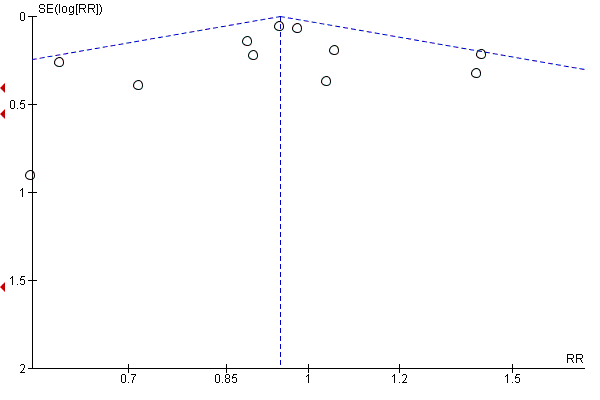
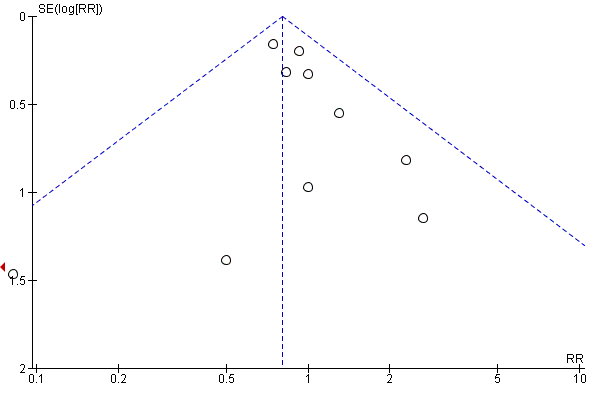
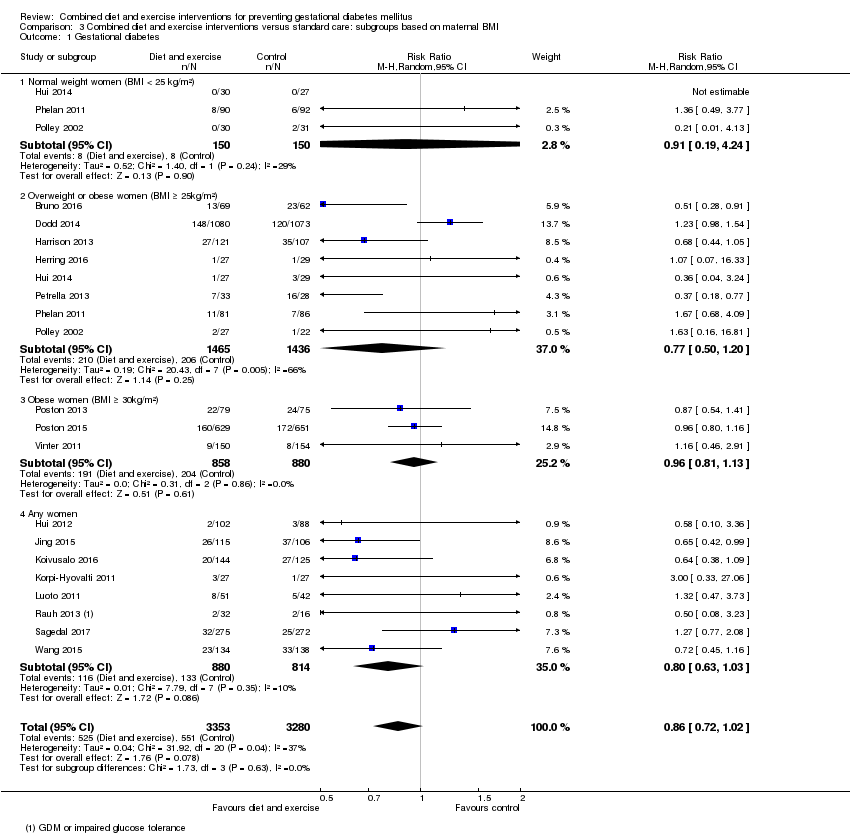
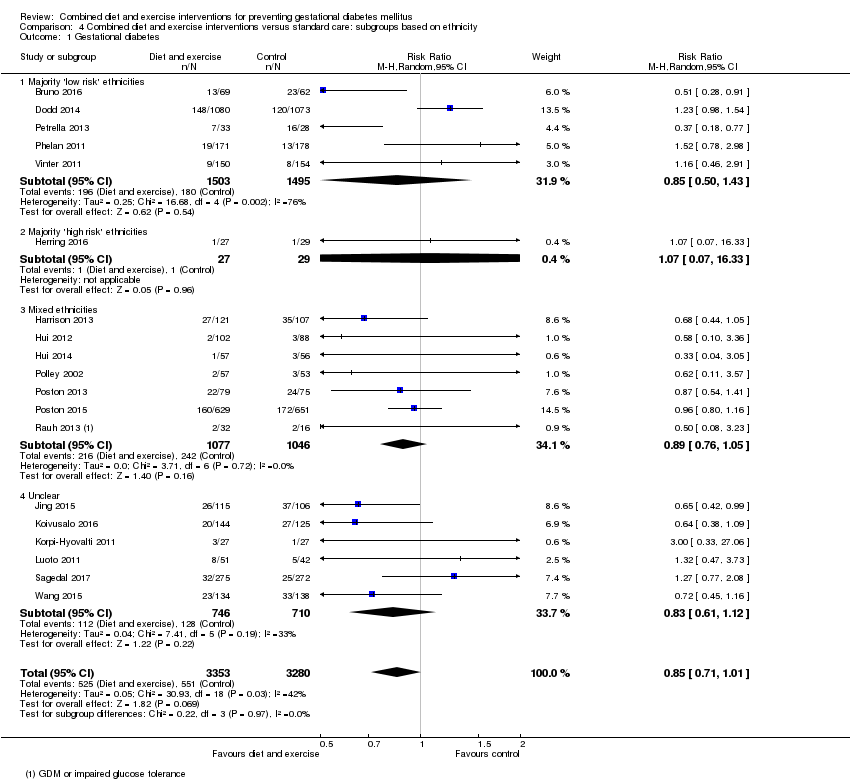
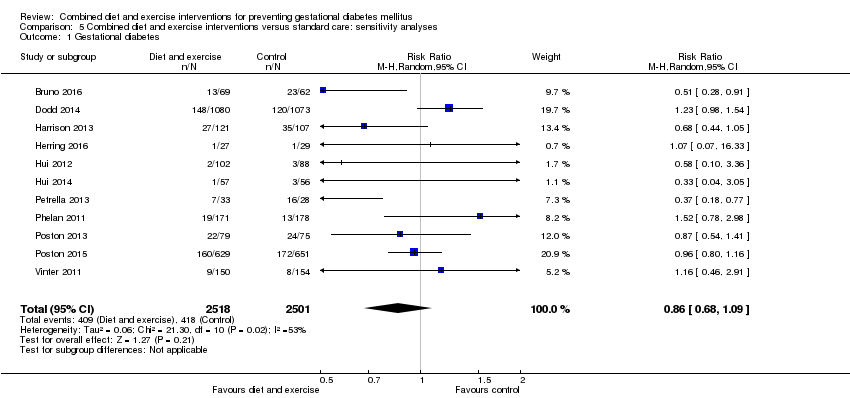
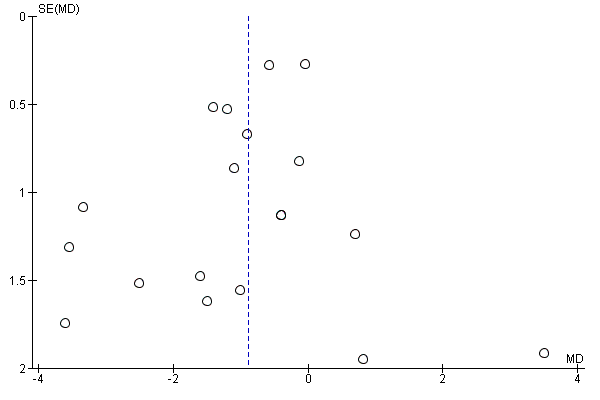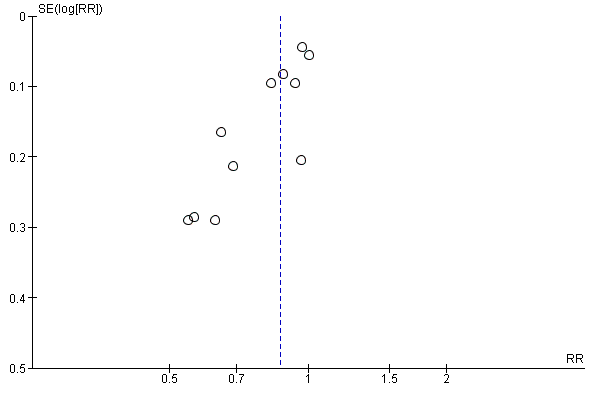| 1 Gestational diabetes Show forest plot | 19 | 6633 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.01] |
|
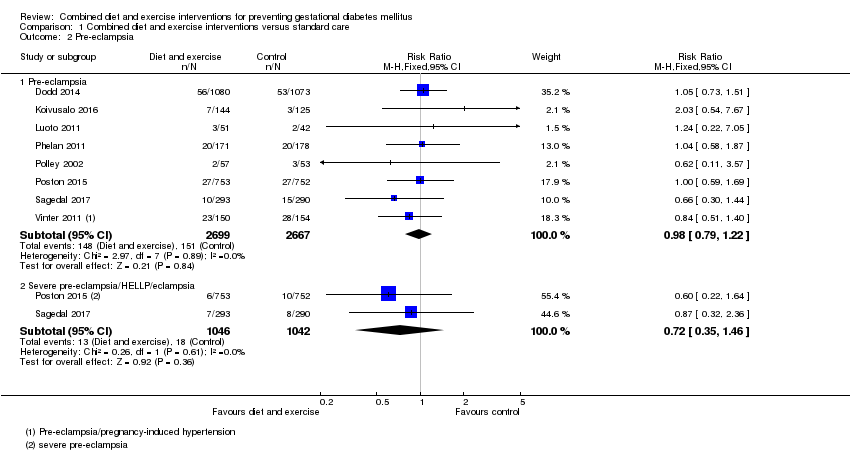
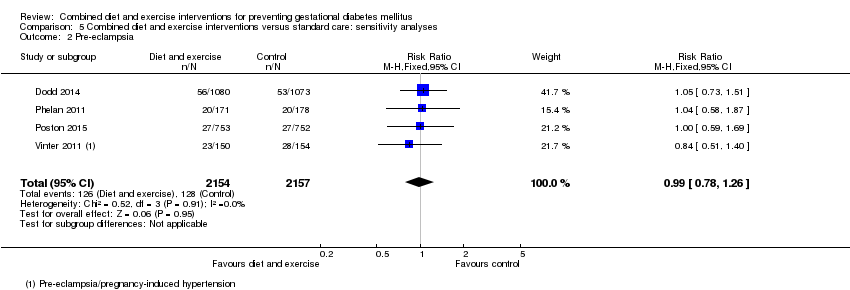
| 2 Pre‐eclampsia Show forest plot | 8 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 2.1 Pre‐eclampsia | 8 | 5366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 2.2 Severe pre‐eclampsia/HELLP/eclampsia | 2 | 2088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.46] |
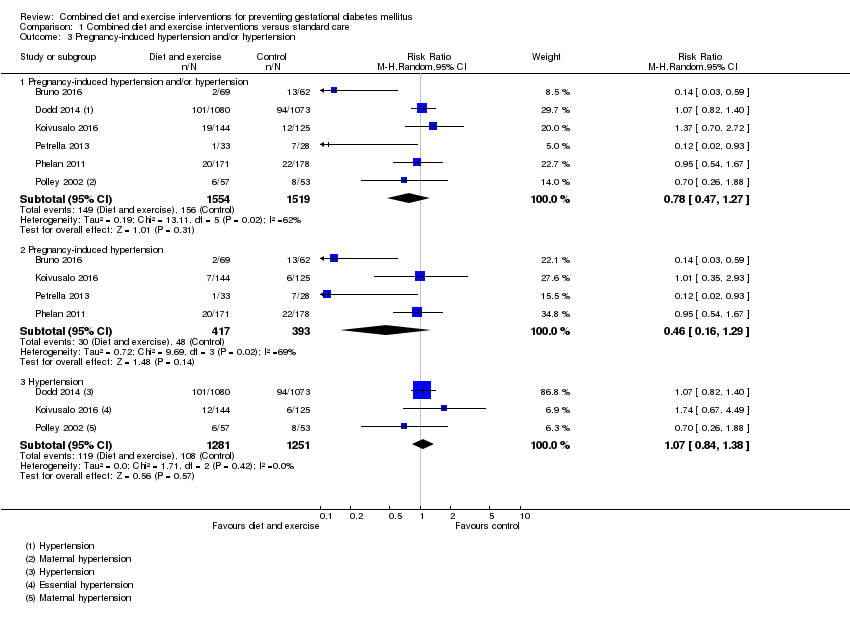
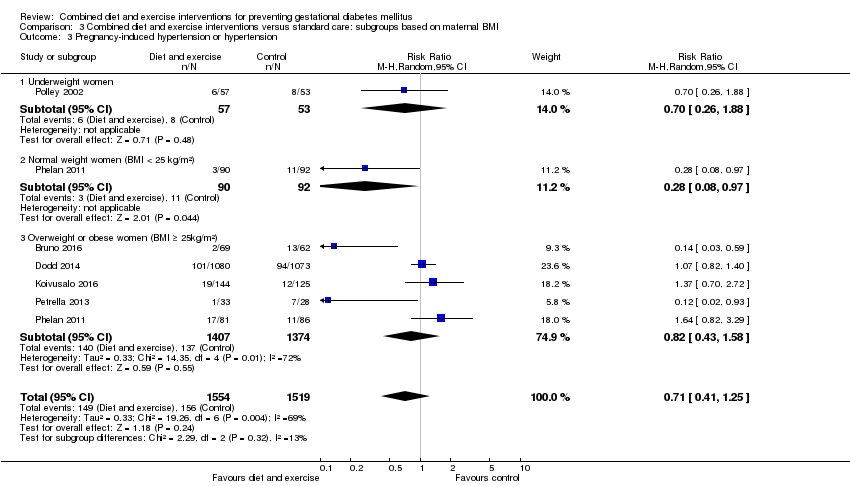
| 3 Pregnancy‐induced hypertension and/or hypertension Show forest plot | 6 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 3.1 Pregnancy‐induced hypertension and/or hypertension | 6 | 3073 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.27] |
| 3.2 Pregnancy‐induced hypertension | 4 | 810 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.29] |
| 3.3 Hypertension | 3 | 2532 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.84, 1.38] |
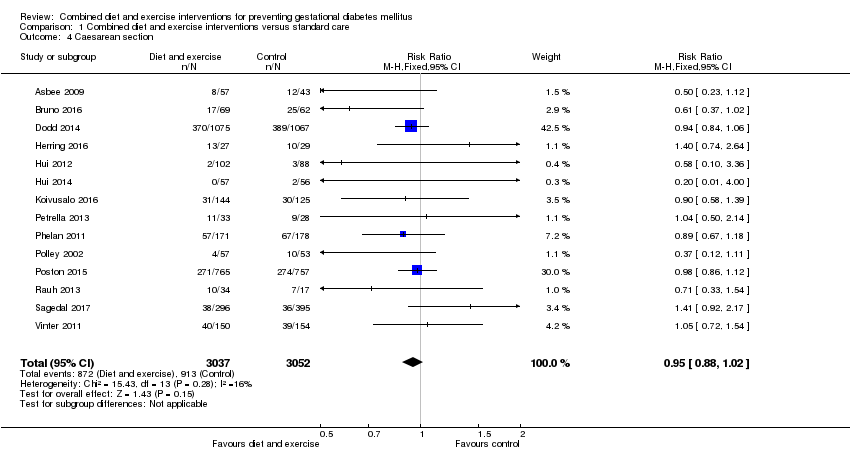
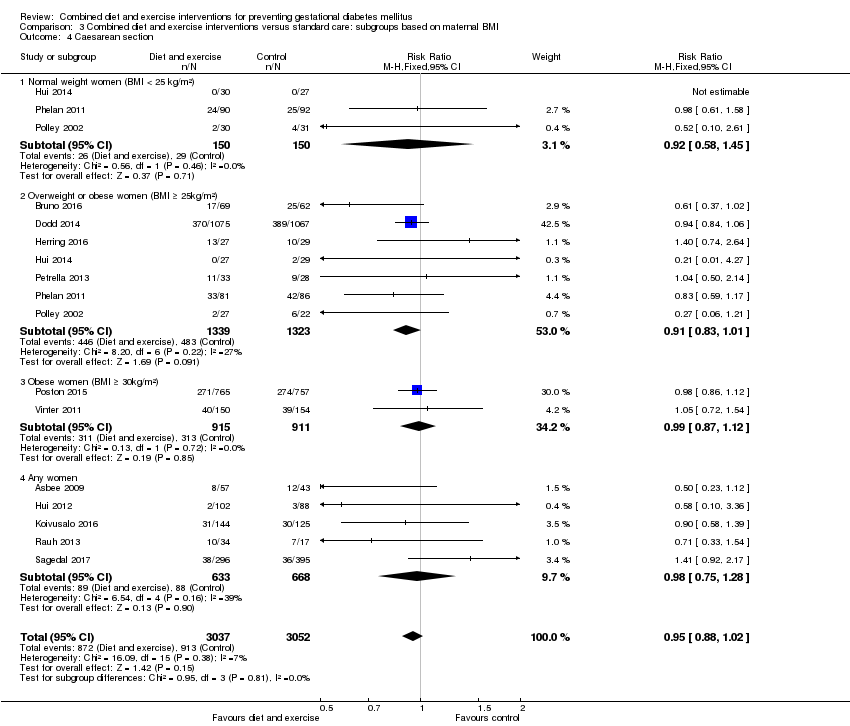
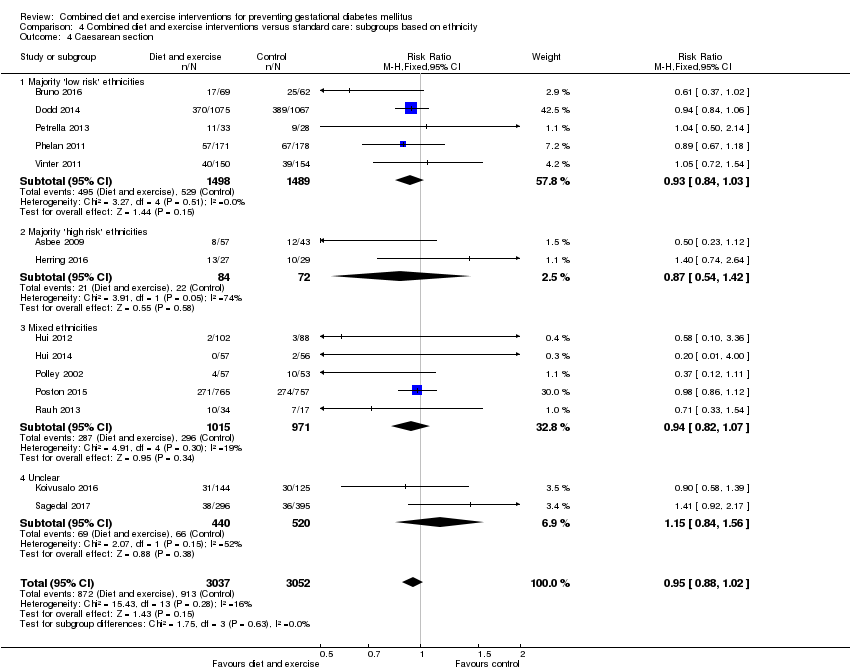
| 4 Caesarean section Show forest plot | 14 | 6089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.02] |
|
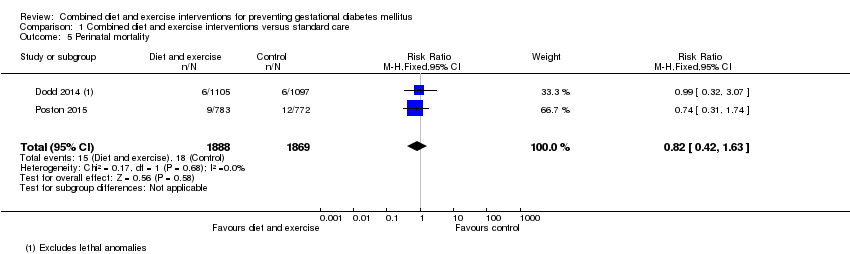
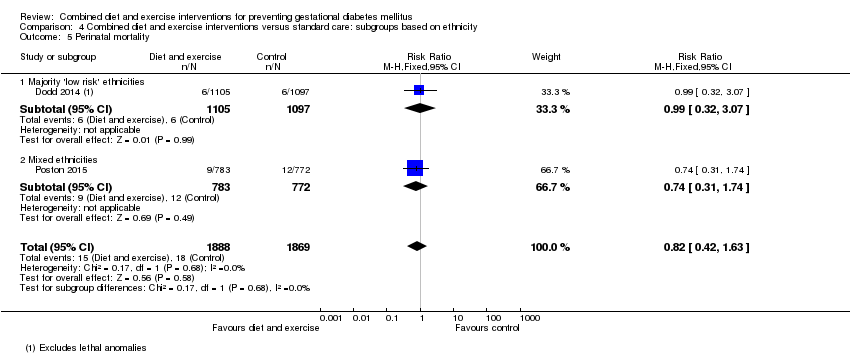
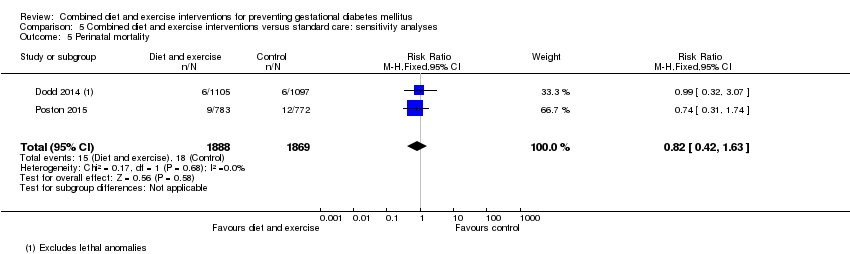
| 5 Perinatal mortality Show forest plot | 2 | 3757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.42, 1.63] |
|
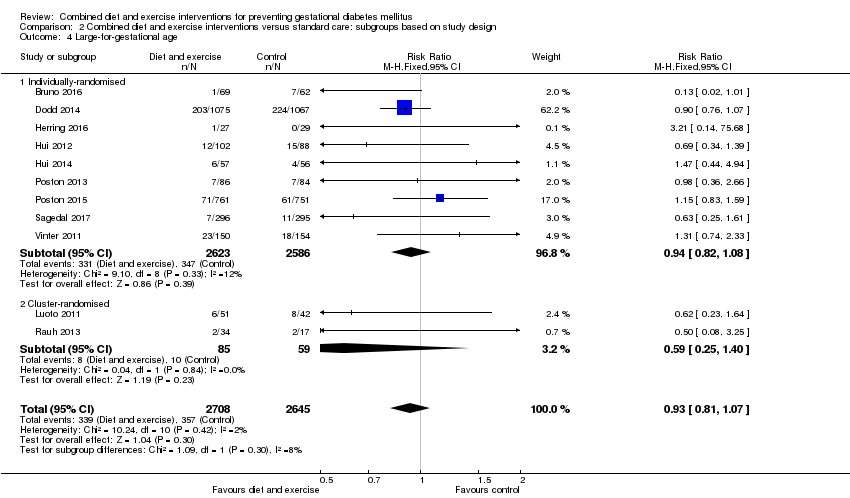
| 6 Large‐for‐gestational age Show forest plot | 11 | 5353 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.07] |
|
| 7 Operative vaginal birth Show forest plot | 3 | 2164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.34] |
|
