Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010318.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 17 October 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Wounds Group
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Jo Dumville: took the lead in writing this review . Performed independent screening, data extraction and risk of bias assessment of included trials. Responded to the peer referee feedback. Approved the final version of the review.
Robert Hinchliffe: Performed independent screening, data extraction and risk of bias assessment of included trials. Responded to the peer referee feedback. Approved the final version of the review.
Nicky Cullum: edited the review, made an intellectual contribution and approved the final version of the review.
Fran Game: edited the review, made an intellectual contribution and approved the final version of the review.
Nikki Stubbs: edited the review, made an intellectual contribution and approved the final version of the review.
Michael Sweeting: undertook analysis for the review; edited the review, made an intellectual contribution and approved the final version of the review.
Frank Peinemann: edited the review, made an intellectual contribution and approved the final version of the review.
Contributions of editorial base:
Joan Webster, Editor: edited the review and approved the final version for submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the protocol and the review.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Rachel Richardson: edited the review.
Sources of support
Internal sources
-
Department of Health Sciences, University of York, UK.
External sources
-
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
-
NIHR Programme Grants for Applied Research, UK.
Declarations of interest
Nicky Cullum and Jo Dumville receive funding from the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme. This study presents independent research funded by the NIHR under its Programme Grants for Applied Research funding scheme (RP‐PG‐0407‐10428). The views expressed in this review are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Nicky Cullum is an NIHR Senior Investigator.
Nicky Cullum declared that Kinetic Concepts Inc (KCI) supplied (free of charge) three VAC therapy units and starter packs for use in a pilot RCT of negative pressure wound therapy for pressure ulcers. They also provided product training, support and access to the KCI 24‐hour advice service for clinical and technical queries. However KCI had no input into the design, conduct, analysis or reporting of that research or this review (which concerns the same technology but in a completely different patient group).
Nikki Stubbs has received funding from pharmaceutical companies to support training and education events in the UK National Health Service. In addition she has received payments for non product‐related educational sessions that are unrelated to the subject matter of this systematic review, and which have not involved product promotion.
Acknowledgements
The authors would like to thank the following people who reviewed the review for clarity, readability and rigour: Wounds Group editors (Andrew Jull; Gill Worthy), peer referees (Rachel Richardson and Janet Gunderson), Managing Editor (Sally Bell Syer) and Trial Search Coordinator (Ruth Foxlee) and Elizabeth Royle who did the copy‐editing for both the protocol and review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Oct 17 | Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus | Review | Zhenmi Liu, Jo C Dumville, Robert J Hinchliffe, Nicky Cullum, Fran Game, Nikki Stubbs, Michael Sweeting, Frank Peinemann | |
| 2013 Oct 17 | Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus | Review | Jo C Dumville, Robert J Hinchliffe, Nicky Cullum, Fran Game, Nikki Stubbs, Michael Sweeting, Frank Peinemann | |
| 2013 Jan 31 | Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus | Protocol | Jo C Dumville, Robert J Hinchliffe, Nicky Cullum, Fran Game, Nikki Stubbs, Michael Sweeting | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
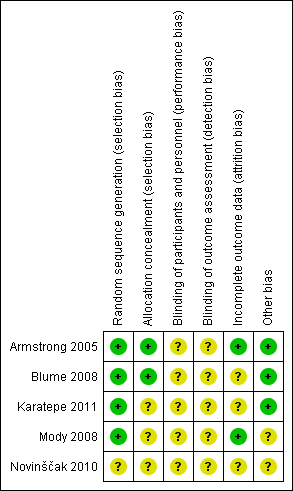
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 NPWT compared with moist (non‐gauze) wound dressings, Outcome 1 Proportion of wounds healed.
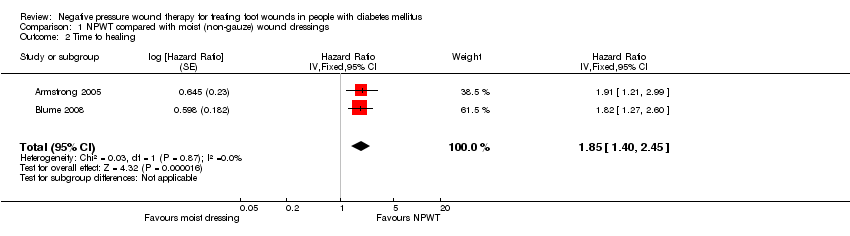
Comparison 1 NPWT compared with moist (non‐gauze) wound dressings, Outcome 2 Time to healing.
