015). One study, Mackey 2006, was not a randomised controlled trial but a prospective study that measured the incidence of myocardial injury in vascular surgery patients. In two studies participants were taking co‐medications and therefore we could not attribute the results to one particular drug (Kouvelos 2013; Neilipovitz 2012). We excluded the DECREASE Study as the integrity of the data was questionable. In a report released by Erasmus MC Follow‐Up Committee in 2012, the principal investigator admitted that written informed consent was not obtained for every participant and that the data were collected in a negligent manner (Erasmus MC Follow‐Up Committee 2012). Finally, one ongoing study tested the effects of curcumin, a natural health product (NCT01225094).
Risk of bias in included studies
See the 'Risk of bias' table in the 'Characteristics of included studies' section, and Figure 2.
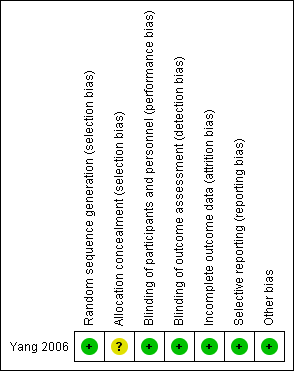
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
A study statistician performed random sequence generation in blocks of four and therefore we judged the study to be at a low risk of selection bias. However, the study authors did not report the methods used to conceal allocation of treatment and therefore the risk of selection bias was unclear.
Blinding
All study participants, investigators, caretakers and data outcome evaluators of Yang 2006 were blinded to treatment. Furthermore, blinding was maintained throughout the study, even if study medication was discontinued.
Incomplete outcome data
The two treatment groups in Yang 2006 were well‐balanced with respect to baseline characteristics, completion of the study protocol, and discontinuation of treatment. Furthermore, the study authors accounted for and reported on all missing data.
Selective reporting
The authors of Yang 2006 specified their hypothesis using results from previously published work. They clearly stated their primary and secondary outcomes and reported data on all outcomes.
Other potential sources of bias
We considered the Yang 2006 study to be at low risk of other potential sources of bias.
Effects of interventions
As only one study, Yang 2006, met the inclusion criteria, we were unable to pool data or perform a meta‐analysis. Therefore, we reported the individual estimates from the study in a narrative synthesis. The included study did not measure mortality at five years but at two shorter time points of 30 days and six months postoperation. Results indicated no clear evidence that metoprolol reduced all‐cause or cardiovascular mortality at 30 days: the incidence of all‐cause mortality was 1/111 in the metoprolol group and 6/116 in the placebo group (odds ratio (OR) 0.17, 95% confidence interval (CI) 0.02 to 1.41) while the incidence of cardiovascular mortality at 30 days was 1/111 and 5/116 in the metoprolol and placebo groups respectively (OR 0.20, 95% CI 0.02 to 1.76). One participant in each treatment group died of causes related to AAA (OR 1.05, 95% CI 0.06 to 16.92). Nonfatal cardiovascular events occurred in 12/111 in the metoprolol group and 9/116 in the placebo group at 30 days (OR 1.44, 95% CI 0.58 to 3.57). At six months, metoprolol did not significantly reduce the rate of all‐cause mortality (OR 0.71, 95% CI 0.26 to 1.95) or cardiovascular deaths (OR 0.73, 95% CI 0.23 to 2.39). The incidence of AAA‐related death was not measured at six months. The incidence of nonfatal cardiovascular events was similar between the two treatment groups at six months (OR 1.41, 95% CI 0.59 to 3.35). For these outcomes, we downgraded the quality of the evidence to low. The quality of evidence was downgraded due to imprecision, as only one study with a small number of participants met the inclusion criteria, the number of events was low, and the result was consistent with benefit and harm. No participant had to undergo an amputation. Quality of life was not reported.
Yang 2006 reported on adverse events in the form of study drug discontinuation (due to bronchospasm, hypotension, or bradycardia) and intraoperative hypotension or bradycardia. However, data on study drug discontinuation and the incidence of intraoperative hypotension or bradycardia were not available for the subgroup of AAA participants. In the overall study of 496 participants, the study authors reported that the incidence of intraoperative complications was significantly higher in the metoprolol group (P < 0.01). Hypotension occurred in 54% of metoprolol participants (46% required treatment) compared to 41% of placebo participants (34% required treatment). Bradycardia occurred in 35% and 10% of metoprolol and placebo participants, respectively, of whom 22% and 7% required treatment. However, given that these outcomes are based on a population of participants who had undergone vascular surgery for other conditions, we cannot generalise the results to participants with AAA.