| 8 Induction of labour Show forest plot | 5 | 3907 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.06] |
|
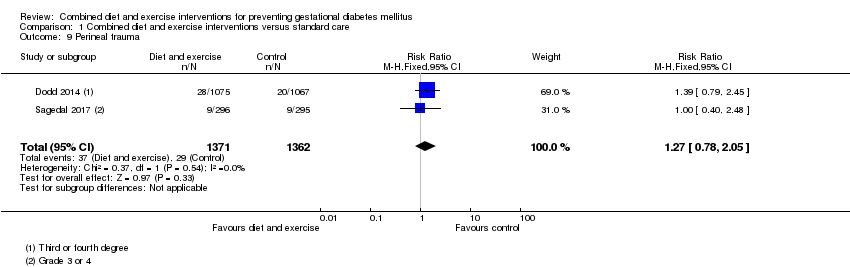
| 9 Perineal trauma Show forest plot | 2 | 2733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.78, 2.05] |
|
| 10 Placental abruption Show forest plot | 1 | 1555 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 72.50] |
|
| 11 Postpartum haemorrhage Show forest plot | 3 | 4235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.18] |
|
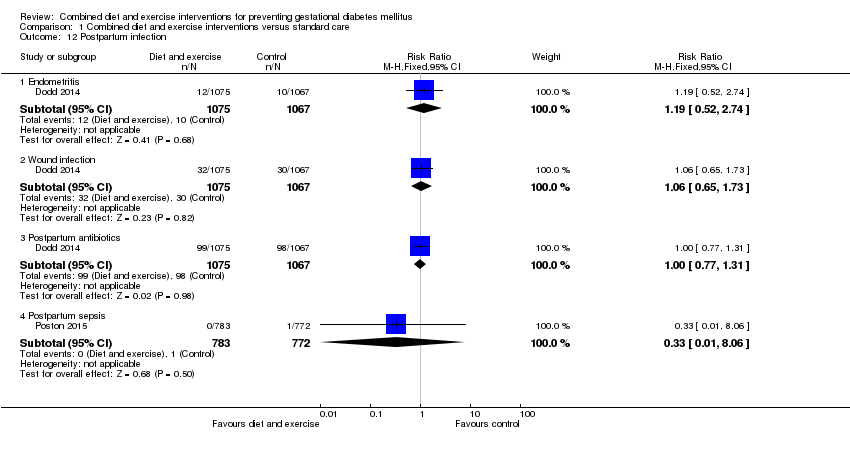
| 12 Postpartum infection Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 12.1 Endometritis | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.52, 2.74] |
| 12.2 Wound infection | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.73] |
| 12.3 Postpartum antibiotics | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.31] |
| 12.4 Postpartum sepsis | 1 | 1555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
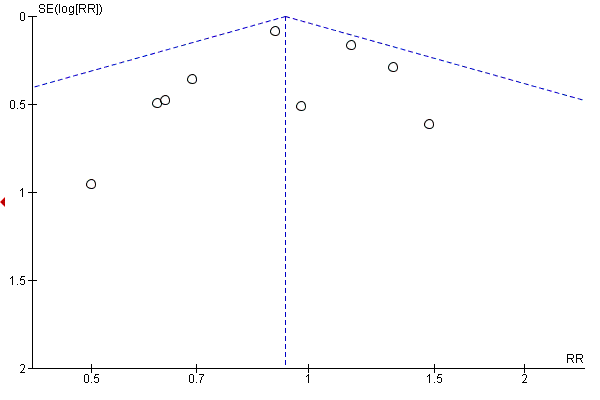
| 13 Gestational weight gain (kg) Show forest plot | 16 | 5052 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.39, ‐0.40] |
|
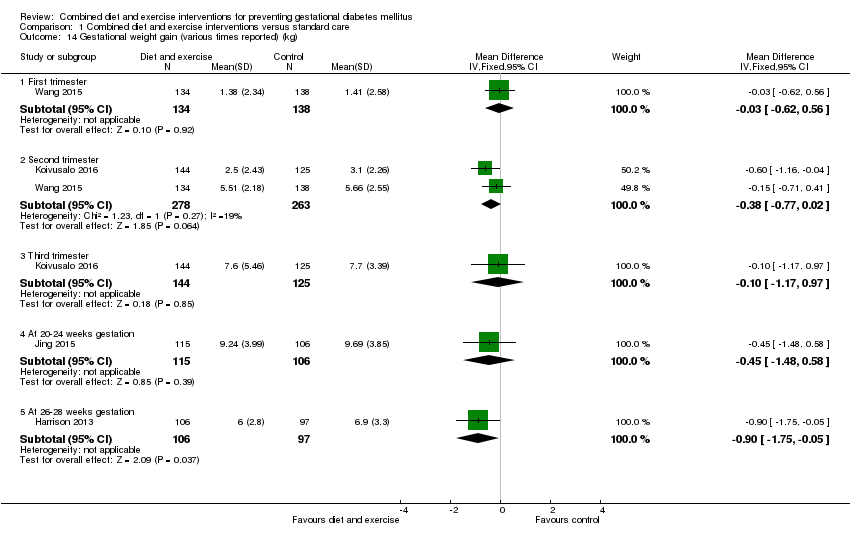
| 14 Gestational weight gain (various times reported) (kg) Show forest plot | 4 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 14.1 First trimester | 1 | 272 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.62, 0.56] |
| 14.2 Second trimester | 2 | 541 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.77, 0.02] |
| 14.3 Third trimester | 1 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.17, 0.97] |
| 14.4 At 20‐24 weeks gestation | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐1.48, 0.58] |
| 14.5 At 26‐28 weeks gestation | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.75, ‐0.05] |
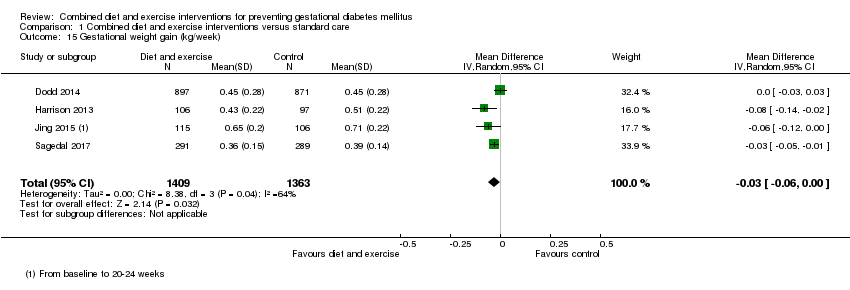
| 15 Gestational weight gain (kg/week) Show forest plot | 4 | 2772 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
|
| 16 Gestational weight gain (above IOM recommendations) Show forest plot | 11 | 4556 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.79, 0.96] |
|
