正在接受物质使用障碍治疗或已经康复人群的戒烟干预措施
摘要
研究背景
酒精和其他药物成瘾者的吸烟率是普通人群的两到四倍。由于担心这些干预措施在该群体中不成功,或者如果戒烟与其他药物依赖治疗相结合,可能会影响从其他成瘾中恢复,因此对烟草依赖的同步治疗受到限制。
研究目的
评估戒烟干预措施是否与同时接受酒精和其他药物依赖治疗或正在康复的人的戒烟有关。
检索策略
我们检索了Cochrane烟草成瘾组专业注册库(Cochrane Tobacco Addiction Group Specialised Register)、Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL)、MEDLINE和美国临床试验注册中心(clinicaltrials.gov)数据库,最新检索日期为2016年8月。对来自尼古丁研究与治疗协会(Society on Nicotine Research and Treatment)和ProQuest数字论文数据库的会议摘要进行灰色文献检索,得出了一项额外的研究,但该研究被排除。
纳入排除标准
我们纳入了评价戒烟干预措施的随机对照试验,这些试验同时接受酒精或其他药物依赖治疗或门诊康复计划。
资料收集与分析
两名综述作者独立评价偏倚风险并提取资料。采用共识方法解决分歧。主要结局是在最长的随访期内戒烟,次要结局是戒酒或戒其他药物,或两者都戒。我们报告了最严格的戒断定义。我们将效果总结为风险比(risk ratios)和95%置信区间(confidence intervals,CI)。两项整群研究未提供组内相关系数,因此被排除在敏感性分析之外。我们使用I 2 统计值来评价异质性。
主要结果
三十五项随机对照试验符合纳入本综述的标准,其中一项正在进行,共涉及5796名受试者。纳入的研究评价了戒烟干预措施的疗效,包括咨询和包含尼古丁替代疗法(nicotine replacement therapy,NRT)或非NRT的药物治疗,或两者结合,在住院和门诊环境中对治疗和康复受试者进行评价。大多数研究没有报告有关评价分配、选择和失访偏倚风险的信息,因此被归类为风险不明确。
分析考虑了干预的性质、受试者是在治疗中还是在康复中,以及依赖的类型。在meta分析纳入的34项研究中,以常规护理或无干预作为对照组,11项评价了咨询,11项评价了药物治疗,12项评价了咨询联合药物治疗。对于两种类型的干预措施,干预措施与戒烟显著相关。在最长随访期间(从六周到18个月不等),药物治疗似乎增加了戒烟率(RR=1.88,95%CI [1.35,2.57],11项研究,1808名受试者,低质量证据),咨询联合药物治疗也是如此(RR=1.74,95%CI [1.39,2.18],12项研究,2229名受试者, 低质量证据)。存在异质性证据为中等质量(药物治疗I 2 =56% ,咨询联合药物治疗I 2 =43%)。咨询干预并未显著增加戒烟率(RR=1.33,95%CI [0.90,1.95])。
对于接受治疗的人(RR=1.99,95%CI [1.59,2.50])、恢复期的人(RR=1.33,95%CI [1.06,1.67])、酒精依赖者(RR=1.47,95%CI [1.20,1.81]),和有其他药物依赖性的人(RR=1.85,95%CI [1.43,2.40])干预措施与戒烟显著相关。
向正在接受其他药物依赖治疗或康复的人提供戒烟疗法与酒精和其他药物戒断率的差异无关(RR=0.97,95%CI [0.91,1.03],11项研究,2231名受试者,异质性证据为中等质量(I 2 = 66%)。
有关干预措施不良影响的资料有限。
作者结论
本综述纳入的研究表明,在酒精和其他药物依赖的治疗和康复过程中,针对吸烟者提供戒烟干预措施可以提高戒烟率。没有证据表明提供戒烟干预措施会影响戒酒和戒毒。戒烟干预和戒烟之间的关联对于药物疗法和咨询联合药物疗法、对于治疗和康复的受试者,以及对于酒精依赖或其他药物依赖的人来说都是一致的。干预措施的证据质量低,主要是因为对治疗性质的偏倚风险和临床异质性的报告不完整。某些结果对随访时间长短或药物治疗类型敏感,这表明需要进一步研究戒烟干预是否与恢复期人群戒烟相关,以及NRT对比非NRT或联合药物治疗相关的结局。总体而言,结果表明应将联合药物疗法的戒烟干预措施纳入临床实践,以减少正在接受酒精和其他药物依赖治疗或正在康复的人群烟草成瘾。
简语概要
在药物滥用治疗或康复期间提供的戒烟干预措施是否有助于吸烟者戒烟?
综述背景
使用烟草是世界范围内主要的可预防死因,在依赖酒精或其他药物的人群中吸烟率尤其高。正在接受酒精或其他药物成瘾治疗的人通常不会同时接受帮助他们戒烟的治疗。有人担心,尝试戒烟可能会减少接受治疗的人其他成瘾中恢复过来的可能性。
研究特征
我们寻找纳入了在医院、门诊或社区诊所中接受药物滥用治疗或已经完成药物滥用治疗的成年吸烟者,并将他们随机分配到帮助戒烟的治疗组或对照组的研究。我们检索证据的时间截止到2016年8月。我们确定了34项已发表的研究。测试的戒烟治疗类型包括:咨询(一次简短的建议谈话或有个人或群体行为支持的多次谈话);药物(称为药物疗法;包括任何类型的尼古丁替代疗法,有或没有其他帮助吸烟者戒烟的药物辅助);或咨询联合药物治疗。尽管不同的试验使用了不同的治疗方法,但我们分别合并了每种治疗类型的试验结果。对照组中的人接受了常规护理:关于戒烟的简短建议,或者被列入等候名单以稍后接受治疗。尽管我们也纳入了一些时间更短的研究,但大多数试验评价了在开始治疗至少六个月后戒烟的人数。
主要结果
涉及1808人的11项研究测试了各种药物疗法的效果。有证据表明接受药物治疗的人在戒烟方面更成功。涉及2229名研究对象的12项研究测试了药物治疗联合咨询的治疗方法。有证据表明接受联合治疗的人在戒烟方面更成功。涉及1759人的11项研究测试了咨询与常规护理相比较的效果。结合这些结果,并没有证据显示单独咨询的益处。
涉及2231人的11项研究报告了人们是否保持了戒酒和戒其他药物。提供戒烟干预措施并没有使人们更有可能重新使用酒精或其他药物。
我们没有发现任何证据表明人们在刚开始接受其他药物使用治疗时或在康复后接受戒烟治疗有差异性。对于因酗酒和海洛因等其他药物接受治疗的人,结果也相似。
证据质量
我们认为证据的质量低。许多研究没有提供足够详细的研究方法。这些研究纳入的治疗类型差异性很大,彼此之间很难比较。
Authors' conclusions
Summary of findings
| Tobacco cessation interventions compared to placebo or usual care for people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Patient or population: people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with tobacco cessation interventions | |||||
| Tobacco abstinence after counselling (counselling) | Study population | RR 1.33 | 1759 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 47 per 1000 | 62 per 1000 | |||||
| Tobacco abstinence after pharmacotherapy (pharmacotherapy) | Study population | RR 1.88 | 1808 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 58 per 1000 | 109 per 1000 | |||||
| Tobacco abstinence after combined counselling and pharmacotherapy (combined) | Study population | RR 1.74 | 2229 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 92 per 1000 | 160 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limited information provided regarding study designs; some cluster‐randomised studies and waiting list controls. 2 Clinical interventions had substantial variance, ranging from one‐time to daily counselling sessions and individual or group therapy. 3 Evidence of publication bias. | ||||||
Background
Tobacco kills up to half its users, accounting for nearly six million deaths annually worldwide (WHO 2016a). Tobacco‐related disease is the leading preventable cause of death in the US (USDHSS 2014), and smoking rates in alcohol and drug‐dependent people, as well as people with mental health disorders, are two to four times that of the general population (Kalman 2005). Estimates suggest these groups account for approximately half of all smoking‐related deaths (Mauer 2006; Schroeder 2009; Williams 2004). In the US, less than one‐quarter of the population smokes and overall smoking rates have declined since the 1960s (Schroeder 2004). Among people with drug dependency and mental health disorders, however, smoking rates have remained constant (Lamberg 2004).
