Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010341.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 01 December 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Oral Health Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Protocol
-
Background: Kelvin Chan (KC), Susan Furness (SF).
-
Methods: KC, SF, Anne‐Marie Glenny (AMG), Helen Worthington (HWo).
Review
-
Screening the search results and retrieving the papers: KC, SF, Helen Wakeford (HWa), Jo Weldon (JW).
-
Developing the background: KC, SF, HWa.
-
Data extraction and risk of bias assessment: SF, HWa, HWo, JW.
-
Developing the 'Methods' section: KC, SF, AMG, HWa, HWo, JW.
-
Analysing the data and interpreting the results: HWa, JW, AMG, HWo, SF, KC.
-
Writing the results, discussion, conclusions and abstract: HWa, KC, SF, JW.
Sources of support
Internal sources
-
School of Dentistry, The University of Manchester, UK.
-
Cochrane Oral Health Group, UK.
-
The University of Dundee, UK.
-
The University of Glasgow, UK.
-
MAHSC, UK.
The Cochrane Oral Health Group is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
External sources
-
National Institutes of Health, National Institute of Dental and Craniofacial Research, USA.
-
Central Manchester & Manchester Children's University Hospitals NHS Trust, UK.
-
Cochrane Oral Health Group Global Alliance, Other.
Through our Global Alliance (http://ohg.cochrane.org/partnerships‐alliances), the Cochrane Oral Health Group has received support from: British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
-
Kelvin Chan: none to declare.
-
Jo Weldon: none to declare. Jo is a salaried member of staff with the Cochrane Oral Health Group.
-
Anne‐Marie Glenny: none to declare. Anne‐Marie is Deputy Co‐ordinating Editor of the Cochrane Oral Health Group.
-
Susan Furness: none to declare. Susan is an Editor with the Cochrane Oral Health Group.
-
Helen Worthington: none to declare. Helen is Co‐ordinating Editor of the Cochrane Oral Health Group.
-
Helen Wakeford: none to declare. Helen is a salaried member of staff with the Cochrane Oral Health Group.
Acknowledgements
Our thanks go to the Cochrane Oral Health Group editorial team and external referees (Anirudha Agnihotry, Rosa Rojo and Andrew Sikora) for their help in conducting this systematic review.
Our thanks go to Toru Naito for assisting with the translation of Fujii 2013, and to Chunjie Li who translated several papers from Chinese and contacted Chinese authors on our behalf.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Dec 01 | Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy | Review | Kelvin KW Chan, Anne‐Marie Glenny, Jo C Weldon, Susan Furness, Helen V Worthington, Helen Wakeford | |
| 2013 Feb 28 | Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy | Protocol | Kelvin KW Chan, Anne‐Marie Glenny, Susan Furness, Helen V Worthington | |
Differences between protocol and review
In the protocol, we did not specify the different comparisons by which we would group the trials. In the review, we grouped the trials by intervention type. We decided that this was the most biologically and clinically logical way to organise these trials as each intervention type (mAb, TKIs, immunotherapy) has a differing mode of action.
We did not specify the creation of subgroups based on standard therapy type (CRT, RT) in the protocol. However, we decided to create these subgroups in the review, given that these different standard therapies may well have differing effects on the efficacy of the targeted therapies and immunotherapies and vice versa (Dittmann 2005; Nakata 2004).
As we had not previously determined how continuous data would be addressed, an amendment has been included under the 'Methods' section (Measures of treatment effect).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antibodies, Monoclonal, Humanized [therapeutic use];
- Antineoplastic Agents [*therapeutic use];
- Carcinoma, Squamous Cell [mortality, *therapy];
- Cetuximab [therapeutic use];
- Cisplatin [therapeutic use];
- Docetaxel;
- ErbB Receptors;
- Gefitinib;
- Immunotherapy [adverse effects, *methods];
- Lapatinib;
- Molecular Targeted Therapy [adverse effects, *methods];
- Mouth Neoplasms [mortality, *therapy];
- Oropharyngeal Neoplasms [mortality, *therapy];
- Protein Kinase Inhibitors [therapeutic use];
- Protein‐Tyrosine Kinases [antagonists & inhibitors];
- Quinazolines [therapeutic use];
- Randomized Controlled Trials as Topic;
- Taxoids [therapeutic use];
Medical Subject Headings Check Words
Humans;
PICOs
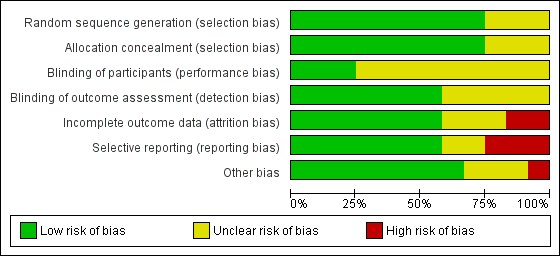
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Monoclonal antibodies (mAb), Outcome 1 Overall survival (5 years).

Comparison 1 Monoclonal antibodies (mAb), Outcome 2 Locoregional control.

Comparison 1 Monoclonal antibodies (mAb), Outcome 3 Progression‐free survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 1 Overall survival.

Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 2 Locoregional control.
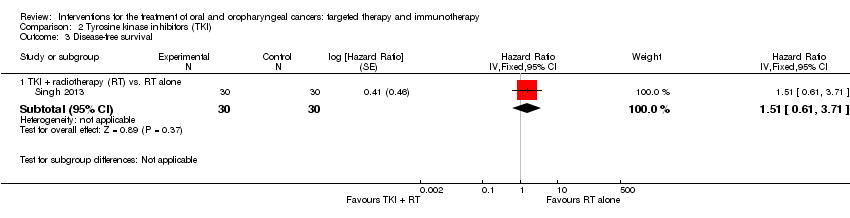
Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 3 Disease‐free survival.
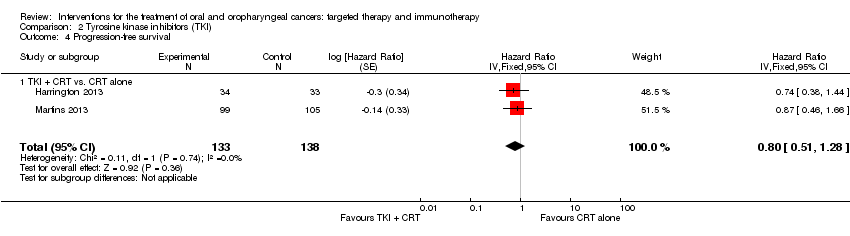
Comparison 2 Tyrosine kinase inhibitors (TKI), Outcome 4 Progression‐free survival.

Comparison 3 Immunotherapy, Outcome 1 Overall survival.

Comparison 3 Immunotherapy, Outcome 2 Disease‐free survival.
| Monoclonal antibodies plus standard therapy versus standard therapy alone for the treatment of people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: RT or CRT alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk mAb | |||||
| Overall survival | Low‐risk population* | HR 0.82 | 1421 | ⊕⊕⊕⊝ moderate1 | There was an 18% reduction in death (during the follow‐up period) in the participants treated with EGFR mAb therapies in addition to standard therapies | |
| 160 per 1000 | 133 per 1000 (113 to 156) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 347 per 1000 (301 to 396) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 577 per 1000 (515 to 639) | |||||
| Locoregional control | Low‐risk population* | HR 0.68 | 424 | ⊕⊕⊕⊝ | There was a 32% reduction in recurrence of cancer (during the follow‐up period) in the participants treated with EGFR mAb therapies (cetuximab) in addition to standard therapies | |
| 160 per 1000 | 112 per 1000 (87 to 144) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 297 per 1000 (237 to 370) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 510 per 1000 (421 to 607) | |||||
| Progression‐free survival | We formed 2 subgroups: mAb therapy + RT versus RT alone and mAb therapy + CRT versus CRT alone. There was a significant difference between these subgroups (P value = 0.008; I2 = 86%) and as a result we were unable to pool the data. The subgroup comparing mAb therapy + RT versus RT alone reported a 30% reduction in the number of people whose disease progresses if treated with EGFR mAb in addition to RT (HR 0.70; 95% CI 0.54 to 0.91; P value = 0.006). However, the subgroup comparing mAb therapy + CRT versus CRT alone reported no evidence of a difference in progression‐free survival (HR 1.08; 95% CI 0.89 to 1.32; P value = 0.76) | |||||
| Adverse effects | A subgroup estimate shows evidence of an increase in skin toxicity/acneiform rash (all grades of adverse effects: RR 6.56, 95% CI 5.35 to 8.03; 1311 participants, 2 studies; adverse effects grades ≥ 3: RR 17.72, 95% CI 8.33 to 37.73; 1403 participants, 3 studies) in people treated with cetuximab in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; EGFR: epidermal growth factor receptor; mAb: monoclonal antibody; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy. | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once for reporting bias (potential publication bias and poor reporting of EGFR and biopsy data in one study). | ||||||
| Tyrosine kinase inhibitors in addition to standard treatments for people with oral cavity and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (either RT or CRT alone) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk Tyrosine kinase inhibitors | |||||