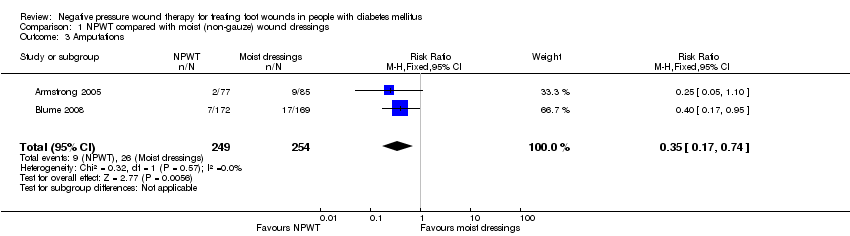
Comparison 1 NPWT compared with moist (non‐gauze) wound dressings, Outcome 3 Amputations.

Comparison 1 NPWT compared with moist (non‐gauze) wound dressings, Outcome 4 Adverse events.
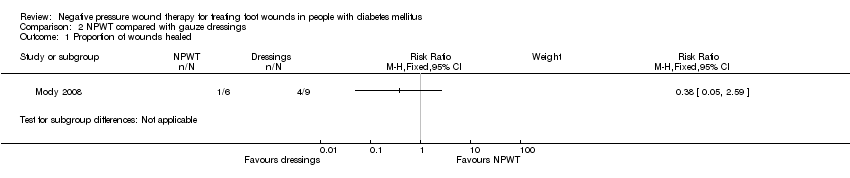
Comparison 2 NPWT compared with gauze dressings, Outcome 1 Proportion of wounds healed.
| NPWT compared to Moist dressings for healing post‐operative wounds in people with diabetes | ||||||
| Patient or population: patients with healing post‐operative wounds in people with diabetes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Moist dressings | NPWT | |||||
| Proportion of wounds healed | Study population | RR 1.44 | 162 | ⊕⊕⊝⊝ | ||
| 388 per 1000 | 559 per 1000 | |||||
| Moderate | ||||||
| Time to ulcer healing | Study population | HR 1.91 | 162 | ⊕⊕⊝⊝ | ||
| 388 per 1000 | 609 per 1000 | |||||
| Moderate | ||||||
| Amputation | Study population | RR 0.25 | 162 | ⊕⊝⊝⊝ | ||
| 106 per 1000 | 26 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 There was the potential for performance bias as unblinded health professionals were able to make decisions about undertaking closure surgery that could then have resulted more wounds being closed (and classed as healed) or amputated in one group compared with the other. | ||||||
| NPWT compared to Moist dressings for debrided foot ulcers in people with diabetes | ||||||
| Patient or population: patients with debrided foot ulcers in people with diabetes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Moist dressings | NPWT | |||||
| Proportion of wounds healed | Low risk of healing1 | RR 1.49 | 341 | ⊕⊕⊝⊝ | ||
| 340 per 1000 | 507 per 1000 | |||||
| Moderate risk of healing1 | ||||||
| 530 per 1000 | 790 per 1000 | |||||
| High risk of healing1 | ||||||
| 650 per 1000 | 968 per 1000 | |||||
| Time to healing | Low risk of healing4 | HR 1.82 | 341 | ⊕⊕⊝⊝ | ||
| 340 per 1000 | 531 per 1000 | |||||
| Moderate risk of healing4 | ||||||
| 530 per 1000 | 747 per 1000 | |||||
| High risk of healing4 | ||||||
| 650 per 1000 | 852 per 1000 | |||||
| Amputation | Study population | RR 0.40 | 341 | ⊕⊕⊝⊝ | ||
| 101 per 1000 | 40 per 1000 | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. | ||||||
| NPWT compared to Gauze dressings for debrided foot ulcers in people with diabetes | ||||||
| Patient or population: patients with debrided foot ulcers in people with diabetes | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Gauze dressings | NPWT | |||||
| Proportion of wounds healed | Low risk of healing1 | RR 0.38 | 15 | ⊕⊝⊝⊝ | ||
| 340 per 1000 | 129 per 1000 | |||||
| Moderate risk of healing1 | ||||||
| 530 per 1000 | 201 per 1000 | |||||
| High risk of healing1 | ||||||
| 650 per 1000 | 247 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Baseline risk of healing obtained from external source in which data from 27,630 patients with a diabetic neuropathic foot ulcer was used to develop a simple prognostic model to predict likelihood of ulcer healing (Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: predicting which ones will not heal. Am J Med. 2003;115:627‐31). It is important to note that given an outcome of ulcer healing, low risk refers to a low risk of healing and thus reflects the most severe patient populations. Conversely high risk refers to a high risk of healing. | ||||||