The health risks of smoking in these high‐risk populations have frequently been viewed as less relevant than the perceived therapeutic benefits of smoking, which were presumed to calm people with psychiatric disorders and reduce the risk of relapse for people recovering from alcohol and other drug dependence. The expectation that smoking was beneficial for these populations has persisted despite empirical findings showing the opposite effects (Guydish 2007; Philip Morris 1994; Psychiatric News 1994), and has discouraged the enactment of policy interventions that would reduce the disproportionate deaths from tobacco use that these high‐risk populations experience (Apollonio 2005; Gudrais 2008).
This review specifically addresses tobacco cessation interventions for people diagnosed with alcohol and other drug dependence (other Cochrane Reviews address populations with mental health disorders, see Tsoi 2013 and Van der Meer 2013). The World Health Organization estimates that over 15 million people worldwide have substance use disorders (WHO 2016b). In the US, studies estimate that nearly 13% of the population is addicted to alcohol, other drugs, or both (CASA 2012; NIDA 2012). The median smoking rate among adults in treatment for drug dependency is 76% (Guydish 2011). Due to high smoking rates, people in these populations face a disproportionate risk of death due to tobacco use. People with alcohol dependency, for example, have a 51% risk of dying from tobacco‐related disease, compared to a 34% risk of dying from alcohol‐related causes (Hurt 1996). Surveys also suggest that participants in treatment for or recovery from alcohol and other drug dependency want to quit smoking and are interested in receiving smoking cessation therapy (Joseph 2003). As a result, increasing numbers of researchers now argue that access to tobacco cessation therapy during treatment and recovery would be clinically appropriate as a means of reducing smoking‐related deaths in these populations (Abrams 2010; Baca 2009; Levy 2010).
Despite these findings, neglect of tobacco addiction in high‐risk populations remains common. This neglect is sometimes attributed to the stigma faced by people experiencing mental health disorders or drug dependency (Schroeder 2008). In addition, questions remain as to how to treat tobacco comorbidity and whether tobacco cessation therapy should be offered during treatment for other drug dependencies or delayed until recovery. Concurrent treatment of tobacco addiction with treatment for other drug dependencies has been limited due to staff fears that recovery could be compromised if clients tried to simultaneously quit smoking (Goldsmith 1993; Richter 2006). For example, in the US, only one‐third of respondents representing alcohol treatment programmes agreed that clients in treatment should be encouraged to quit smoking (Bobo 1995); similar results have been reported for providers in Australia and Switzerland (Walsh 2005; Zullino 2000).
Description of the condition
Tobacco use in populations dealing with alcohol and other drug dependency causes significant morbidity and mortality. It is not clear how or when to address tobacco addiction in these populations. Alcohol and other drug dependency is highly correlated with mental health disorders (dual diagnosis); 60% of people with substance use disorder also experience mental illness (NIDA 2007). Smokers with a history of alcoholism are more nicotine dependent than smokers without a history of alcoholism (Hurt 2003; Ward 2012), and these people are also less likely to quit smoking (Hays 1999). Former alcoholics that seek to quit smoking request more pharmacotherapy than smokers without a history of alcoholism (Hughes 2000).
Description of the intervention
Tobacco cessation treatment can be in the form of counselling, pharmacotherapy, both, or other interventions (e.g. contingency payments, increased doses of medications intended to treat other diagnoses). In this review, we assess the effects of different types of interventions: counselling, pharmacotherapy consisting of nicotine replacement therapy (NRT) with or without non‐NRT pharmacotherapy, or a combination of these. Counselling could include individual or group (or both) counselling to encourage behavioural change, for single or multiple sessions, based on methods including the trans‐theoretical model of readiness to change, motivational interventions (5‐A framework), cognitive behavioural therapy (CBT), and behavioural counselling, which may include education or the provision of information. Pharmacotherapy could include NRT, offered by prescription with tapering under physician supervision or ad libitum, using gum, lozenge, inhaler, or transdermal patch, or non‐NRT drugs that reduce the nicotine cravings such as varenicline or bupropion. Combined therapy could include any combination of the treatments included under counselling and pharmacotherapy.
How the intervention might work
Tobacco cessation treatments provide: motivation and support for change through counselling, treatment for withdrawal symptoms using NRT or non‐NRT pharmacotherapy, or a combination of these. Counselling can include a clear request to quit, identification of the risks of tobacco use, identification of strategies that reduce barriers to quitting, and organisation of people in comparable situations to discuss concerns and quit strategies. NRT is an alternative delivery system for nicotine that reduces cravings for nicotine that lead to the desire to smoke. Non‐NRT pharmacotherapy reduces cravings for nicotine; varenicline is a nicotinic receptor partial agonist and bupropion is a nicotinic antagonist.
The rates at which the general population achieves tobacco abstinence using counselling combined with pharmacotherapy range from 11% to 30% (Campbell 2003). Counselling combined with pharmacotherapy, and combined use of NRT and non‐NRT, is more successful than pharmacotherapy alone (Bornemann 2016), and thus combination therapy is recommended in the general population (Ebbert 2007). In some cases, combined treatments can achieve success rates as high as 65% (Bornemann 2016). For people with more severe tobacco dependence, a group that encompasses most people with drug dependency, some research suggests both combination therapy and the use of multiple pharmacological agents (Bornemann 2016; Hurt 2009).
Why it is important to do this review
It is not known whether adding tobacco cessation therapy to drug dependency treatment programmes yields higher overall abstinence from tobacco, alcohol, and other drugs. We systematically reviewed studies that provided tobacco cessation therapy to people in treatment for or recovery from alcohol and other drug dependence and conducted a meta‐analysis of the results. Our analyses considered what type of tobacco cessation therapy is associated with increased tobacco abstinence, whether tobacco cessation therapy should be offered concurrently with treatment for other addictive drugs or delayed, and whether the type of drug dependency affects the association between tobacco cessation therapy and tobacco abstinence.