| Overall survival | Low‐risk population* | HR 0.99 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in participant survival in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 159 per 1000 (102 to 239) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 402 per 1000 (275 to 557) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 646 per 1000 (478 to 808) | |||||
| Locoregional control | Low‐risk population* | HR 0.89 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in locoregional control in people treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 144 per 1000 (88 to 229) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 370 per 1000 (241 to 539) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 607 per 1000 (429 to 791) | |||||
| Disease‐free Survival | Low‐risk population* | HR 1.51 | 60 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants survived without signs or symptoms of oral cavity cancer when treated with tyrosine kinase inhibitors (gefitinib) in addition to standard therapies | |
| 160 per 1000 | 231 per 1000 (101 to 476) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 543 per 1000 (271 to 854) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 795 per 1000 (473 to 980) | |||||
| Progression‐free Survival | Low‐risk population* | HR 0.8 | 271 | ⊕⊝⊝⊝ | There was no evidence of a difference in the length of time that participants stayed alive with stable disease when treated with tyrosine kinase inhibitors in addition to standard therapies | |
| 160 per 1000 | 130 per 1000 (85 to 200) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 340 per 1000 (233 to 486) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 568 per 1000 (415 to 739) | |||||
| Adverse effects | A subgroup estimate showed evidence of an increase in gastrointestinal complaints (all grades of adverse effects: RR 15.53, 95% CI 2.18 to 110.55; 67 participants, 1 study) in people treated with lapatinib in addition to standard therapy | |||||
| CI: confidence interval; CRT: chemoradiotherapy; HR: hazard ratio; OIS: optimal information size; RR: risk ratio; RT: radiotherapy | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010). 1 Downgraded once due reporting bias (data from a large study (Gregoire 2011) was not available). 2 Downgraded once due to imprecision 3 Downgraded once due to unclear risk of bias across multiple domains. 4 Downgraded once for applicability ‐ only participants receiving RT as a standard therapy were included in this subgroup (no CRT). | ||||||
| Recombinant Interleukin (rIL‐2) in addition to surgery for the treatment of people with oral and oropharyngeal cancers | ||||||
| Patient or population: people with oral cavity or oropharyngeal cancers Comparison: standard therapy (surgery) alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk Standard therapy | Corresponding risk rIL‐2 | |||||
| Overall survival | Low‐risk population* | HR 0.52 (0.31 to 0.87) | 201 | ⊕⊝⊝⊝ | There was an 48% reduction in death in the groups treated with rIL‐2 in addition to standard therapy | |
| 160 per 1000 | 87 per 1000 | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 237 per 1000 (149 to 363) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 421 per 1000 (278 to 599) | |||||
| Disease‐free survival | Low‐risk population* | HR 0.66 | 201 | ⊕⊝⊝⊝ | There is no evidence of a difference in the length of time that participants survived without signs or symptoms of cancer when treated with rIL‐2 in addition to standard therapies | |