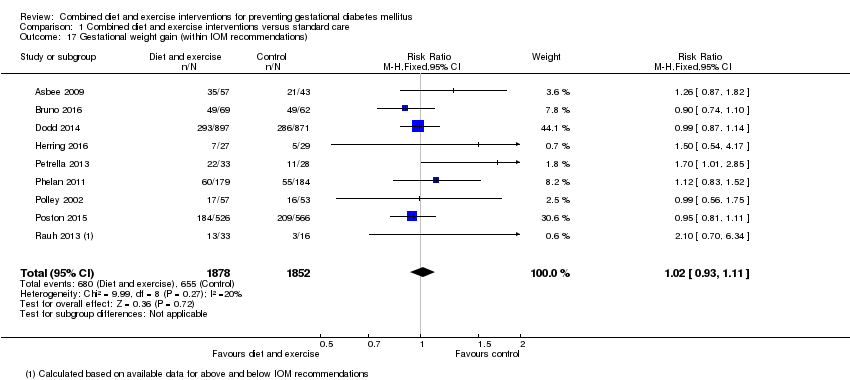
| 17 Gestational weight gain (within IOM recommendations) Show forest plot | 9 | 3730 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
|
| 18 Gestational weight gain (below IOM recommendations) Show forest plot | 7 | 3499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.24] |
|
| 19 Behaviour changes associated with the intervention Show forest plot | | | Other data | No numeric data |
|
| 20 Relevant biomarker changes associated with the intervention Show forest plot | | | Other data | No numeric data |
|
| 21 Sense of well‐being and quality of life Show forest plot | | | Other data | No numeric data |
|
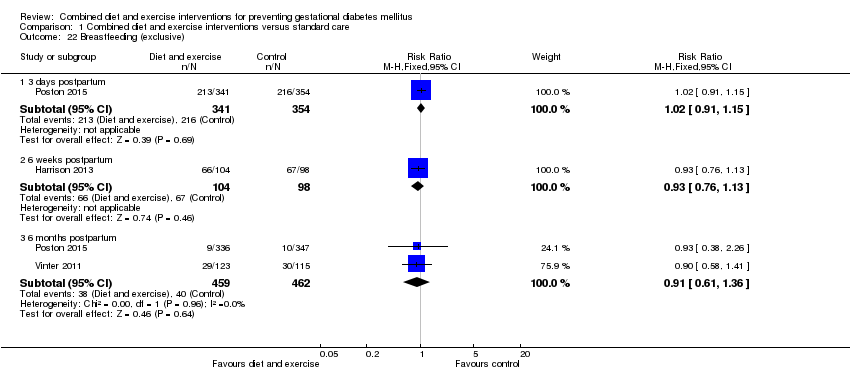
| 22 Breastfeeding (exclusive) Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 22.1 3 days postpartum | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.15] |
| 22.2 6 weeks postpartum | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.13] |
| 22.3 6 months postpartum | 2 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.61, 1.36] |
| 23 Breastfeeding (partial) Show forest plot | 3 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 23.1 3 days postpartum | 1 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.40, 0.66] |
| 23.2 6 weeks postpartum | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.80, 2.60] |
| 23.3 6 months postpartum | 2 | 921 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.18] |
| 24 Breastfeeding Show forest plot | | | Other data | No numeric data |
|
| 25 Postnatal weight retention (latest time reported) (kg) Show forest plot | 6 | 1673 | Mean Difference (IV, Fixed, 95% CI) | ‐0.94 [‐1.52, ‐0.37] |
|
| 26 Return to pre‐pregnancy weight (latest time reported) Show forest plot | 3 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.08, 1.45] |
|
| 27 Postnatal BMI (latest time reported) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 27.1 BMI | 2 | 902 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.85, 0.55] |
| 27.2 BMI change from baseline to 6 weeks postpartum | 1 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.12, ‐0.00] |
| 28 Maternal cardiovascular health (latest time reported) Show forest plot | | | Other data | No numeric data |
|
| 29 Stillbirth Show forest plot | 5 | 4783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.35, 1.36] |
|
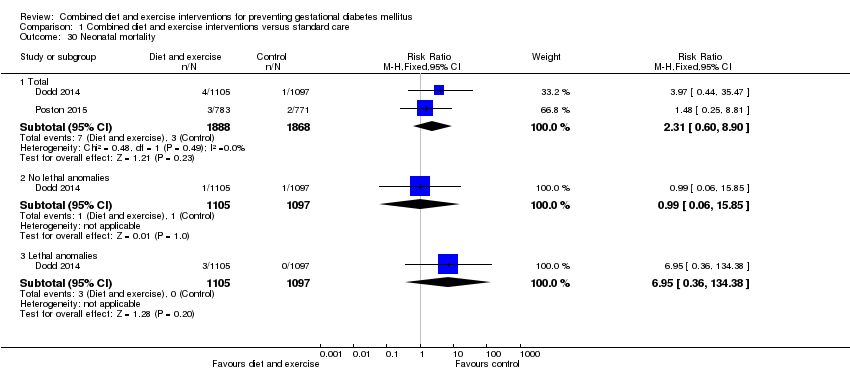
| 30 Neonatal mortality Show forest plot | 2 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 30.1 Total | 2 | 3756 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.60, 8.90] |
| 30.2 No lethal anomalies | 1 | 2202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.85] |
| 30.3 Lethal anomalies | 1 | 2202 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.95 [0.36, 134.38] |
| 31 Gestational age at birth (weeks) Show forest plot | 11 | 5658 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.05, 0.15] |
|
| 32 Gestational age at birth (days or weeks) Show forest plot | | | Other data | No numeric data |
|
| 33 Preterm birth Show forest plot | 11 | 5398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.98] |
|
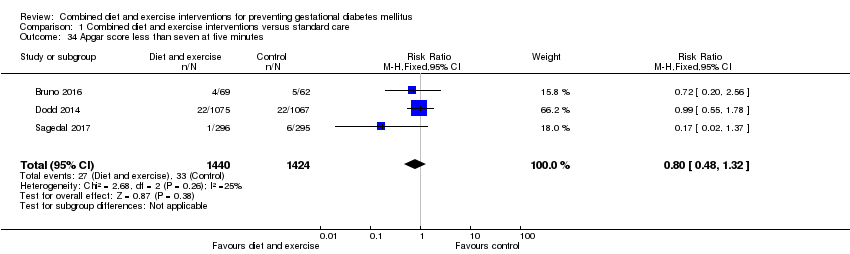
| 34 Apgar score less than seven at five minutes Show forest plot | 3 | 2864 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.32] |
|
| 35 Macrosomia Show forest plot | 10 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 35.1 > 4000 g | 9 | 5368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 35.2 > 4500 g | 4 | 3061 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.42, 0.94] |
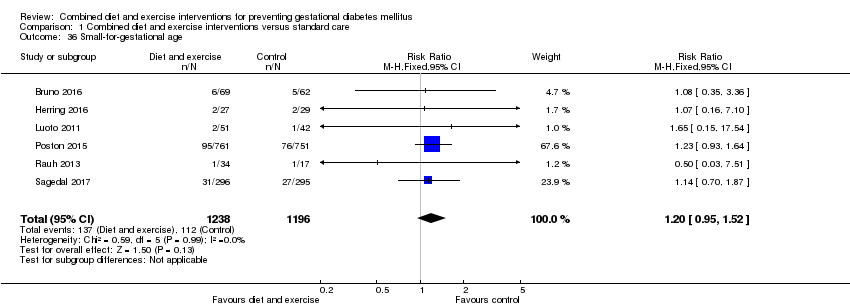
| 36 Small‐for‐gestational age Show forest plot | 6 | 2434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.95, 1.52] |
|
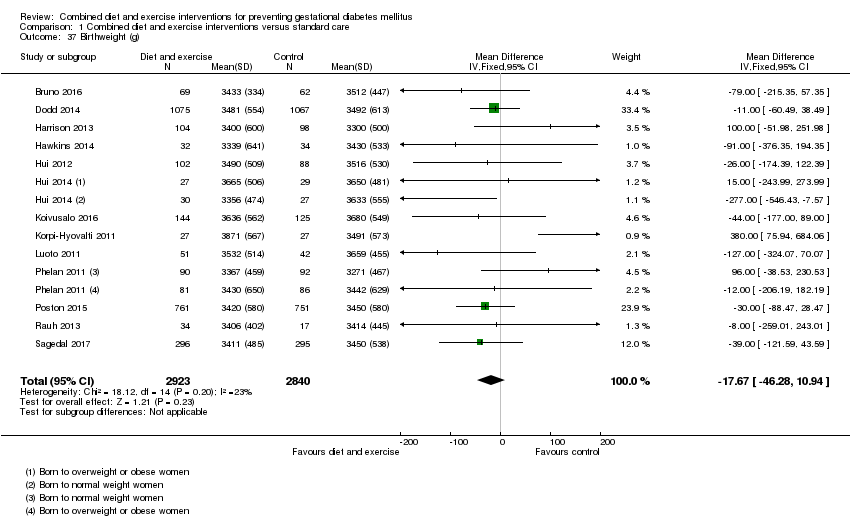
| 37 Birthweight (g) Show forest plot | 13 | 5763 | Mean Difference (IV, Fixed, 95% CI) | ‐17.67 [‐46.28, 10.94] |
|
| 38 Birthweight (g) Show forest plot | | | Other data | No numeric data |
|
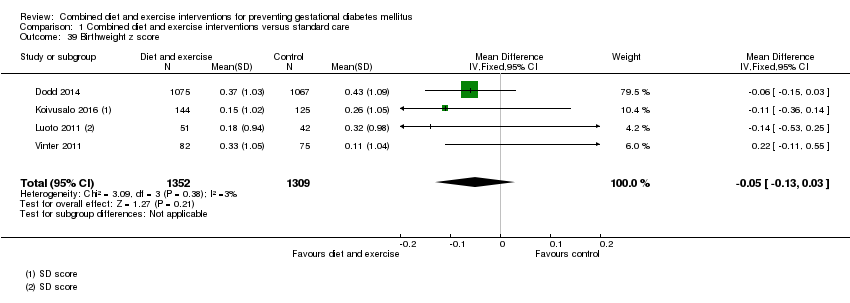
| 39 Birthweight z score Show forest plot | 4 | 2661 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.13, 0.03] |
|
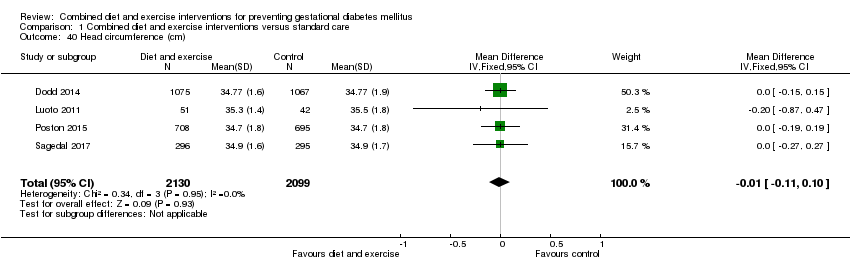
| 40 Head circumference (cm) Show forest plot | 4 | 4229 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.11, 0.10] |
|
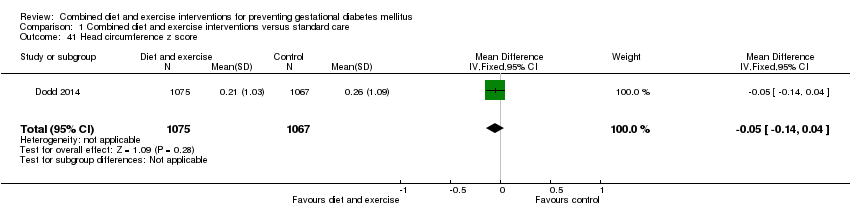
| 41 Head circumference z score Show forest plot | 1 | 2142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.14, 0.04] |
|
| 42 Length (cm) Show forest plot | 6 | 3303 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.09] |
|
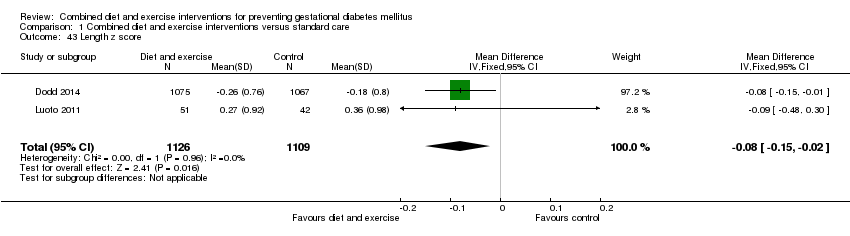
| 43 Length z score Show forest plot | 2 | 2235 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.15, ‐0.02] |
|
| 44 Ponderal index (kg/m3) Show forest plot | 3 | 2826 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.16, 0.25] |
|
| 45 Adiposity (sum of skinfold thickness) (mm) Show forest plot | 2 | 1472 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.33, 0.50] |
|
| 45.1 Sum of biceps, triceps, subscapular, suprailiac, abdominal and thigh skinfold thickness | 1 | 970 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.86, 0.92] |
| 45.2 Sum of triceps and subscapular skinfold thickness (mm) | 1 | 502 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.36, 0.56] |
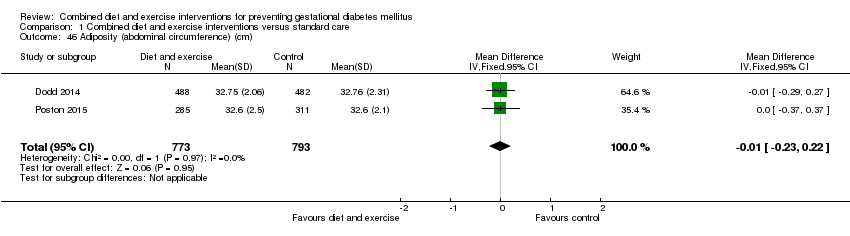
| 46 Adiposity (abdominal circumference) (cm) Show forest plot | 2 | 1566 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.23, 0.22] |
|
| 47 Adiposity Show forest plot | | | Other data | No numeric data |
|
| 48 Shoulder dystocia Show forest plot | 2 | 2733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.79, 1.83] |
|
| 49 Nerve palsy Show forest plot | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.36, 10.82] |
|
| 50 Bone fracture Show forest plot | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.36, 10.82] |
|
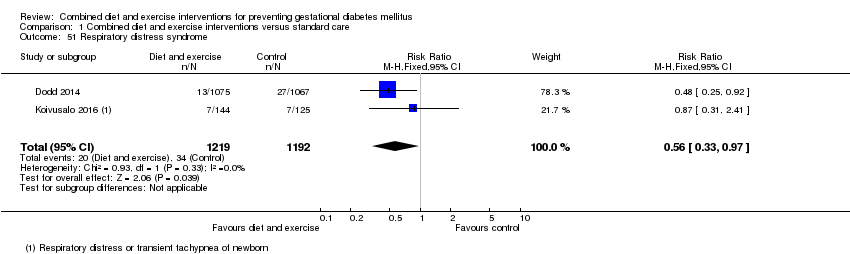
| 51 Respiratory distress syndrome Show forest plot | 2 | 2411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.33, 0.97] |
|
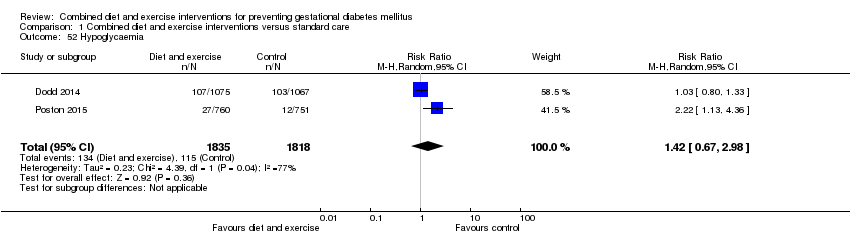
| 52 Hypoglycaemia Show forest plot | 2 | 3653 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.67, 2.98] |
|
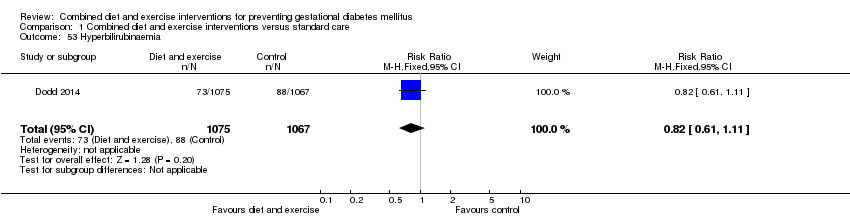
| 53 Hyperbilirubinaemia Show forest plot | 1 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
|
| 54 Childhood weight (latest time reported) (kg) Show forest plot | 3 | 882 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.22] |
|
| 54.1 6 months | 1 | 677 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 54.2 10‐12 months | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.96, 0.24] |
| 54.3 2.8 years | 1 | 157 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.19, 0.79] |
| 55 Childhood weight z score (latest time reported) Show forest plot | 1 | 643 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.08] |
|
| 56 Childhood height (latest time reported) (cm) Show forest plot | 2 | 816 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.58, 1.25] |
|
| 56.1 6 months | 1 | 659 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐0.58, 2.66] |
| 56.2 2.8 years | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.11, 1.11] |
| 57 Childhood height z score (latest time reported) Show forest plot | 1 | 622 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.31, 0.27] |
|
| 58 Childhood head circumference (latest time reported) (cm) Show forest plot | 1 | 670 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.70, 0.46] |
|
| 59 Childhood adiposity (latest time reported) (BMI z score) Show forest plot | 2 | 794 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.29, 0.40] |
|
| 59.1 6 months | 1 | 637 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.39, 0.17] |
| 59.2 2.8 years | 1 | 157 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.10, 0.58] |
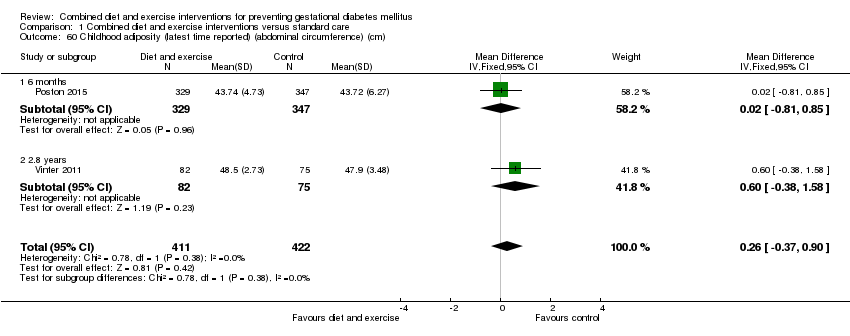
| 60 Childhood adiposity (latest time reported) (abdominal circumference) (cm) Show forest plot | 2 | 833 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.37, 0.90] |
|
| 60.1 6 months | 1 | 676 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.81, 0.85] |
| 60.2 2.8 years | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.38, 1.58] |
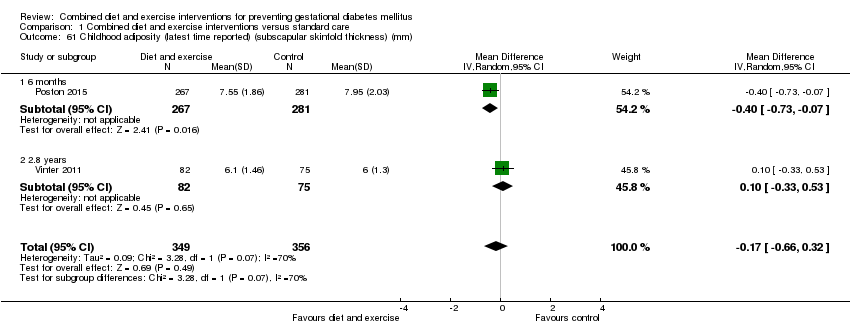