| Armstrong 2005 | 16 weeks | Diabetic foot amputation to trans‐metatarsal level | Group A: moist wound therapy with alginates, hydrocolloid, foam or hydrogel dressings (n = 85) Group B: NPWT (VAC system, dressing changes every 48 h. Treatment conducted until wound closure or completion of 112‐day assessment (n = 77) | Number of wounds completely healed Group A: 33/85 (38.8%) Group B: 43/77 (55.8%) Of healed wounds —healed by secondary intention (without primary/surgical wound closure) Group A: 25/33 (75.8%) Group B: 31/43 (72.1%) Remaining wounds were closed following surgery. Time to wound healing median time to healing Group A: 77 days (IQR 40 to 122) Group B: 56 days (IQR 26 to 92) Log rank = p = 0.005 Amputation Number of participants undergoing further amputation Group A: 9/85 (10.6%) Major = 5/Minor = 4 Group B: 2/77 (2.3%) Major = 0/Minor = 2 There was no difference noted in time to healing for acute or chronic wounds. | Adverse events Participants who had one or more adverse events Group A: 46/85 (54.1%) Group B: 40/77 (51.9%) Participants who had one or more treatment‐related adverse events Group A: 11/85 (12.9%) 5 classified as serious Group B: 9/77 (11.7%) 1 classified serious Resource use Average total cost per participant Group A: USD 36,887 Group B: USD26,972 Average total direct cost per participants for those treated for 8 weeks or longer Group A: USD 36,096 Group B: USD 27,270 Average per participant cost to achieve 100% healing Group A: USD 38,806 Group B: USD 25,954 |
| Blume 2008 | 16 weeks | Ulceration of the foot in people with diabetes | Group A: advanced moist wound therapy dressings used according to guidelines/local protocols (n = 169) Group B: NPWT (VAC system), applied according to manufacturer’s instructions. (n = 172) | Number of wounds completely healed (six participants excluded in paper as did not receive treatment, added back into denominator here) Group A: 48/169 (28.4%) Group B: 73/172 (42.4%) Proportion of wounds closed using surgery (unclear if considered part of healed group) Group A: 14/169 (8.3%) Group B: 16/172 (9.3%) Time to wound healing median time to healing Group A: could not be estimated Group B: 96 days (95% CI 75.0 to 114.0) Log rank taken as P value 0.001 Amputation Number of participants undergoing amputation* Group A: 17/169 (10.1%) Major = 4; minor = 13 Group B: 7/172 (4.1%) Major = 5; minor = 2 | Adverse events Limited data: not extracted Resource use – taken from conference abstract that we think is related to this main publication. Mean estimated total costs of inpatient services per participant Group A: USD 8570 (95%CI USD 5922 to USD 11,432) Group B: USD 5206 (95%CI USD 3172 to USD 7561) |
| Karatepe 2011 | Not specified. Last assessment one month after healing | Diabetic foot ulcers | Group A: conventional wound care treatment: based on text in report taken to be dry gauze (n = 37) Group B: NPWT (VAC system) (n = 30) | Time to healing Median time to healing Group A: 4.4 weeks Group B: 3.9 weeks Mean value presented but not extracted. No specific P value presented | Health‐related quality of life SF‐36: Data not presented. |
| Mody 2008 | Not specified: until healing or loss to follow‐up | Diabetic foot ulcers | Group A: wet‐to‐dry gauze (n = 9) Group B: locally‐constructed NPWT (n = 6) | Number of wounds completely healed By secondary intention: Group A: 1/9 (11.0%) Group B: 1/6 (16.6%) By delayed primary closure: Group A: 3/9 (33%) Group B: 0/6 (0%) | |
| Novinščak 2010 | 2 months | Complicated diabetic foot ulcers | Group A: classic gauze (n = 8) Group B: dressings (moist) (n = 12) | Healing rate (percentage with wound closure – defined by author on contact) Group A: 4/8* (50%) Group B: 9/12* (75%) Group C: * could not be calculated (90%) *Figure calculated by review author as only proportions obtained from study author |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.18, 1.84] |
| 2 Time to healing Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | 1.85 [1.40, 2.45] | |
| 3 Amputations Show forest plot | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.17, 0.74] |
| 4 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 All adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Treatment‐related adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |