Electronic cigarettes for smoking cessation and reduction
Information
- DOI:
- https://doi.org/10.1002/14651858.CD010216.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 17 December 2014see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Tobacco Addiction Group
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
All authors contributed to the writing of this review.
JHB and HM extracted data, with discrepancies and disagreements referred to PH.
As principal investigator of one of the included trials, CB was not involved with data extraction or assessment of study quality.
Sources of support
Internal sources
-
Queen Mary University of London, UK.
provides salary, office space and library resources for HM and PH
-
The University of Auckland, New Zealand.
provides salary, office space and library resources for CB
External sources
-
No sources of support supplied
Declarations of interest
Within the last three years HM has undertaken educational sessions sponsored by Pfizer and Johnson & Johnson, manufacturers of smoking cessation medications.
Within the last three years PH has provided consultancy to GSK, Pfizer, and Johnson & Johnson, manufacturers of smoking cessation medications.
Due to these two interests, this review is not compliant with the Cochrane commercial sponsorship policy, as updated in 2014. At the time the protocol was published it was compliant.
Two authors (HM, CB) have additional declarations:
CB and HM were investigators on a study of ECs from an EC manufacturer (Ruyan Group, Beijing and Hong Kong). Ruyan supplied the ECs used in the trial and contracted with Health NZ Ltd. to undertake the study. Health New Zealand Ltd funded The University of Auckland to conduct the trial, independently of Ruyan Group (Holdings) Ltd. The trial design conduct, analysis and interpretation of results were conducted independently of the sponsors.
CB and HM were investigators on the ASCEND EC trial funded by the Health Research Council of New Zealand that used product supplied at no charge from PGM international, a retailer of ECs.
JHB has no conflicts of interest to declare.
Acknowledgements
JHB is funded by the National Institute of Health Research School for Primary Care Research.
Version history
| Published | Title | Stage | Authors | Version |
| 2024 Jan 08 | Electronic cigarettes for smoking cessation | Review | Nicola Lindson, Ailsa R Butler, Hayden McRobbie, Chris Bullen, Peter Hajek, Rachna Begh, Annika Theodoulou, Caitlin Notley, Nancy A Rigotti, Tari Turner, Jonathan Livingstone-Banks, Tom Morris, Jamie Hartmann-Boyce | |