Discussion
Summary of main results
Only one study fulfilled the inclusion criteria of this review. The study was a randomised controlled trial in which 496 participants undergoing non‐cardiac vascular surgery received either metoprolol or placebo (Yang 2006). We received data on a subgroup of 227 participants who underwent AAA repair from the study author. Results of the study indicate that metoprolol is not associated with a reduction in the rate of all‐cause or cardiovascular mortality at either 30 days or six months. No participant had to undergo an amputation. Quality of life was not reported. Adverse drug effects were reported for the whole study population and were not available for the subgroup of participants with AAA. We downgraded the quality of the evidence due to imprecision, as only one study with a small number of participants met the inclusion criteria, the number of events was low, and the result was consistent with benefit and harm.
Overall completeness and applicability of evidence
Currently, there is a severe lack of evidence concerning the effectiveness of pharmacological prophylaxis in the prevention of cardiovascular events in AAA patients. The one included study was relatively small and tested one beta‐blocker against a placebo at 30 days and six months follow‐up. Therefore, the results of this study are not widely applicable to the AAA population and the follow‐up period was relatively short to study mortality and cardiovascular events in such participants. Recent evidence has questioned whether beta‐blockers are of any perioperative value and suggests they may be harmful (Bolsin 2013). As there are many different drugs available, it is important to test these drugs, not just against a placebo but also against each other. Furthermore, it is important to establish if a combination of drugs would yield a better outcome than one drug alone.
Quality of the evidence
The quality of reporting in the single included study was good. With the exception of failing to report the methods used to conceal allocation of treatments, the study authors provided adequate information on the process of randomisation and blinding. As such, we deemed the study to be at a low risk of selection, performance, and detection bias. Additionally, the study authors accounted for all missing data and reported data on all primary and secondary outcomes, and therefore minimised the chances of attrition and performance bias. For all outcomes, we downgraded the quality of the evidence to low. We downgraded the quality of evidence for imprecision, as there was only one included study with a small number of participants, the number of events was small, and the confidence intervals (CI) indicated both benefit and harm.
Potential biases in the review process
We, the authors of this Cochane review, were neither involved in the included study nor in any of the excluded studies. Furthermore, we do not have any commercial or other conflict of interest. The search was as comprehensive as possible and two review authors independently assessed all studies for inclusion. We are confident that we have included all relevant studies and attempted to reduce bias in the review process. However, the possibility remains that we may have missed studies that have not been published.
Agreements and disagreements with other studies or reviews
This is an update of a Cochrane review first published in 2014 and the first systematic review to measure the effectiveness of pharmacological prophylaxis in reducing cardiovascular morbidity and mortality in AAA patients. One prospective study of AAA participants who were followed up over a median of 4.7 years determined that, in those who survived AAA repair, beta‐blocker use was associated with a significantly lower incidence of all‐cause mortality (hazard ratio (HR) 0.6, 95% CI 0.5 to 0.9) and cardiovascular mortality (HR 0.7, 95% CI 0.4 to 0.9) (Kertai 2004). After adjusting for clinical risk factors and beta‐blocker use, the same study showed that long‐term use of statins showed a reduction in both all‐cause and cardiovascular mortality (HR 0.4, 95% CI 0.3 to 0.6; and HR 0.3, 95% CI 0.2 to 0.6 respectively). Therefore, it would appear that statins reduce cardiovascular risk regardless of beta‐blocker use. However, this was a prospective cohort study with no randomisation and therefore likely to be at high risk of bias.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
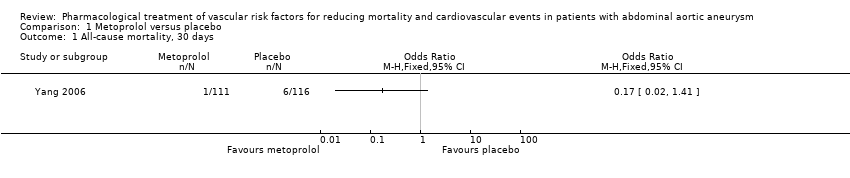
Comparison 1 Metoprolol versus placebo, Outcome 1 All‐cause mortality, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 2 Cardiovascular death, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 3 AAA‐related death, 30 days.