Two earlier reviews have been conducted in this area (Prochaska 2004; Thurgood 2016). This analysis updates and expands on these previous reviews by considering multiple interventions, assessing abstinence from tobacco and other drugs, conducting meta‐analyses of treatment effects, and providing a subgroup analysis of follow‐up and analysing type of drug dependency.
Objectives
To evaluate whether interventions for tobacco cessation are associated with tobacco abstinence for people in concurrent treatment for or in recovery from alcohol and other drug dependence.
Methods
Criteria for considering studies for this review
Types of studies
Studies were randomised controlled trials (RCTs) and cluster‐RCTs, with no exclusions based on language of publication or publication status.
Types of participants
Participants were adults aged 15 years or older undergoing inpatient or outpatient treatment for alcohol or other drug dependence, or in recovery from alcohol and other drug dependence, and participating in a study to encourage tobacco cessation. Interventions could target either groups (e.g. the population of a single clinic) or participants (e.g. people at a single clinic). We distinguished between studies that randomised participants within clinics and studies that randomised by clinic site (cluster randomisation). We included information on the type of dependency for which the person originally sought treatment (e.g. alcohol or other drugs, or both). Participants in the included studies did not need to have been selected based on type of tobacco product, level of smoking (e.g. daily smokers) or their presumed suitability for interventions.
Types of interventions
We included counselling and pharmacotherapy interventions designed to encourage tobacco cessation. We organised interventions by type in the following categories:
-
counselling only: brief or extended sessions, and individual or group sessions, delivered in a clinic setting for tobacco cessation purposes during the course of existing addictions treatment, in addition to usual care interventions;
-
pharmacotherapy: NRT of all modalities (e.g. gum, patch, lozenge), both prescription and non‐prescription, offered to participants for tobacco cessation purposes during the course of existing addictions treatment, or non‐NRT pharmacology (e.g. varenicline) offered to participants for tobacco cessation purposes during the course of existing addictions treatment, in addition to usual care interventions;
-
counselling plus pharmacotherapy: a combination of any of the above methods.
The controls in these studies were participants in substance abuse treatment who were offered different tobacco cessation therapies, delayed therapy, lower levels of treatment, or no tobacco‐related cessation therapy. We excluded interventions that did not rely on counselling or tobacco cessation‐related pharmacotherapy (e.g. higher doses of methadone).
Types of outcome measures
Primary outcomes
-
Point prevalence tobacco abstinence, defined by self‐reported tobacco use or through biochemical validation (e.g. breath carbon monoxide, urinary cotinine) (or both) at the longest follow‐up period reported in each study. Results were measured as the number of participants abstinent in each condition (treatment or control) at final follow‐up relative to the number of participants enrolled in the study. Biochemical validation of self‐reported abstinence was not required but was recorded and used where available. We relied on point prevalence abstinence rather than continuous abstinence, when both were reported, due to the difficulty of follow‐up within this population. No minimum length of follow‐up was required; the period of longest follow‐up ranged from 6 weeks to 18 months.
We recorded the definition of tobacco use as defined by each study. These included current daily use and current occasional use. We excluded studies reporting reduced smoking rather than abstinence from the analysis. We also excluded studies that measured interventions included in the criteria above, but that did not report tobacco abstinence.
Secondary outcomes
-
Point prevalence abstinence from alcohol and other drugs as defined by self‐reported drug use or through biochemical validation (or both) at the longest follow‐up period reported in the study.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Tobacco Addiction Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), and MEDLINE. The Specialised Register includes reports of trials identified from systematic and sensitive searches of resources, including MEDLINE, EMBASE, and PsycINFO, for reports of trials of interventions for smoking cessation and prevention (see the Tobacco Addiction Group Module in the Cochrane Library for full details). The Specialised Register search used topic related keywords and free text terms covering alcohol abuse and drug dependence. The CENTRAL search combined topic‐related terms and terms related to smoking cessation. Key search criteria combined study design (e.g. RCT, double blind method), smoking cessation (e.g. tobacco, nicotine), and substance abuse (e.g. alcohol abuse, drug dependence). See Appendix 1 for the full MEDLINE search strategy. We conducted an initial CENTRAL and MEDLINE search on 14 February 2012 with search dates ranging from 1970 to 2011. We completed additional searches on 2 August 2016 with search dates updated to 1 August 2016.
Searching other resources
We searched the grey literature, including conference abstracts from the Society for Research on Nicotine and Tobacco, World Health Organization, and the ProQuest database of digital dissertations, and all registered trials through the National Institutes of Health's ClinicalTrials.gov website.
Data collection and analysis
Selection of studies
Three review authors (DA, RP, and LB) independently reviewed the literature searches from the title, abstract, or descriptors, to identify potentially relevant trials.
Data extraction and management
Two review authors (DA and RP) independently extracted data for the trials using a standardised data extraction form prior to entry into Review Manager 5 (RevMan 2014). Two review authors (DA and RP) corresponded with authors in efforts to obtain missing or raw data. We excluded all studies that clearly did not meet the inclusion criteria in terms of study design, population, or interventions. Two review authors (DA and RP) independently extracted the data, which was checked by a second review author (DA or LB). Two review authors independently extracted data for risk of bias for all included studies.
We extracted the following information, when reported, using a tool developed by one review author (LB) and modified by a second review author (DA).
-
Methods, including the setting of the trial, study design, study objectives, study site(s), definition of tobacco use, methods of participant recruitment, types of treatment interventions, proposed outcome measures, and methods of analysis.
-
Participant data, including age, gender, ethnicity, socioeconomic status, and numbers of participants recruited and assessed.
-
Interventions, including descriptions of interventions, duration of treatment, delivery of intervention, type and duration of behavioural support (if applicable) and components of treatment in the control group.
-
Outcomes, including methods of data collection for results, definitions of abstinence, abstinence from tobacco, abstinence from other drugs, validation, follow‐up period, other follow‐ups in the course of the study, and other data as defined under Types of outcome measures.
-
Risks of bias, including methods of sequence generation for randomisation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, clustering by clinic site, imbalance of outcome measures at baseline, comparability of intervention and control group characteristics at baseline, selective recruitment of participants, and other potential threats to validity.
Assessment of risk of bias in included studies
Two review authors (DA and RP) independently evaluated risk of bias, in line with recommendations made in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The criteria included allocation sequence, allocation concealment, blinding for participants and personnel, selective outcome reporting, and incomplete outcome data. We noted additional criteria recommended by the Cochrane Effective Practice and Organisation of Care (EPOC) group: assessing threats to validity including: imbalance of outcome measures at baseline and comparability of intervention and control group characteristics at baseline (EPOC 2009). For cluster study designs, when relevant we also assessed the risk of bias associated with selective recruitment of participants through choice of site. We assessed risk of bias in each domain as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias', based on the guidelines from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with notes indicating the reasons for each assessment included in the 'Risk of bias' table. We resolved conflicts in the assessments either by consensus or by referring to a third review author (LB).
Measures of treatment effect
We calculated risk ratios (RR) for the primary and secondary outcomes with their 95% confidence intervals (CI). The RR was defined as (number of participants abstinent from tobacco in the intervention group/total number randomised to the intervention group)/(number of participants abstinent from tobacco in the control group/total number randomised to the control group). The RR is greater than 1 if more participants remain abstinent from tobacco in the intervention group than in the control group. We used an intention to treat analysis for all studies that reported the numbers of participants assigned to each study condition, classifying participants lost to follow‐up as non‐abstinent. Of the 34 included studies, two provided no information on loss to follow‐up (Kalman 2001; Karam‐Hage 2011), one study had no participants lost to follow‐up (Heydari 2013), and one study independently verified abstinence for participants that dropped out (Cooney 2009).
Unit of analysis issues
There were two cluster RCTs, for which the analysis was performed at the individual level (Bobo 1996; Bobo 1998). These studies did not adjust for clustering and the incidence rate ratio (IRR) was unavailable. They were included in the meta‐analysis given that a sensitivity analysis found their inclusion did not affect the results.
Dealing with missing data
We evaluated missing information regarding participants on an available case analysis basis as described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If information needed for the meta‐analysis was missing (e.g. if numbers abstinent in the treatment and control groups were not reported) or could not be calculated, we sought to contact the authors to gain access to these data. If there was loss of participants before baseline assessment, this review assumed that these missing data had no effect on the final results of the analysis. Two review authors (DA and RP) assessed and discussed attrition after baseline assessments. The main considerations were differential attrition between the intervention and control groups, and differential attrition within groups that were correlated with baseline characteristics.
Whenever possible, we recorded the extent of participants lost to follow‐up in each condition. Because loss to follow‐up in the case of tobacco cessation treatment is typically associated with continued tobacco use, participants lost to follow‐up were coded as non‐abstinent.
Assessment of heterogeneity
We classified trials according to the subgroups listed in Types of interventions. We combined studies within these categories of intervention. There can be heterogeneity due to different factors, including level of tobacco use (e.g. number of cigarettes smoked per day), demographics, time to follow‐up measures, and measurement tools (e.g. self‐report versus clinical assessment). If the confidence intervals of studies have poor overlap, this usually indicates the presence of statistical heterogeneity.
In addition to visually inspecting data, we used I2 statistic to identify inconsistencies between studies and groups (Higgins 2011). The Chi2 test has low power when studies have small sample sizes, or when there are few studies. Recognising that some level of statistical heterogeneity is inevitable, the I2 statistic instead attempts to quantify the potential impact of this heterogeneity on a meta‐analysis. It describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling. We also considered Chi2; outcomes were similar. The review used a fixed‐effect model throughout the analyses.
Assessment of reporting biases
There are limited statistical methods to detect within‐study selective reporting. If non‐significant results were mentioned but not reported adequately, we assumed that there was risk of bias. Unfortunately, information sought from authors of studies may be incomplete or unreliable (Chan 2004a; Chan 2004b). Our analysis assessed whether a small number of key outcomes were present in all the included studies, and reported which studies included these outcomes and which did not. We assessed the risk of bias due to selective reporting of outcomes for each study rather than for individual outcomes. Where we suspected selective outcome reporting, we contacted study authors for additional information. We created funnel plots for included studies by outcome. Based on prior research, we assumed that studies that considered pharmacotherapy had a high risk of publication bias.
Data synthesis
We conducted meta‐analyses for the primary outcome of point prevalence tobacco abstinence based on the type of intervention, stage of treatment or recovery, and the type of addiction. We analysed data using Review Manager 5 (RevMan 2014). We included multi‐arm trials, but extracted only data from the relevant comparisons. We also conducted meta‐analysis for the secondary outcome of point prevalence abstinence from alcohol and other drugs.
We used the GRADE approach to assess overall quality of evidence. Given that this review included only RCTs, evidence was downgraded from 'high quality' by one level for study limitations including risk of bias, inconsistency, indirectness, imprecision, or risk of publication bias. We generated the 'Summary of findings' table for each type of intervention (counselling, pharmacotherapy, or combined) in GRADEpro and imported into Review Manager 5 (RevMan 2014). The table provides information for each outcome regarding the overall quality of evidence, the magnitude of the effects, and the overall data.
Subgroup analysis and investigation of heterogeneity
In studies that offered extended follow‐up of participants, we presented results for several periods of follow‐up including short‐term (four weeks or less), medium‐term (four weeks to six months), and long‐term (greater than six months). In studies with more than one follow‐up assessment, we reported outcomes at the longest follow‐up period. We conducted subgroup analysis for people in treatment relative to people in recovery and by type of addiction. We conducted subgroup analysis for NRT versus non‐NRT pharmacotherapy, and for people in treatment versus recovery.
Sensitivity analysis
We conducted sensitivity analysis on studies that were cluster randomised (Bobo 1996; Bobo 1998). The studies included in this review were all RCTs and this restriction limits concern about several methodological issues unique to the cluster RCTs.
Results
Description of studies
Results of the search
The review included 34 studies involving 5796 participants (Baltieri 2009; Bobo 1996; Bobo 1998; Breland 2014; Burling 1991; Burling 2001; Campbell 1995; Carmody 2012; Cooney 2007; Cooney 2009; Cooney 2015; Gariti 2002; Grant 2003; Grant 2007; Hays 2009; Heydari 2013; Hughes 2003; Joseph 2004; Kalman 2001; Kalman 2011; Karam‐Hage 2011; Martin 1997; Mooney 2008; Mueller 2012; Nahvi 2014; Nieva 2011; Patten 1998; Reid 2008; Rohsenow 2014; Rohsenow 2015a; Shoptaw 2002; Stein 2006; Stein 2013; Winhusen 2014). All the included studies addressed cigarette smoking, one study also included hookah use (Heydari 2013). Details are listed in the Characteristics of included studies table.
The electronic searches yielded 1966 citations and no relevant citations were identified from the additional searches. Searching all registered trials through the National Institutes of Health's ClinicalTrials.gov website yielded 11 records, four of which were relevant (Alessi 2006; O'Malley 2012; Rohsenow 2014; Tsoh 2008). Of these four studies, one had associated publications included in the review (Rohsenow 2014), one was ongoing (O'Malley 2012), and two had posted no results and listed no associated publications (Alessi 2006; Tsoh 2008). A search through the grey literature, including conference abstracts from the Society on Nicotine Research and Treatment and the ProQuest database of digital dissertations yielded one additional study, a conference abstract with no results available from the authors (Higley 2014).
After removing duplicates, 552 studies remained. From those, 248 records were potentially relevant based on title and abstract. Of these, we excluded 194 for not meeting study criteria (including not an RCT, post‐hoc analysis of a prior study, methodological description of an existing study). We assessed the 54 remaining articles for eligibility and excluded 19 of these studies. The Characteristics of excluded studies table contains the reasons for exclusion (e.g. measured reduction in smoking rather than abstinence, incomplete outcome data, intervention that was not tobacco cessation pharmacotherapy or counselling).
The review included 35 studies (Figure 1). One of these studies was ongoing; it assesses pharmacotherapy (varenicline) and is described in Characteristics of ongoing studies (O'Malley 2012). The results of the ongoing study were not included in the analysis due to incomplete reporting of results (O'Malley 2012).
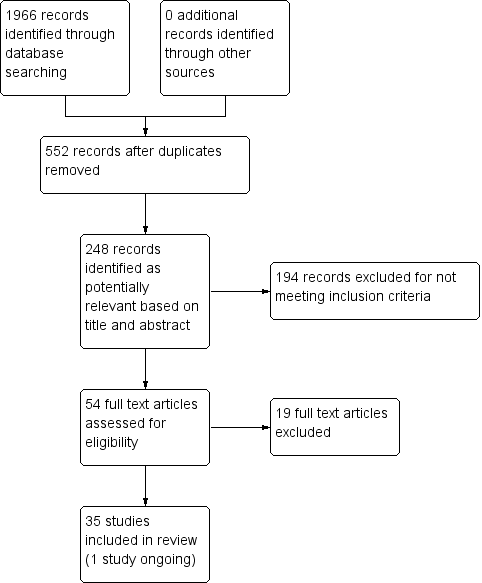
Study flow diagram.
Included studies
The 34 studies included in the meta‐analysis involved results from five countries; 30 of the studies were conducted in the US, one in Brazil (Baltieri 2009), one in Iran (Heydari 2013), one in Switzerland (Mueller 2012), and one in Spain (Nieva 2011). Details of included studies can be found in the Characteristics of included studies table. The characteristics of studies included in each subgroup analysis for the primary outcome of tobacco abstinence are described below.
Tobacco abstinence by intervention type
The inclusion criteria identified relevant interventions as counselling, pharmacotherapy, or a combination of these.
Of the 34 studies included in the analysis, 11 studies involving 1759 participants offered counselling for treatment relative to usual care; usual care could include pharmacotherapy. Of the 11 counselling studies, two offered one‐time individual counselling for 10 to 15 minutes (Bobo 1996; Bobo 1998), one offered a 30‐minute motivational interviewing intervention (Breland 2014), one offered 15‐minute daily counselling (Burling 1991), one offered an individual counselling session and encouragement to attend group sessions (Gariti 2002), one offered five 30‐minute CBT sessions specifically targeted to smoking cessation (Mueller 2012), one offered tobacco‐specific therapy as part of an existing series of eight × two‐hour group counselling sessions (Patten 1998), two offered motivational interviewing intervention sessions with boosters or contingent vouchers (Rohsenow 2014; Rohsenow 2015a), one offered behavioural interventions including 12 × 60‐minute group counselling sessions and nicotine patches relative to a control group receiving only nicotine patches (Shoptaw 2002), and one offered four motivational interviewing sessions combined with skills training follow‐ups (Stein 2006).
An additional 11 studies involving 1808 participants considered pharmacotherapy for tobacco cessation, relative to usual care, which could include both counselling and other pharmaceutical interventions. Of these, one study offered either naltrexone or topiramate (Baltieri 2009), one offered ab libitum nicotine gum in addition to nicotine patches (Cooney 2009), two offered bupropion in addition to the usual care provision of nicotine patches (Grant 2007; Kalman 2011), two offered bupropion (Hays 2009; Karam‐Hage 2011), two offered nicotine patches and gum (Heydari 2013; Hughes 2003), one offered bupropion, buprenorphine, and counselling relative to a control group that received only buprenorphine and counselling (Mooney 2008), one offered varenicline (Nahvi 2014), and one had two intervention arms, one offering varenicline and one offering NRT patches and ad libitum nicotine rescue (Stein 2013).
The remaining 12 studies involving 2229 participants offered tobacco cessation therapy that combined counselling and pharmacotherapy, relative to usual care. Of the 12 studies, 11 offered counselling in combination with NRT (Campbell 1995; Carmody 2012; Cooney 2007; Cooney 2015; Grant 2003; Joseph 2004; Kalman 2001; Martin 1997; Nieva 2011; Reid 2008), and one offered counselling in combination with both NRT and bupropion (Winhusen 2014).
Tobacco abstinence by in treatment or in recovery
The review included studies that enrolled participants both in treatment for and in recovery from addiction to alcohol and other drugs.
Of the 34 studies included in the analysis, 12 studies were conducted with 2134 participants in treatment for alcohol or other drug dependency (or both) (Cooney 2015; Hays 2009; Heydari 2013; Hughes 2003; Kalman 2011; Karam‐Hage 2011; Martin 1997; Mueller 2012; Patten 1998; Rohsenow 2014; Rohsenow 2015a; Winhusen 2014). The remaining 22 studies were conducted with 3792 participants in recovery from alcohol or other drug dependency (or both) (Baltieri 2009; Bobo 1996; Bobo 1998; Breland 2014; Burling 1991; Burling 2001; Campbell 1995; Carmody 2012; Cooney 2007; Cooney 2009; Gariti 2002; Grant 2003; Grant 2007; Joseph 2004; Kalman 2001; Mooney 2008; Nahvi 2014; Nieva 2011; Reid 2008; Shoptaw 2002; Stein 2006; Stein 2013).
Tobacco abstinence by type of dependency
Participants in the included studies were diagnosed with alcohol dependence or dependence on other drugs.
Of the 34 studies included in the analysis, 17 studies enrolled 2467 participants in treatment for or in recovery from alcohol dependence (Baltieri 2009; Bobo 1996; Carmody 2012; Cooney 2007; Cooney 2009; Cooney 2015; Grant 2007; Hughes 2003; Joseph 2004; Kalman 2001; Kalman 2011; Karam‐Hage 2011; Martin 1997; Mueller 2012; Nieva 2011; Patten 1998; Rohsenow 2014). The remaining 17 students enrolled 3329 participants in treatment for or in recovery from other drug dependence, or combined dependence (Bobo 1998; Breland 2014; Burling 1991; Burling 2001; Campbell 1995; Gariti 2002; Grant 2003; Hays 2009; Heydari 2013; Mooney 2008; Nahvi 2014; Reid 2008; Rohsenow 2015a; Shoptaw 2002; Stein 2006; Stein 2013; Winhusen 2014).
Other study characteristics
Randomisation: in two of the studies, participants were cluster randomised by clinic site (Bobo 1996; Bobo 1998), while in the remaining studies the participants were the unit of randomisation.
Controls: three of the 34 studies used waiting list controls (Campbell 1995; Cooney 2015; Nieva 2011). This means that participants were randomised to receive the intervention immediately or after a defined time.
Follow‐up: the 34 included studies had varied lengths of maximum follow‐up; 16 studies with approximately six months of longest follow‐up, and 11 studies following participants for one year or longer. Participants were followed for a maximum of six weeks (Breland 2014), eight weeks (Karam‐Hage 2011), 12 weeks (Baltieri 2009; Mooney 2008), 13 weeks (Cooney 2015), 16 weeks (Campbell 1995), 20 weeks (Kalman 2001), and periods of approximately six months, including 24 weeks (Kalman 2011; Nahvi 2014), 26 weeks (Reid 2008), and six months (Bobo 1996; Burling 1991; Cooney 2007; Gariti 2002; Grant 2007; Hays 2009; Heydari 2013; Hughes 2003; Mueller 2012; Nieva 2011; Stein 2006; Stein 2013; Winhusen 2014). The remaining studies had their longest follow‐ups at 12 months (Bobo 1998; Burling 2001; Carmody 2012; Cooney 2009; Grant 2003; Patten 1998; Martin 1997; Rohsenow 2014; Rohsenow 2015a; Shoptaw 2002), and 18 months (Joseph 2004).
Validation: biochemical verification (breath or urinary cotinine level) was used to validate self‐reported abstinence in 31 of 34 studies, collateral contacts were used to validate self‐reported abstinence in two of 34 studies (Grant 2003; Grant 2007), and one study did not validate self‐reported abstinence (Baltieri 2009).
Excluded studies
Of the 56 full text articles assessed for eligibility, we excluded 19. Of these 19 excluded studies, seven measured smoking reduction rather than abstinence (Diehl 2006; Haug 2004; Laaksonen 2013; Leggio 2015; Meszaros 2013; Poling 2010; Wiseman 2005), and an additional five studies assessed contingency management for tobacco cessation rather than counselling or pharmacotherapy (Alessi 2008; Alessi 2014; Dunn 2008; Dunn 2010; Rohsenow 2008). We excluded the remaining seven studies for the following reasons: three reported no findings (Alessi 2006; Higley 2014; Tsoh 2008), one did not provide sufficient outcomes data for analysis (Covey 1993), and three intervened with pharmacotherapy that did not vary across study arms or was not targeted to tobacco cessation (Kalman 2006; Rohsenow 2015b; Story 1991).
Details of excluded studies can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
The rationale for risk of bias judgments can be found in the Characteristics of included studies table. Overall, most studies contained inadequate information to assess risk of bias.
Allocation
The risk of selection bias, judged on the basis of allocation concealment, was low in five studies (Joseph 2004; Nahvi 2014; Reid 2008; Rohsenow 2014; Stein 2006), and high in seven studies, including those that randomised by clinics or used waiting list controls (Bobo 1996; Bobo 1998; Campbell 1995; Cooney 2015; Martin 1997; Nieva 2011; Patten 1998). The remaining 22 studies did not describe methods for concealment of allocation and were at unclear risk of bias.
Selection bias, as assessed through random sequence generation for assignment to treatment and control groups, was low in 13 studies (Bobo 1998; Breland 2014; Carmody 2012; Cooney 2009; Cooney 2015; Joseph 2004; Kalman 2011; Mooney 2008; Nahvi 2014; Reid 2008; Rohsenow 2014; Rohsenow 2015a; Shoptaw 2002), and high in two studies (Martin 1997; Patten 1998). The remaining 19 studies did not describe methods of randomisation and were at unclear risk of bias.
Blinding
The risk of performance bias, as measured by blinding of participants and personnel, was low in 12 studies (Baltieri 2009; Bobo 1996; Bobo 1998; Cooney 2009; Kalman 2001; Kalman 2011; Mooney 2008; Nahvi 2014; Rohsenow 2014; Rohsenow 2015a; Stein 2006; Stein 2013), and high in three studies (Campbell 1995; Cooney 2015; Nieva 2011). The remaining 19 studies did not describe methods for blinding and were at unclear risk of bias.
Incomplete outcome data
The risk of attrition bias was low in 11 studies (Bobo 1996; Cooney 2009; Grant 2007; Heydari 2013; Karam‐Hage 2011; Nahvi 2014; Nieva 2011; Reid 2008; Rohsenow 2015a; Stein 2006; Stein 2013), and high in three studies (Baltieri 2009; Carmody 2012; Mooney 2008). The remaining 20 studies did not adequately describe loss to follow‐up or the differences between treatment and control groups and were at unclear risk of bias.
Other potential sources of bias
Thirty‐one of 34 studies used biochemical verification to validate self‐reported abstinence (Bobo 1996; Bobo 1998; Breland 2014; Burling 1991; Burling 2001; Campbell 1995; Carmody 2012; Cooney 2007; Cooney 2009; Cooney 2015; Gariti 2002; Hays 2009; Heydari 2013; Hughes 2003; Joseph 2004; Kalman 2001; Kalman 2011; Karam‐Hage 2011; Martin 1997; Mooney 2008; Mueller 2012; Nahvi 2014; Nieva 2011; Patten 1998; Reid 2008; Rohsenow 2014; Rohsenow 2015a; Shoptaw 2002; Stein 2006; Stein 2013; Winhusen 2014). An additional two studies used reports by collateral contacts to validate self‐reported abstinence (Grant 2003; Grant 2007), and one study did not verify self‐reported abstinence (Baltieri 2009).
We created funnel plots for included studies by outcome; all were symmetrical other than a slight asymmetry towards treatment for pharmacotherapy interventions, suggesting the possibility of publication bias.
Only 11 of 34 studies reported outcomes for abstinence from alcohol or other drugs. As the reasons for failing to report abstinence from other drugs could relate to the costs of assessment, the demands of working with participants in recovery rather than treatment, independent reporting requirements, baseline imbalance, and selective recruitment due to cluster randomisation. The failure to report outcomes for abstinence from alcohol or other drugs was not assumed to be a potential source of bias.
Effects of interventions
Tobacco abstinence by type of intervention
Offering a counselling intervention relative to usual care was not significantly associated with an increase in tobacco abstinence (RR 1.33, 95% CI 0.90 to 1.95), based on data from 1759 people in 11 studies (Analysis 1.1). A sensitivity analysis excluded the two included studies that had randomised by clinic site rather than at the participant level (Bobo 1996; Bobo 1998); the effects were not sensitive to the exclusion of the cluster RCTs (RR 1.16, 95% CI 0.74 to 1.84). The outcome was downgraded from high to low quality due to the potential for risk of bias due to limited information regarding allocation, blinding, and incomplete outcome data, as well as cluster randomisation and the use of waiting list controls; and clinical heterogeneity in the nature of the interventions, which ranged from a single counselling session to multiple sessions and which could include individual or group therapy (summary of findings Table for the main comparison).
Providing pharmacotherapy for tobacco cessation relative to placebo or usual care was significantly associated with tobacco abstinence (RR 1.88, 95% CI 1.37 to 2.57), based on data from 1808 people in 11 studies (Analysis 1.2). Multiple types of pharmacotherapy were included in the main analysis. When the analysis was limited to those studies assessing only NRT, the treatment effect remained significant (RR 7.74, 95% CI 3.00 to 19.94,) 3 studies, 635 participants). When the analysis was limited to those studies assessing either non‐NRT pharmacotherapy or studies that combined NRT and non‐NRT pharmacotherapy, there was no significant treatment effect (RR 1.25, 95% CI 0.89 to 1.77, 8 studies, 1173 participants). The outcome was downgraded from high to low quality due to the potential for risk of bias and the well‐documented risk of publication bias in drug studies (summary of findings Table for the main comparison).
Providing combined counselling and pharmacotherapy relative to placebo or usual care (or both) was significantly associated with tobacco abstinence (RR 1.74, 95% CI 1.39 to 2.18) based on data from 2229 people in 12 studies (Analysis 1.3). The outcome was downgraded from high to low quality due to the potential for risk of bias and clinical heterogeneity in the nature of interventions (summary of findings Table for the main comparison).