| 160 per 1000 | 109 per 1000 (72 to 163) | |||||
| Moderate‐risk population* | ||||||
| 405 per 1000 | 290 per 1000 (200 to 411) | |||||
| High‐risk population* | ||||||
| 650 per 1000 | 500 per 1000 (363 to 657) | |||||
| Adverse effects | There was no evidence of an increase or reduction of nausea/vomiting, stomatitis or leukopenia in participants treated with rIL‐2 in addition to standard therapy (1 study, 30 participants) | |||||
| CI: confidence interval; HR: hazard ratio; OIS: optimal information size | ||||||
| GRADE Working Group grades of evidence | ||||||
| * Assumed risk based on 5‐year survival data (Pulte 2010) 1 Downgraded twice due to high/unclear risk of bias across domains. | ||||||
| Inclusion criteria | Exclusion criteria | |
| Location of cancer | People with primary cancer of the oral cavity (ICD‐O: C01‐06) or oropharynx (ICD‐O: C09‐10 ‐ includes tonsil) | Lip (ICD‐O: C00) cancers |
| Type of cancer | Squamous cell carcinoma | Parotid gland (ICD‐O: C07), unspecified major salivary gland (ICD‐O: C08) |
| ICD‐O: International Classification of Diseases for Oncology. | ||
| ITT analysis | Docetaxel + cisplatin + cetuximab (n = 48) | Docetaxel + cisplatin (n = 44) |
| OS (3 years) | 88% (n = 42) | 74% (n = 33) |
| PFS (3 years) | 70% (n = 34) | 56% (n = 25) |
| ITT: intention to treat; n: number of participants; OS: overall survival; PFS: progression‐free survival. | ||
| Outcome | Grade | No. of studies | No. of patients | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 1.6 Mucositis | All grades | 3 | 1417 | Not estimable due to high heterogeneity (P value = 0.0009; I2 = 86%) between subgroups (cetuximab; nimotuzumab), and within the cetuximab subgroup (P value = 0.0002) I2 = 93%) | |
| Grades ≥ 3 | 4 | 1495 | 1.20 (0.96 to 1.51) (random effects) P value = 0.12 | P value = 0.07; I2 = 58% | |
| 1.7 Dysphagia | All grades | 3 | 1403 | 0.97 (0.92 to 1.03) (fixed effect) P value = 0.37 | P value = 0.60; I2 = 0% |
| Grades ≥ 3 | 3 | 1403 | 0.93 (0.83 to 1.04) (fixed effect) P value = 0.19 | P value = 0.26; I2 = 26% | |
| 1.8 Xerostomia | All grades | 3 | 1417 | 0.97 (0.91 to 1.04) (fixed effect) P value = 0.46 | P value = 0.61; I2 = 0% |
| Grades ≥ 3 | 2 | 1311 | 1.36 (0.80 to 2.31) (fixed effect) P value = 0.25 | P value = 0.60; I2 = 0% | |
| 1.9 Skin toxicity/ acneiform rash | All grades | 3 | 1403 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 99.3%) | |
| 2 (cetuximab) | 1311 | 6.56 (5.35 to 8.03) (fixed effect) P value < 0.00001 | P value = 0.080; I2 = 66% | ||
| 1 (nimotuzumab) | 92 | 1.06 (0.85 to 1.31) P value = 0.61 | Not applicable | ||
| Grades ≥ 3 | 4 | 1495 | Not estimable due to significant difference between subgroups (P value = 0.005; I2 = 87.5%) | ||
| 3 (cetuximab) | 1403 | 17.72 (8.33 to 37.73) (fixed effect) P value < 0.00001 | P value = 0.56; I2 = 0% | ||
| 1 (nimotuzumab) | 92 | 0.20 (0.01 to 4.05) P value = 0.29 | Not applicable | ||
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 2.5 Mucositis | All grades | 2 | 286 | 1.03 (0.94 to 1.11) (fixed effect) P value = 0.55 | P value = 0.44; I2 = 0% |
| Grades ≥ 3 | 2 | 286 | 1.22 (0.96 to 1.56) (fixed effect) P value = 0.10 | P value = 0.53; I2 = 0% | |
| 2.6 Skin toxicity | All grades | 4 | 544 | Not estimable due to significant difference between subgroups (P value < 0.00001; I2 = 95.5%) | |
| 2 (gefitinib) | 286 | 1.03 (0.82 to 1.28) (fixed effect) P value = 0.82 | P value = 0.42; I2 = 0% | ||
| 1 (lapatinib) | 67 | 2.02 (1.23 to 3.32) P value = 0.005 | Not applicable | ||
| 1 (erlotinib) | 191 | 6.57 (3.60 to 12.00) P value < 0.00001 | Not applicable | ||
| Grades ≥ 3 | 4 | 544 | 1.25 (0.54 to 2.88) (random effects) P value = 0.61 | P value = 0.09; I2 = 54% | |
| 2.7 Gastrointestinal | All grades | 2 | 293 | Not estimable due to significant difference between subgroups (P value = 0.007; I2 = 86.2%) | |
| 1 (gefitinib) | 226 | 1.04 (0.98 to 1.11) P value = 0.18 | Not applicable | ||
| 1 (lapatinib) | 67 | 15.53 (2.18 to 110.55) P value = 0.006 | Not applicable | ||
| Grades ≥ 3 | 3 | 484 | 1.11 (0.83 to 1.49) (fixed effect) P value = 0.47 | P value = 0.53; I2 = 0% | |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome | BCG‐cell wall preparation + surgery | Surgery alone |
| Overall survival (3 years) | 10/10 | 9/10 |
| Recurrence (3 years) | 3/10 | 4/10 |
| BCG: Bacillus Calmette‐Guérin. | ||
| Outcome | Neoadjuvant chemotherapy (n = 17) | Neoadjuvant chemotherapy + rIL‐2 (n = 16) |
| Locoregional control Complete response | 3/15 | 4/13 |
| Locoregional control Partial response | 9/15 | 6/13 |
| rIL‐2: recombinant interleukin. | ||
| Outcome | Grade | No. of studies | No. of participants | Risk ratio (MH, 95% CI, P value) | Heterogeneity (P value; I2) |
| 3.3 Nausea/vomiting | All grades | 1 | 30 | 1.24 (0.90 to 1.70) P value = 0.19 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 3.40 (0.15 to 77.34) P value = 0.44 | Not applicable | |
| 3.4 Stomatitis | All grades | 1 | 30 | 1.47 (0.75 to 2.90) P value = 0.27 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 1.71 (0.33 to 8.83) P value = 0.52 | Not applicable | |
| 3.5 Leukopenia | All grades | 1 | 30 | 0.57 (0.22 to 1.50) P value = 0.25 | Not applicable |
| Grades ≥ 3 | 1 | 30 | 0.38 (0.04 to 3.26) P value = 0.38 | Not applicable | |
| 3.6 Increased temperature | All grades | 1 | 24 | 0.67 (0.13 to 3.30) P value = 0.62 | Not applicable |
| 3.7 Moderate‐severe chills | All grades | 1 | 24 | 11.00 (0.67 to 179.29) P value = 0.09 | Not applicable |
| 3.8 Gastrointestinal | All grades | 1 | 24 | 5.00 (0.27 to 94.34) P value = 0.28 | Not applicable |
| CI: confidence interval; MH: Mantel‐Haenszel. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival (5 years) Show forest plot | 3 | 1421 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 1.1 mAb therapy + radiotherapy (RT) vs. RT alone | 2 | 530 | Hazard Ratio (Fixed, 95% CI) | 0.73 [0.58, 0.91] |
| 1.2 mAb therapy + chemoradiotherapy (CRT) vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.74, 1.23] |
| 2 Locoregional control Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.68 [0.52, 0.89] |
| 3 Progression‐free survival Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 mAb therapy + RT vs. RT alone | 1 | 424 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 3.2 mAb therapy + CRT vs. CRT alone | 1 | 891 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.89, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 1.1 TKI + chemoradiotherapy (CRT) vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.62, 1.57] |
| 2 Locoregional control Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 2.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 3 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 TKI + radiotherapy (RT) vs. RT alone | 1 | 60 | Hazard Ratio (Fixed, 95% CI) | 1.51 [0.61, 3.71] |
| 4 Progression‐free survival Show forest plot | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| 4.1 TKI + CRT vs. CRT alone | 2 | 271 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.51, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Surgery ± radiotherapy (RT) + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.52 [0.31, 0.87] |
| 2 Disease‐free survival Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Surgery ± RT + rIL‐2 vs. surgery ± RT alone | 1 | 201 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.43, 1.02] |
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Ang 2014 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) | Acute period : ≤ 90 days from start of RT (Gp A n = 444; Gp B n = 447) | ||
| Any event | All grades: n = 431 (97%); | All grades: n = 434 (97%); | |
| Dysphagia | All grades: n = 364 (82%); | All grades: n = 384 (86%); | |
| Mucositis | All grades: n = 364 (82%); | All grades: n = 322 (72%); | |
| Skin reaction outside portal (pruritus; dermatitis exfoliative NOS; acne NOS; nail disorder NOS) | All grades: n = 364 (82%); | All grades: n = 63 (14%); | |
| Skin reaction inside portal (radiation dermatitis NOS; radiation recall syndrome) | All grades: n = 346 (78%); | All grades: n = 353 (79%); | |
| Fatigue | All grades: n = 289 (65%); | All grades: n = 268 (60%); | |
| Late period : > 90 days from start of RT (Gp A n = 415; Gp B n = 432) | |||
| Any event | All grades: n = 403 (97%); Grades 3‐4: n = 249 (60%) | All grades: n = 419 (97%); Grades 3‐4: n = 233 (54%) | |
| Dysphagia | All grades: n = 357 (86%); Grades 3‐4: n = 154 (37%) | All grades: n = 359 (83%); Grades 3‐4: n = 156 (36%) | |
| Dry mouth | All grades: n = 311 (75%); Grades 3‐4: n = 21 (5%) | All grades: n = 324 (75%); Grades 3‐4: n = 17 (4%) | |
| Skin fibrosis | All grades: n = 311 (46%); Grades 3‐4: n = 8 (2%) | All grades: n = 190 (44%); Grades 3‐4: n = 4 (1%) | |
| Fatigue | All grades: n = 170 (41%); Grades 3‐4: n = 12 (3%) | All grades: n = 194 (45%); Grades 3‐4: n = 13 (3%) | |
| Laryngeal oedema | All grades: n = 166 (40%); Grades 3‐4: n = 17 (4%) | All grades: n = 181 (42%); Grades 3‐4: n = 13 (3%) | |
| Bonner 2006 Comparison: mAb RT + cetuximab vs. RT (no placebo) (Gp A n = 208; Gp B n = 212) | Mucositis | All grades: n = 193 (93%); Grades 3‐5: n = 116 (56%) | All grades: n = 199 (94%); Grades 3‐5: n = 110 (52%) |
| Acneiform rash | All grades: n = 181 (87%); Grades 3‐5: n = 35 (17%) | All grades: n = 21 (10%); Grades 3‐5: n = 2 (1%) | |
| Radiation dermatitis | All grades: n = 179 (86%); Grades 3‐5: n = 48 (23%) | All grades: n = 191 (90%); Grades 3‐5: n = 38 (18%) | |
| Weight loss | All grades: n = 175 (84%); Grades 3‐5: n = 23 (11%) | All grades: n = 153 (72%); Grades 3‐5: n = 15 (7%) | |
| Xerostomia | All grades: n = 150 (72%); Grades 3‐5: n = 10 (5%) | All grades: n = 151 (71%); Grades 3‐5: n = 6 (3%) | |
| Dysphagia | All grades: n = 135 (65%); Grades 3‐5: n = 54 (26%) | All grades: n = 134 (63%); Grades 3‐5: n = 64 (30%) | |