| 2022 Nov 17 | Electronic cigarettes for smoking cessation | Review | Jamie Hartmann-Boyce, Nicola Lindson, Ailsa R Butler, Hayden McRobbie, Chris Bullen, Rachna Begh, Annika Theodoulou, Caitlin Notley, Nancy A Rigotti, Tari Turner, Thomas R Fanshawe, Peter Hajek | |
| 2021 Sep 14 | Electronic cigarettes for smoking cessation | Review | Jamie Hartmann-Boyce, Hayden McRobbie, Ailsa R Butler, Nicola Lindson, Chris Bullen, Rachna Begh, Annika Theodoulou, Caitlin Notley, Nancy A Rigotti, Tari Turner, Thomas R Fanshawe, Peter Hajek | |
| 2021 Apr 29 | Electronic cigarettes for smoking cessation | Review | Jamie Hartmann-Boyce, Hayden McRobbie, Ailsa R Butler, Nicola Lindson, Chris Bullen, Rachna Begh, Annika Theodoulou, Caitlin Notley, Nancy A Rigotti, Tari Turner, Thomas R Fanshawe, Peter Hajek | |
| 2020 Oct 14 | Electronic cigarettes for smoking cessation | Review | Jamie Hartmann-Boyce, Hayden McRobbie, Nicola Lindson, Chris Bullen, Rachna Begh, Annika Theodoulou, Caitlin Notley, Nancy A Rigotti, Tari Turner, Ailsa R Butler, Thomas R Fanshawe, Peter Hajek | |
| 2016 Sep 13 | Electronic cigarettes for smoking cessation | Review | Jamie Hartmann‐Boyce, Hayden McRobbie, Chris Bullen, Rachna Begh, Lindsay F Stead, Peter Hajek | |
| 2014 Dec 17 | Electronic cigarettes for smoking cessation and reduction | Review | Hayden McRobbie, Chris Bullen, Jamie Hartmann‐Boyce, Peter Hajek | |
| 2012 Nov 14 | Electronic cigarettes for smoking cessation and reduction | Protocol | Hayden McRobbie, Chris Bullen, Peter Hajek | |
Differences between protocol and review
Originally, the protocol did not specify a minimum follow‐up period for data on adverse events. The Methods section has been changed to clarify that we will exclude follow‐up data at less than a week.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs
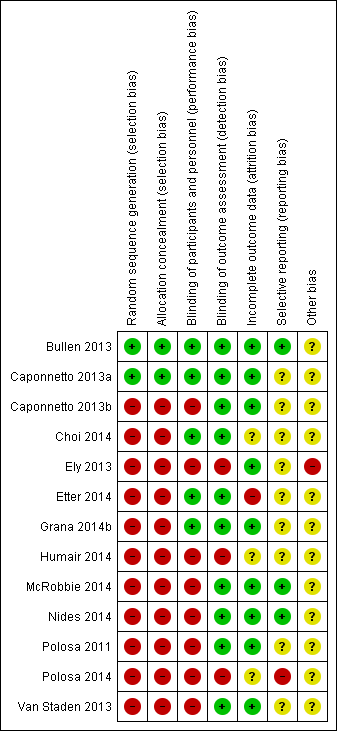
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Smoking cessation, Outcome 1 Nicotine EC versus placebo EC.

Comparison 1 Smoking cessation, Outcome 2 Nicotine EC versus nicotine replacement therapy.
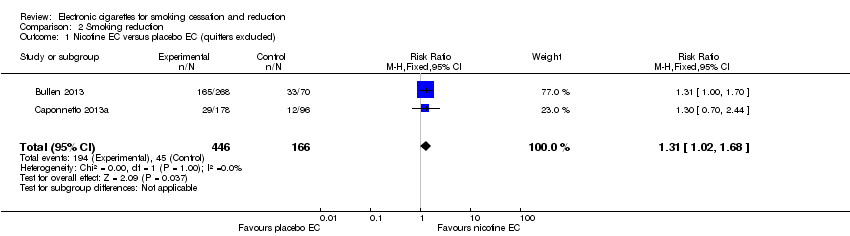
Comparison 2 Smoking reduction, Outcome 1 Nicotine EC versus placebo EC (quitters excluded).

Comparison 2 Smoking reduction, Outcome 2 Nicotine EC versus nicotine replacement therapy (quitters excluded).
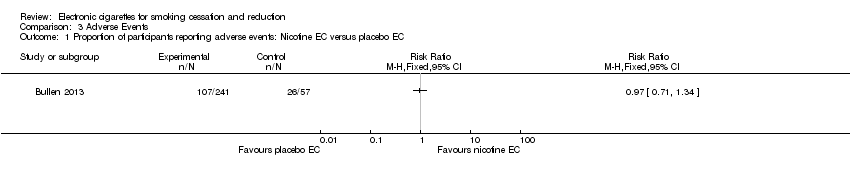
Comparison 3 Adverse Events, Outcome 1 Proportion of participants reporting adverse events: Nicotine EC versus placebo EC.