There were no notable differences in risk of bias between counselling and pharmacotherapy interventions; the studies that addressed combined intervention had slightly higher risks of bias. Most studies had unclear risks of bias for some or all domains. Funnel plots were symmetrical for counselling and combined interventions, and slightly asymmetrical toward treatment for pharmacotherapy, suggesting the possibility of publication bias.
Tobacco abstinence by treatment or recovery group
Offering tobacco cessation therapy relative to usual care or placebo was significantly associated with tobacco abstinence at the length of longest follow‐up (Analysis 2.1) both for participants in treatment (RR 1.99, 95% CI 1.59 to 2.50) based on data from 2134 people in 12 studies, and for participants in recovery (RR 1.42, 95% CI 1.11 to 1.82) based on data from 3662 people in 22 studies. The effect size was greater for participants in treatment than it was for participants in recovery. The test for subgroup differences showed Chi2 = 3.98, degrees of freedom (df) = 1 (P = 0.05), I2 = 74.9%. The clinical significance of this finding is difficult to assess given that studies of participants in treatment could offer tobacco cessation therapies immediately upon enrolment or after a delay (e.g. seven days after admission, 30 days after admission) without indicating whether the delay was expected to influence the outcome of the intervention. Not all studies assessing participants in treatment indicated the point in treatment at which the tobacco cessation intervention occurred. Two studies explicitly addressed the question of concurrent treatment relative to delayed treatment by imposing a six‐month/180‐day delay in treatment as the intervention (Joseph 2004; Nieva 2011); the results of these studies were inconsistent with each other with respect to abstinence from tobacco. Overall these studies do not provide sufficient evidence to determine whether the observed difference in effect size is clinically relevant. There were no notable differences in risk of bias between the treatment and recovery groups, but most studies had unclear risks of bias for some or all domains. Funnel plots were symmetrical for both participants in treatment and participants in recovery.
Tobacco abstinence by type of dependency
Offering tobacco cessation therapy relative to usual care or placebo was significantly associated with tobacco abstinence at the length of longest follow‐up for both participants with alcohol dependence (RR 1.57, 95% CI 1.27 to 1.95) based on data from 2467 people in 17 studies, and participants with other drug dependence or combined alcohol and other dependence (RR 1.85, 95% CI 1.43 to 2.40) based on data from 3329 people in 17 studies (Analysis 3.1). There were no notable differences in risk of bias between the alcohol or other drug dependency groups, but most studies had unclear risks of bias for some or all domains.
A sensitivity analysis considered the effects of excluding the seven studies with less than six months of follow‐up from all analyses (Baltieri 2009; Breland 2014; Campbell 1995; Cooney 2015; Kalman 2001; Karam‐Hage 2011; Mooney 2008). The effects were not sensitive to the exclusion of studies with less than six months' follow‐up except for participants in recovery from alcohol and other drug dependence; when studies with less than six months of follow‐up were excluded, tobacco cessation interventions were no longer associated with increased tobacco abstinence in this population. Funnel plots were symmetrical for both types of dependence.
Secondary outcome ‐ abstinence from alcohol or other drugs
Offering tobacco cessation therapy for participants in treatment or recovery for other drug dependence was not associated with a difference in abstinence rates from alcohol and other drugs (RR 0.97, 95% CI 0.91 to 1.03), based on data from 2231 people in 11 studies (Analysis 4.1). All studies included in this analysis had unclear risk of bias for at least one domain. The funnel plot for this outcome was symmetrical.
Discussion
Summary of main results
Tobacco cessation therapy that includes pharmacotherapy appears to be associated with increased tobacco abstinence for participants diagnosed with alcohol and other drug dependence, although the quality of evidence supporting these findings was low. The results of this review are consistent with those of previous reviews (Prochaska 2004; Thurgood 2016). Abstinence rates in this population are low relative to the general population.
The anticipated absolute effects of treatment on tobacco abstinence for this population were 109 per 1000 participants (95% CI 80 to 150) for pharmacotherapy, relative to 58 per 1000 participants for placebo or usual care, for a period of follow‐up ranging from eight weeks to six months. The anticipated absolute effects of treatment were 160 per 1000 participants (95% CI 128 to 201) for combined counselling and pharmacotherapy, relative to 92 per 1000 participants for placebo or usual care, for a period of follow‐up ranging from 13 weeks to 18 months.
The anticipated absolute effects of treatment on tobacco abstinence for this population were 62 per 1000 participants (95% CI 42 to 91) for counselling alone, relative to 47 per 1000 participants for placebo or usual care, for a period of follow‐up ranging from six weeks to 12 months; these results were not statistically significant.
Participation in tobacco cessation therapy does not appear to influence the success of treatments for alcohol and other drug dependence.
Overall completeness and applicability of evidence
The results reported here are based on a greater body of research relative to earlier systematic reviews of tobacco cessation therapy in people in treatment for or recovery from substance abuse (Prochaska 2004; Thurgood 2016). The findings suggest tobacco cessation interventions based on pharmacotherapy or combined counselling and pharmacotherapy increase rates of tobacco abstinence without influencing rates of abstinence from alcohol or other drugs. These interventions were associated with tobacco abstinence for both participants in treatment as well as participants in recovery, suggesting that intervention during treatment could offer an earlier opportunity to reduce tobacco use in this population. Tobacco cessation was achieved across a wide variety of interventions involving pharmacotherapy alone or pharmacotherapy plus counselling, suggesting that the choice of intervention is less important than ensuring that people in recovery are offered some type of smoking cessation intervention. Despite earlier expectations that interventions to promote tobacco cessation could compromise treatment for other addictions (SAMHSA 2011), tobacco cessation therapy interventions do not appear to affect abstinence rates for alcohol and other drugs. Not all studies assessed these outcomes.
Study limitations include the inability to assess the effects of multiple treatment providers. People in treatment for drug dependency do not receive care from a single source; they may begin with residential care and move to outpatient care over time or complete all treatment as outpatients. As either inpatients or outpatients, people seeking treatment for drug dependency may be counselled on tobacco cessation either by staff dealing with other addictions or by staff dealing specifically with tobacco‐related disease. Pharmacotherapy is typically prescribed by a physician that handles medical issues for the client, but not issues relating to addictions. Staff acceptance is a key factor, some staff members smoke themselves, and changing staff attitudes is a first major step towards eventually changing staff behaviour (Hurt 1996). Given existing literature, it was not possible to assess the effects of receiving treatment for drug dependency from multiple care providers or from staff who may themselves smoke.
Quality of the evidence
We found the overall quality of evidence for all outcomes, based on 5796 people in 34 studies, to be low quality, primarily due to the risk of bias arising from incomplete reporting, potentially inconsistent results due to heterogeneity in the nature of interventions, and the risk of publication bias. It was not possible to assess the potential sources of bias for most included studies; there was incomplete reporting for at least 19 of 34 included studies in every category assessed. There was also an absence of reporting of adverse events for the interventions included in this review. Results should be viewed cautiously given unclear methods of treatment allocation, unclear methods of blinding of participants and personnel, and incomplete outcome data regarding loss of participants to attrition. Given the sensitivity of the findings to heterogeneity in the length of follow‐up and the nature of pharmacotherapy, further research is warranted to identify whether tobacco cessation interventions would be best targeted to people in treatment or in recovery, and whether NRT or non‐NRT pharmacotherapy, or a combination, would lead to greater tobacco abstinence in this population.