Comparison 1 Metoprolol versus placebo, Outcome 4 Nonfatal cardiovascular event, 30 days.
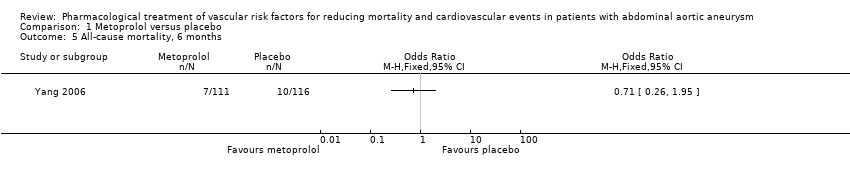
Comparison 1 Metoprolol versus placebo, Outcome 5 All‐cause mortality, 6 months.
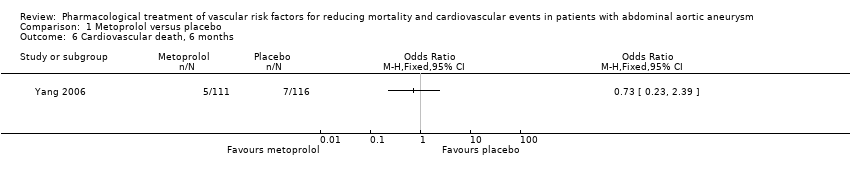
Comparison 1 Metoprolol versus placebo, Outcome 6 Cardiovascular death, 6 months.
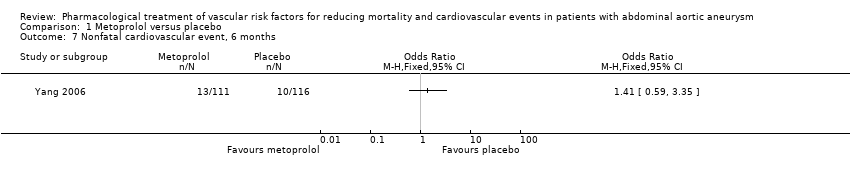
Comparison 1 Metoprolol versus placebo, Outcome 7 Nonfatal cardiovascular event, 6 months.
| Metoprolol compared to placebo for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm (AAA) | ||||||
| Patient or population: patients of any age with AAA less than 30 mm in diameter | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with metoprolol | |||||
| All‐cause mortality, 30 days1 | Study population | OR 0.17 | 227 | ⊕⊕⊝⊝ | — | |
| 52 per 1000 | 9 per 1000 | |||||
| Cardiovascular death, 30 days3 | Study population | OR 0.20 | 227 | ⊕⊕⊝⊝ | — | |
| 43 per 1000 | 9 per 1000 | |||||
| AAA‐related death, 30 days4 | Study population | OR 1.05 | 227 | ⊕⊕⊝⊝ | — | |
| 9 per 1000 | 9 per 1000 | |||||
| Nonfatal cardiovascular event, 30 days5 | Study population | OR 1.44 | 227 | ⊕⊕⊝⊝ | — | |
| 78 per 1000 | 108 per 1000 | |||||
| All‐cause mortality, 6 months1 | Study population | OR 0.71 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 63 per 1000 | |||||
| Cardiovascular death, 6 months3 | Study population | OR 0.73 | 227 | ⊕⊕⊝⊝ | — | |
| 60 per 1000 | 45 per 1000 | |||||
| AAA‐related death, 6 months4 | See comments | See comments | See comments | See comments | The incidence of AAA‐related death was not measured at six months. | |
| Nonfatal cardiovascular event, 6 months5 | Study population | OR 1.41 | 227 | ⊕⊕⊝⊝ | — | |
| 86 per 1000 | 117 per 1000 | |||||
| *The risk with placebo was the average risk in the placebo group (i.e. the number of participants with events divided by total number of participants of the placebo group included in the meta‐analysis). The risk in the metoprolol group (and its 95% CI) is based on the assumed risk in the placebo group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Death from all causes. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Cardiovascular death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 AAA‐related death, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Nonfatal cardiovascular event, 30 days Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 All‐cause mortality, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Cardiovascular death, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Nonfatal cardiovascular event, 6 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