| 61 Childhood adiposity (latest time reported) (subscapular skinfold thickness) (mm) Show forest plot | 2 | 705 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.66, 0.32] |
|
| 61.1 6 months | 1 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.73, ‐0.07] |
| 61.2 2.8 years | 1 | 157 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.33, 0.53] |
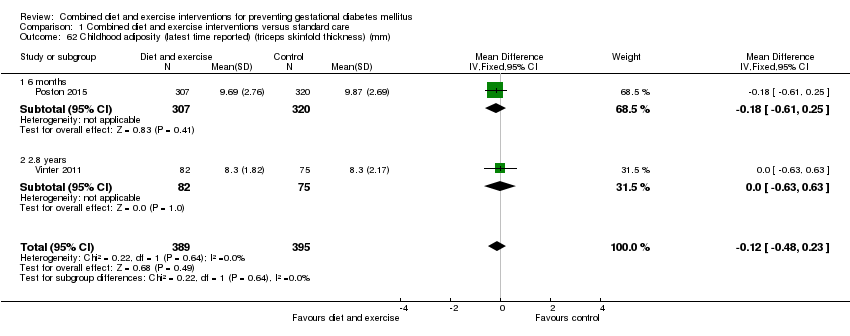
| 62 Childhood adiposity (latest time reported) (triceps skinfold thickness) (mm) Show forest plot | 2 | 784 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.23] |
|
| 62.1 6 months | 1 | 627 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.61, 0.25] |
| 62.2 2.8 years | 1 | 157 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.63, 0.63] |
| 63 Childhood adiposity (latest time reported) (total body fat) (%) Show forest plot | 2 | 614 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.56, 0.07] |
|
| 63.1 6 months | 1 | 547 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.64, 0.04] |
| 63.2 2.8 years | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.03, 3.03] |
| 64 Childhood adiposity (latest time reported) Show forest plot | | | Other data | No numeric data |
|
| 65 Childhood cardiovascular health (latest time reported) Show forest plot | | | Other data | No numeric data |
|
| 66 Antenatal visits Show forest plot | 1 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
|
| 67 Antenatal admissions Show forest plot | 1 | 2153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.04] |
|
| 68 Length of antenatal stay (days) Show forest plot | 2 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 68.1 Antenatal stay (days) | 1 | 2153 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.49, ‐0.05] |
| 68.2 Antenatal inpatient stay (nights), if admitted | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.00, 1.00] |
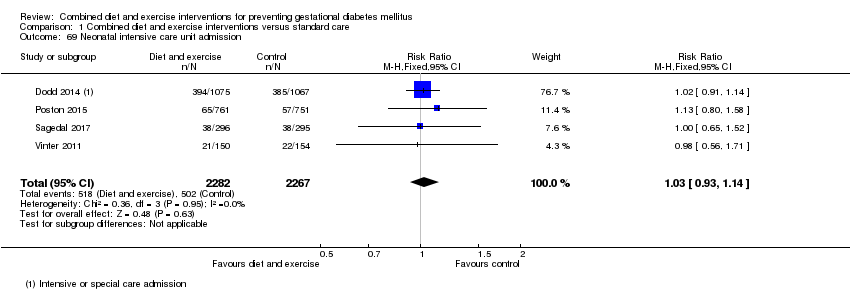
| 69 Neonatal intensive care unit admission Show forest plot | 4 | 4549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.14] |
|
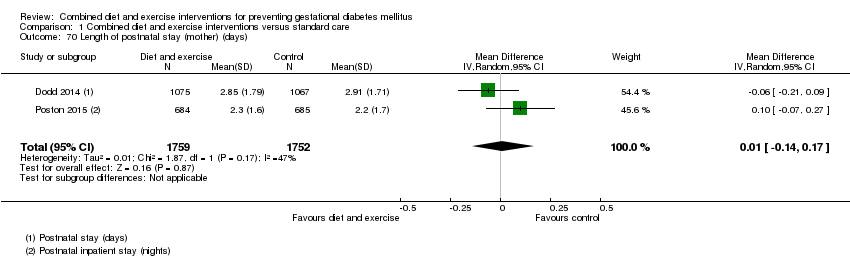
| 70 Length of postnatal stay (mother) (days) Show forest plot | 2 | 3511 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.14, 0.17] |