Potential biases in the review process
Potential biases in the review process include the risk that the search did not identify all relevant studies. Although the search included both published and unpublished sources of data, it is not possible to conclude that it identified all relevant studies. In addition, multiple studies identified through clinical trials registries and abstract searches could not be included due to the failure to report findings. Given the risk of publication bias, the studies that did not report their findings may have had negative results that indicated that tobacco cessation interventions did not promote tobacco abstinence. In that event, not all relevant data were obtained and review findings would be biased to show a treatment effect. Study selection methods also excluded a potentially relevant intervention (contingency management). A funnel plot suggested the possibility of publication bias in the studies assessing pharmacotherapy interventions.
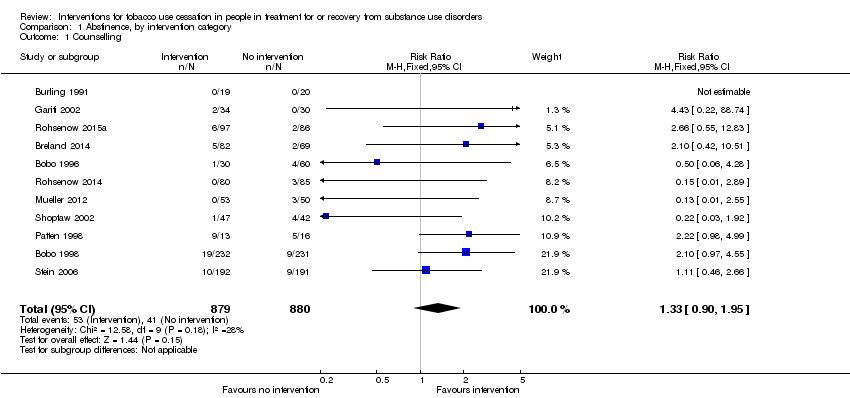
Comparison 1 Abstinence, by intervention category, Outcome 1 Counselling.

Comparison 1 Abstinence, by intervention category, Outcome 2 Pharmacotherapy.

Comparison 1 Abstinence, by intervention category, Outcome 3 Combined counselling and pharmacotherapy.
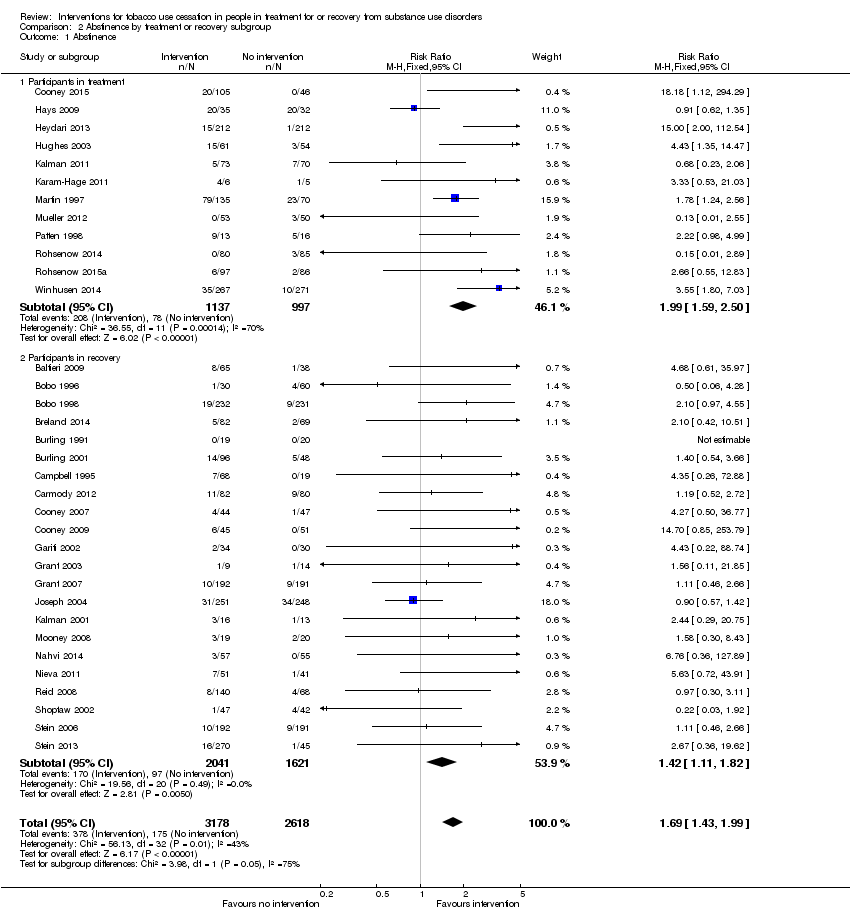
Comparison 2 Abstinence by treatment or recovery subgroup, Outcome 1 Abstinence.

Comparison 3 Abstinence by type of dependency, Outcome 1 Abstinence.

Comparison 4 Alcohol or other drug abstinence, Outcome 1 Abstinence at longest follow‐up.
| Tobacco cessation interventions compared to placebo or usual care for people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Patient or population: people in treatment for or recovery from alcohol or other drug dependency | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with tobacco cessation interventions | |||||
| Tobacco abstinence after counselling (counselling) | Study population | RR 1.33 | 1759 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 47 per 1000 | 62 per 1000 | |||||
| Tobacco abstinence after pharmacotherapy (pharmacotherapy) | Study population | RR 1.88 | 1808 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 58 per 1000 | 109 per 1000 | |||||
| Tobacco abstinence after combined counselling and pharmacotherapy (combined) | Study population | RR 1.74 | 2229 | ⊕⊕⊝⊝ | Baseline risk assessed in study outcomes | |
| 92 per 1000 | 160 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limited information provided regarding study designs; some cluster‐randomised studies and waiting list controls. 2 Clinical interventions had substantial variance, ranging from one‐time to daily counselling sessions and individual or group therapy. 3 Evidence of publication bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Counselling Show forest plot | 11 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.90, 1.95] |
| 2 Pharmacotherapy Show forest plot | 11 | 1808 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.37, 2.57] |
| 2.1 Nicotine replacement therapy (NRT) | 3 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.74 [3.00, 19.94] |
| 2.2 Other pharmacotherapy or combined NRT and other pharmacotherapy | 8 | 1173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.77] |
| 3 Combined counselling and pharmacotherapy Show forest plot | 12 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.39, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| 1.1 Participants in treatment | 12 | 2134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.59, 2.50] |
| 1.2 Participants in recovery | 22 | 3662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.11, 1.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence Show forest plot | 34 | 5796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 1.99] |
| 1.1 Alcohol dependence | 17 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.95] |
| 1.2 Other drug (or combined) dependence | 17 | 3329 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.43, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Abstinence at longest follow‐up Show forest plot | 11 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.91, 1.03] |