Comparison 3 Adverse Events, Outcome 2 Proportion of participants reporting adverse events: nicotine EC versus nicotine replacement therapy.
| Electronic cigarettes (EC) for smoking cessation and reduction | ||||||
| Patient or population: people defined as current smokers at enrolment into trials, motivated or unmotivated to quit Comparison: placebo electronic cigarettes or nicotine replacement therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Control | Electronic cigarettes | |||||
| Cessation: Nicotine EC versus placebo EC2 | 40 per 1000 | 93 per 1000 | RR 2.29 | 662 | ⊕⊕⊝⊝ | Only RCTs reported here. Some cohort data also available (see full review) but only RCTs provide efficacy data |
| Cessation: Nicotine EC versus nicotine replacement therapy | 58 per 1000 | 73 per 1000 | RR 1.26 | 584 | ⊕⊝⊝⊝ | As above |
| Reduction: Nicotine EC versus placebo EC | 271 per 1000 | 355 per 1000 | RR 1.31 | 612 | ⊕⊕⊝⊝ | As above. Analysis excludes quitters |
| Reduction: Nicotine EC versus nicotine replacement therapy | 435 per 1000 | 614 per 1000 | RR 1.41 | 546 | ⊕⊝⊝⊝ | As above. Analysis excludes quitters |
| Adverse events (AEs) Follow‐up: 6 ‐ 12 months | Summary data not available. None of the studies reported any serious AEs that were related to EC use. Neither RCT detected a significant difference in AEs between intervention and control groups. Cohort studies found mouth and throat irritation, dissipating over time, to be the most frequently reported AEs in EC users | 1090 (8 studies (2 RCTs, 6 cohort)) | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 'Assumed risk' calculated as risk in control groups. 2 'Placebo EC' refers to ECs which do not contain nicotine. 3 Downgraded one level due to indirectness. The electronic cigarette used in Bullen 2013 was not very effective at delivering nicotine 6 Downgraded due to risk of bias. 6/8 included studies (cohort studies) judged to be at high risk of bias 7 Downgraded due to imprecision. Only one trial provided data for nicotine EC versus nicotine replacement therapy | ||||||
| Study | Smokers motivated or unmotivated to quit? | Intervention vs relevant Control | % abstinent | ||||
| Cohort studies | 6 month | 12 months | 18 months | 24 months | Notes | ||
| Unmotivated to quit | Nicotine EC | 14% (2/14) | |||||
| Motivated to quit | Nicotine EC¹ | 44% (21/48) | |||||
| Unmotivated to quit | Nicotine EC | 23% (9/40) | 15% (6/40) | 13% (5/40) | |||
| Cohort studies not allowing inclusion of non‐responders | |||||||
| Not defined | Daily EC users at baseline | 46% (16/35) | Response rate: 47% (367/773) completed follow‐up survey | ||||
| Not defined | Used EC in the past 30 days (even once) at baseline | 10% (9/88) | Response rate: 81% completed follow‐up Abtsinence rate was 14% (119/861) in non‐EC users | ||||
| Not defined | Used EC for ≥ 1 day in the past 30 days at baseline | 11% | Response rate: unknown Abstinence rate was 17% in non‐EC users | ||||
| 1 All participants (N = 48) used an EC, but 16 also used bupropion and 2 used varenicline | |||||||
| Study | Smokers motivated or unmotivated to quit? | Intervention vs. Control | % reduced by ≥ 50% of baseline cigarette consumption | |||
| 6 month | 12 months | 18 months | 24 months | |||
| Unmotivated to quit | Nicotine EC | 50% (7/14) | ||||
| Motivated to quit | Nicotine EC¹ | 27% (13/48) | ||||
| Unmotivated to quit | Nicotine EC | 33% (13/40) | 28% (11/40) | 28% (11/40) | ||
| 1 All participants (N = 48) used an EC, but 16 also used bupropion and 2 used varenicline | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nicotine EC versus placebo EC Show forest plot | 2 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.05, 4.96] |
| 2 Nicotine EC versus nicotine replacement therapy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nicotine EC versus placebo EC (quitters excluded) Show forest plot | 2 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.02, 1.68] |
| 2 Nicotine EC versus nicotine replacement therapy (quitters excluded) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants reporting adverse events: Nicotine EC versus placebo EC Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Proportion of participants reporting adverse events: nicotine EC versus nicotine replacement therapy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


