Progesterone receptor modulators for endometriosis
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009881.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 25 July 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecology and Fertility Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Selection of studies: JF, YW, MZ, HZ.
Data extraction and management: JF, HZ.
Assessment of risk of bias in included studies: JF, HS.
Consultation: WH.
Review writing: JF, HC.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
JF, HS, MZ, HZ, YW, HC and WH have no interests to declare.
Acknowledgements
Review authors thank the Cochrane Gynaecology and Fertility Group for its support.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jul 25 | Progesterone receptor modulators for endometriosis | Review | Jing Fu, Hao Song, Min Zhou, Huili Zhu, Yuhe Wang, Hengxi Chen, Wei Huang | |
| 2012 May 16 | Progesterone receptor antagonists and progesterone receptor modulators for endometriosis | Protocol | Jing Fu, Lina Hu, Wei Huang, Huili Zhu, Qiushi Wang, Fan He, Lingxia Xie, Xiaoling Gan | |
Differences between protocol and review
Change to title
Upon consultation with the Cochrane Gynaecology and Fertility Group, we changed the title of this review from "Progesterone receptor antagonists and progesterone receptor modulators for endometriosis" to "Progesterone receptor modulators for endometriosis" as PRMs include PRA and SPRM. We added "asoprisnil" or "CBD 2914" or "CDB‐2914" or "CDB‐4124" or "Ulipristal" or "ulipristal acetate" to the search strategies.
Change to objectives
We edited the objectives to make it clear that comparisons with no treatment or with placebo were eligible for the review, as this was unclear from objectives stated in the protocol.
Change to types of interventions in the methods
We edited the types of interventions section to make it clear that surgical interventions were not eligible.
We added decreases in dysmenorrhoea and dyspareunia to the primary outcomes, as these are the types of endometriosis pain reported in most reports.
We added dose or regimen comparisons of PRMs. This was previously a subgroup analysis, but we wished to allow inclusion of studies in which this was the main comparison.
Change to data synthesis
We removed progesterone receptor antagonists because they are a type of progesterone receptor modulator. We added dose or regimen comparisons of PRMs as a main comparison.
We edited the data synthesis section to make it clear that surgical interventions were not eligible.
Change to subgroup analysis and investigation of heterogeneity
As the data synthesis section states that different comparisons will be made for different drugs, we kept 'different course or dosage' in the subgroup and deleted 'different drug'.
Change to types of outcome measures
The protocol stated that pain scores would be used to measure the primary outcome, and this was our preferred measure. However, we also included other pain‐related data if reported by the included studies because we determined that this type of measure would be informative.
Change to measures of treatment effect
The protocol stated that Peto odds ratios would be calculated. Instead, we used Mantel‐Haenszel odds ratios, as Peto odds ratios are not recommended as a default approach for meta‐analysis (Higgins 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Danazol [therapeutic use];
- Dysmenorrhea [drug therapy, epidemiology];
- Dyspareunia [drug therapy, epidemiology];
- Endometriosis [*drug therapy];
- Estrenes [therapeutic use];
- Gestrinone [adverse effects, therapeutic use];
- Gonadotropin‐Releasing Hormone [analogs & derivatives];
- Hormone Antagonists [administration & dosage, adverse effects, *therapeutic use];
- Leuprolide [adverse effects, therapeutic use];
- Mifepristone [administration & dosage, adverse effects, *therapeutic use];
- Norpregnadienes [therapeutic use];
- Oximes [therapeutic use];
- Prevalence;
- Randomized Controlled Trials as Topic;
- Receptors, Progesterone [*antagonists & inhibitors];
Medical Subject Headings Check Words
Female; Humans;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
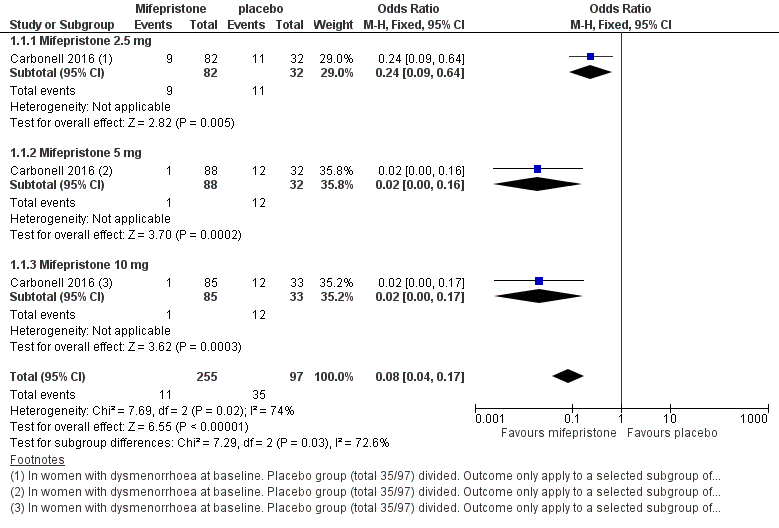
Forest plot of comparison: 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, outcome: 1.1 Dysmenorrhoea at three months.