| Koh 2013 Comparison: mAb (CRT + cetuximab vs. CRT (no placebo)) Gp A n = 48; Gp B n = 44 | Partial narrative reporting for Gp A toxicities relating to attrition rationale in abstract: "Reason for incompletion in CDP arm included hypersensitivity (1), septic shock (1), skin rash (1), seizure (1), arterial thrombosis (1), unexplained death (1), unsatisfactory response (1), and withdrawal of informed consent (1)". No detail reported for Gp B. However, poster presented alongside abstract indicates similar frequency of Grade 3‐4 toxicities in both Gps | ||
| Neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 13 (27%) Grades 3‐4: n = 5 (10%) | Grades 3‐4: n = 5 (11%) Grades 3‐4: n = 4 (9%) | |
| Anorexia CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 6 (13%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 4 (9%) | |
| Mucositis CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 9 (19%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 3 (7%) | |
| Febrile neutropenia CRT induction Concurrent CRT | Grades 3‐4: n = 7 (%) Grades 3‐4: n = 3 (6%) | Grades 3‐4: n = 4 (9%) Grades 3‐4: n = 0 (0%) | |
| Skin toxicity CRT induction Concurrent CRT | Grades 3‐4: n = 3 (6%) Grades 3‐4: n = 4 (8%) | Grades 3‐4: n = 0 (0%) Grades 3‐4: n = 1 (2%) | |
| Diarrhoea CRT induction Concurrent CRT | Grades 3‐4: n = 4 (8%) Grades 3‐4: n = 0 (0%) | Grades 3‐4: n = 3 (7%) Grades 3‐4: n = 0 (0%) | |
| Reddy 2014 Comparison: mAb CRT + nimotuzumab vs. CRT vs. RT + nimotuzumab vs. RT (Gp A n = 23; Gp B n = 23; Gp C n = 23; Gp D n = 23) **Note: control arm mucositis events Gp D n = 27 (+4 than Gp n), therefore, all grade mucositis data not used in analysis | Mucositis | All grades: n = 44 (96%); Grade 3: n = 21 (46%) | All grades: n = 49 (106%)** ; Grade 3: n = 21 (46%) |
| Skin reaction | All grades: n = 37 (80%); Grade 3: n = 0 (0%) | All grades: n = 35 (76%); Grade 3: n = 2 (4%) | |
| Nausea/vomiting | All grades: n = 34 (74%); Grade 3: n = 1 (2%) | All grades: n = 30 (65%); Grade 3: n = 0 (0%) | |
| Salivary gland disorder | All grades: n = 27 (59%); Grade 3: n = 0 (0%) | All grades: n = 29 (63%); Grade 3: n = 2 (4%) | |
| Dysphagia | All grades: n = 16 (35%); Grade 3: n = 5 (%) | All grades: n = 16 (35%); Grade 3: n = 1 (2%) | |
| Candidiasis | All grades: n = 13 (28%); Grade 3: n = 5 (11%) | All grades: n = 19 (41%); Grade 3: n = 7 (15%) | |
| Rodriguez 2010 Comparison: mAb RT + nimotuzumab vs. RT + placebo (Gp A n = 54; Gp B n = 52) | Any adverse event | n = 38 (70%) | n = 30 (58%) |
| Mucositis | n = 11 (20%) | n = 9 (17%) | |
| Dry mouth | n = 9 (17%) | n = 12 (23%) | |
| Dry radio‐dermatitis | n = 6 (11%) | n = 6 (12%) | |
| Odynophagia | n = 4 (7%) | n = 6 (12%) | |
| CRT: chemoradiotherapy; Gp: group; mAb: monoclonal antibody; NOS: not otherwise specify; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| De Stefani 2002 Comparison: interleukin rIL‐2 + RT + surgery vs. RT + surgery (no placebo) (Gp A n = 100; Gp B n = 101) | Narrative commentary only: "Complication and toxicity rates for the surgery arm and the surgery plus radiotherapy arm were the same in the control group and in the rIL‐2 group. Postoperative radiotherapy originates well‐known side effects, but they were independent of the preoperative rIL‐2 treatment. [...] Neoadjuvant rIL‐2 injections did not complicate the surgical treatment, and adjuvant rIL‐2 injections did not increase distant side effects due to previous surgery or radiotherapy" | ||
| Mantovani 1998 Comparison: interleukin rIL‐2 + CRT ± surgery vs. CRT ± surgery (no placebo) (Gp A n = 14; Gp B n = 16) | Nausea/vomiting | All grades: n = 13 (93%); Grades 3‐4: n = 1 (7%) | All grades: n = 12 (75%); Grades 3‐4: n = 0 (0%) |
| Stomatitis | All grades: n = 9 (64%); Grades 3‐4: n = 3 (21%) | All grades: n = 7 (44%); Grades 3‐4: n = 2 (13%) | |
| Leukopenia | All grades: n = 4 (29%); Grades 3‐4: n = 1 (7%) | All grades: n = 8 (50%); Grades 3‐4: n = 3 (19%) | |
| Fever | All grades: n = 6 (43%); Grades 3‐4: n = 0 (0%) | All grades: n = 0 (0%); Grades 3‐4: n = 0 (0%) | |
| Diarrhoea | All grades: n = 2 (14%); Grades 3‐4: n = 1 (7%) | All grades: n = 3 (19%); Grades 3‐4: n = 1 (6%) | |
| Anaemia | All grades: n = 3 (21%); Grades 3‐4: n = 1 (7%) | All grades: n = 2 (13%); Grades 3‐4: n = 0 (0%) | |
| Bier 1981 Comparison: BCG‐CWP + surgery vs. surgery (no placebo) (Gp A n = 12; Gp B n = 12) | Increased temperature | n = 2 (17%) | n = 3 (25%) |
| Moderate‐severe chills | n = 5 (42%) | n = 0 (0%) | |
| Gastrointestinal complaints (including nausea/vomiting) | n = 2 (17%) | n = 0 (0%) | |
| BCG‐CWP: Bacillus Calmette‐Guérin ‐ cell wall preparation; Gp: group; n: number of participants; rIL‐2: recombinant interleukin; RT: radiotherapy. | |||
| Study ID | Specified adverse effect | Intervention arm | Control arm |
| Gregoire 2011 Comparison: TKI (CRT + gefitinib (250/500 mg) vs. Gp B n = 116 (Gps A+F+G)) | Mucositis | All grades: n = 96 (87%); Grades 3‐5: n = 51 (%) | All grades: n = 98 (84%); Grades 3‐5: n = 42 (36%) |
| Nausea | All grades: n = 50 (45%); Grades 3‐5: n = 4 (4%) | All grades: n = 55 (47%); Grades 3‐5: n = 3 (3%) | |
| Vomiting | All grades: n = 56 (51%); Grades 3‐5: n = 6 (5%) | All grades: n = 52 (45%); Grades 3‐5: n = 9 (8%) | |
| Dysphagia | All grades: n = 29 (26%); Grades 3‐5: n = 5 (5%) | All grades: n = 43 (37%); Grades 3‐5: n = 13 (11%) | |
| Dry mouth (xerostomia) | All grades: n = 33 (30%); | All grades: n = 30 (26%); Grades 3‐5: n = 2 (2%) | |
| Radiation skin injury | All grades: n = 29 (26%); Grades 3‐4: n = 2 (2%) | All grades: n = 29 (25%); Grades 3‐5: n = 4 (3%) | |
| Harrington 2013 Comparison: TKI (CRT + lapatinib vs. CRT + placebo) Gp A n = 34; Gp B n = 33 | Diarrhoea | All grades: n = 16 (46%); Grade 3: n = 2 (6%) | All grades: n = 1 (3%); Grade 3: n = 0 (0%) |
| Rash | All grades: n = 10 (29%); Grade 3: n = 3 (9%) | All grades: n = 5 (16%); Grade 3: n = 1 (3%) | |
| Other skin reactions | All grades: n = 15 (43%); Grade 3: n = 2 (6%) | All grades: n = 7 (23%); Grade 3: n = 5 (16%) | |
| Martins 2013 (Gp A n = 95; Gp B n = 96) | Pain | All grades: n = 50 (53%); Grades 3‐4: n = 18 (19%) | All grades: n = 54 (56%); Grades 3‐4: n = 18 (19%) |
| Gastrointestinal | Grades 3‐4: n = 46 (48%) | Grades 3‐4: n = 41 (43%) | |
| Rash | All grades: n = 65 (68%); Grade 3: n = 12 (13%) | All grades: n = 10 (10%); Grade 3: n = 2 (2%) | |
| Serious adverse events* | n = 38 (40%) | n = 32 (33%) | |
| Haematological | Grades 3‐4: n = 15 (16%) | Grades 3‐4: n = 25 (26%) | |
| Metabolic | Grades 3‐4: n = 7 (7%) | Grades 3‐4: n = 5 (5%) | |
| Singh 2013 Comparison TKI RT + gefitinib vs. RT (no placebo) (Gp A n = 30; Gp B n = 30) | Mucositis | All grades: n = 30 (100%); Grades 3‐4: n = 21 (70%) | All grades: n = 30 (100%); Grades 3‐4: n = 19 (63%) |
| Skin reaction | All grades: n = 30 (100%); Grades 3‐4: n = 12 (40%) | All grades: n = 30 (100%); Grades 3‐4: n = 11 (36%) | |
| CRT: chemoradiotherapy; Gp: group; n: number of participants; TKI: tyrosine kinase inhibitor. | |||