|
| 71 Length of postnatal stay (baby) (days) Show forest plot | 2 | 3618 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.90, 0.20] |
|
| 72 Costs to families associated with the management provided (unit cost, €) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 72.1 Delivery cost to the patient | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐10.82, 16.82] |
| 72.2 Neonatal care cost to the patient | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [‐13.67, 19.67] |
| 73 Costs associated with the intervention (unit cost, €) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 769.0 [‐1032.23, 2570.23] |
|
| 73.1 Total costs | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 769.0 [‐1032.23, 2570.23] |
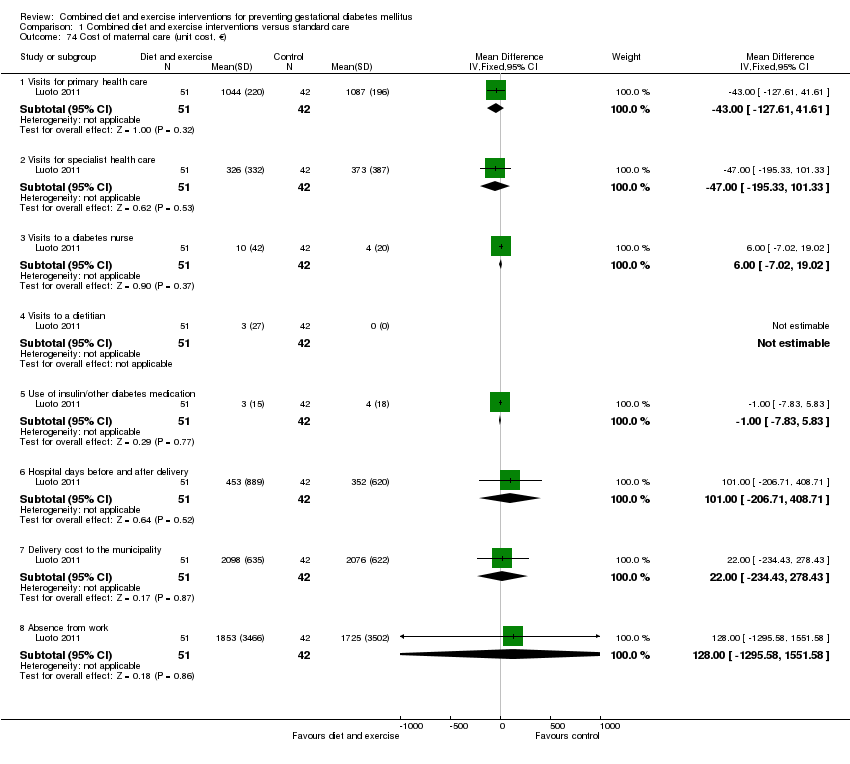
| 74 Cost of maternal care (unit cost, €) Show forest plot | 1 | | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
|
| 74.1 Visits for primary health care | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐43.0 [‐127.61, 41.61] |
| 74.2 Visits for specialist health care | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐47.0 [‐195.33, 101.33] |
| 74.3 Visits to a diabetes nurse | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐7.02, 19.02] |
| 74.4 Visits to a dietitian | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 74.5 Use of insulin/other diabetes medication | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.83, 5.83] |
| 74.6 Hospital days before and after delivery | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 101.00 [‐206.71, 408.71] |
| 74.7 Delivery cost to the municipality | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 22.0 [‐234.43, 278.43] |
| 74.8 Absence from work | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 128.0 [‐1295.58, 1551.58] |
| 75 Cost of infant care (unit cost, €) Show forest plot | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 453.0 [‐298.20, 1204.20] |
|
| 75.1 Neonatal care cost to municipality | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 453.0 [‐298.20, 1204.20] |