Forest plot of comparison.
2 Mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.1 Amenorrhoea at three months.

Forest plot of comparison: side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.2 Hot flushes at three months.
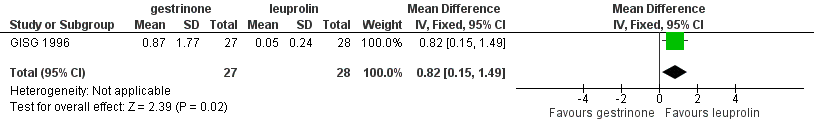
Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.1 Painful periods, visual analogue scale.

Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.3 Pain on intercourse, visual analogue scale.

Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Dysmenorrhoea at 3 months.

Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Dyspareunia at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Amenorrhoea at 3 months.
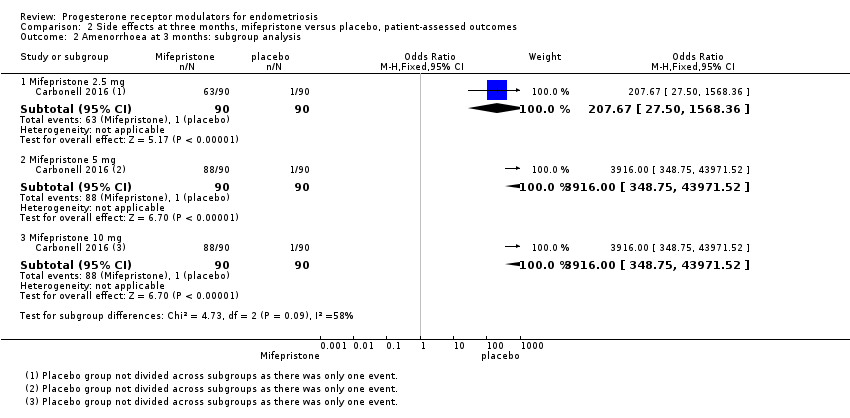
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Amenorrhoea at 3 months: subgroup analysis.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 3 Hot flushes at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 4 Hot flushes at 3 months: subgroup analysis.
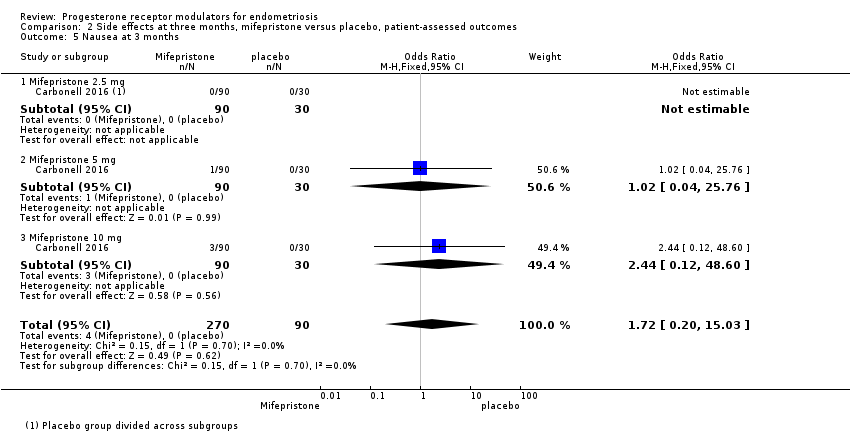
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 5 Nausea at 3 months.
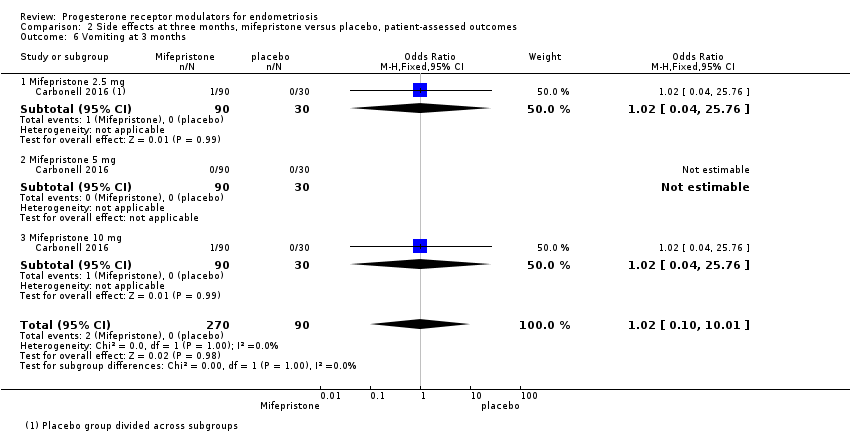
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 6 Vomiting at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 7 Fatigue/Tiredness at 3 months.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 1 Prevalence of dysmenorrhoea.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 2 Dysmenorrhoea score 0‐10 VAS scale.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 3 Prevalence of dyspareunia.
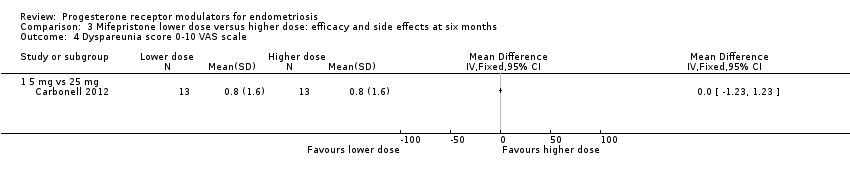
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 4 Dyspareunia score 0‐10 VAS scale.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 5 Prevalence of pelvic pain.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 6 Prevalence of amenorrhoea.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 7 Prevalence of hot flushes.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 8 Prevalence of nausea.
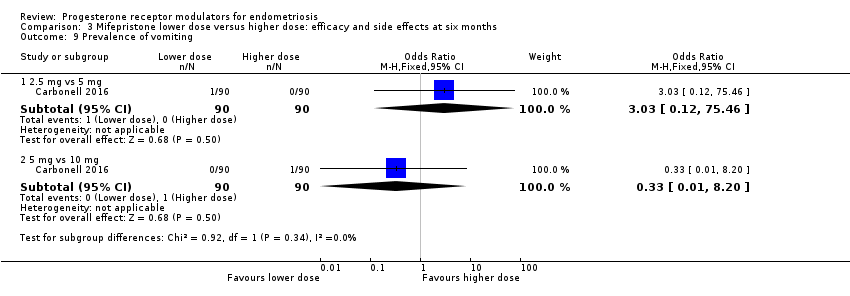
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 9 Prevalence of vomiting.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 10 Prevalence of fatigue/tiredness.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 11 Prevalence of endometrial thickness > 20 mm.

Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 1 None or mild pelvic pain.
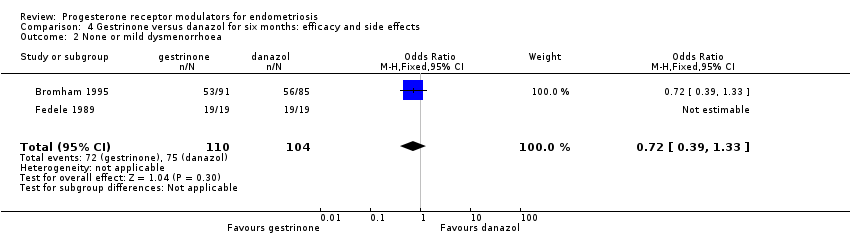
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 2 None or mild dysmenorrhoea.

Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 3 None or mild dyspareunia.

Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 4 Adverse effects.
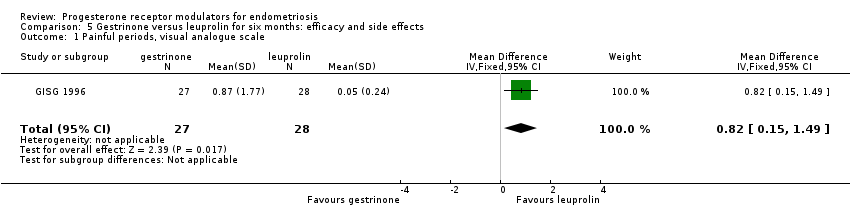
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 1 Painful periods, visual analogue scale.
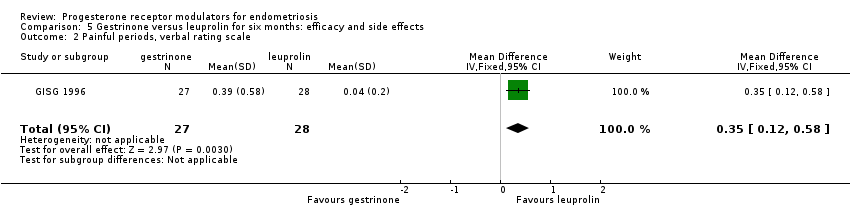
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 2 Painful periods, verbal rating scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 3 Pain on intercourse, visual analogue scale.
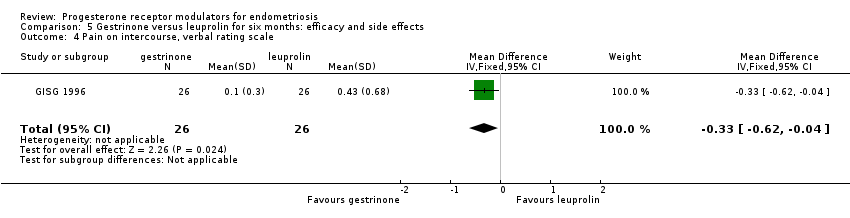
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 4 Pain on intercourse, verbal rating scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 5 Non‐menstrual pain, visual analogue scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 6 Side effects.

Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 1 improvement in pain.
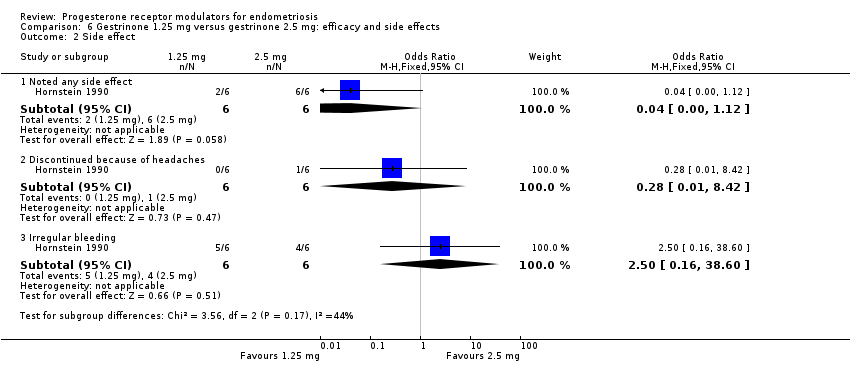
Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 2 Side effect.
| Mifepristone versus placebo for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (mifepristone) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mifepristone | |||||
| Prevalence of dysmenorrhoea Follow‐up: 3 months | 402 per 1000 | 51 per 1000 | OR 0.08 (0.04 to 0.17) | 352 | ⊕⊕⊕⊝ | |
| Prevalence of dyspareunia Follow‐up: 3 months | 288 per 1000 | 85 per 1000 | OR 0.23 (0.10 to 0.51) | 223 | ⊕⊕⊝⊝ | |
| Side effects: amenorrhoea Follow‐up: 3 months | 11 per 1000 | 884 per 1000 | OR 686.16 (92.29 to 5101.33) | 360 | ⊕⊕⊕⊝ High | 239/270 events in the mifepristone group vs 1/90 in the placebo group |
| Side effects: hot flushes Follow‐up: 3 months | 11 per 1000 | 243 per 1000 | OR 28.79 (3.93 to 210.73) | 360 | ⊕⊕⊕⊝ High | 66/270 events in the mifepristone group vs 1/90 in the placebo group |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious imprecision (wide confidence intervals and/or very few events) bOutcome applied only to women with dysmenorrhoea at baseline, but this was 352/360 women randomised, so not downgraded for indirectness cOutcome applied only to women with dyspareunia at baseline, which was 223/360 women randomised. Downgraded one level for serious indirectness | ||||||
| Gestrinone versus danazol for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: danazol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Danazol | Gestrinone | |||||
| Pelvic pain: none or mild | 890 per 1000 | 852 per 1000 (727 to 927) | OR 0.71 (0.33 to 1.56) | 230 (2) | ⊕⊕⊝⊝ | |
| Dysmenorrhoea: none or mild Follow‐up: 6 months | 721 per 1000 | 650 per 1000 | OR 0.72 (0.39 to 1.33) | 214 | ⊕⊝⊝⊝ | |
| Dyspareunia: none or mild Follow‐up: 6 months | 889 per 1000 | 869 per 1000 | OR 0.83 (0.37 to 1.86) | 222 | ⊕⊝⊝⊝ | |
| Side effects: hirsutism Follow‐up: 6 months | 248 per 1000 | 464 per 1000 | OR 2.63 (1.60 to 4.32) | 302 | ⊕⊝⊝⊝ | I2 = 68% |
| Decreased breast size Follow‐up: 6 months | 477 per 1000 | 360 per 1000 | OR 0.62 (0.38 to 0.98) | 302 | ⊕⊕⊝⊝ | |
| Side effects: hot flushes Follow‐up: 6 months | 425 per 1000 | 368 per 1000 (270 to 482) | OR 0.79 (0.50 to 1.26) | 302 (2 studies) | ⊕⊝⊝⊝ | I2 = 72% |
| Side effects: acne Follow‐up: 6 months | 556 per 1000 | 644 per 1000 (529 to 744) | OR 1.45 (0.90 to 2.33) | 302 (2 studies) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAssessed in all randomised participants. Not all were symptomatic at baseline (although results show no significant differences in baseline symptoms between groups). Outcome therefore applies only to a select subgroup of participants: downgraded one level for serious indirectness bDowngraded one level for serious risk of bias associated with poor reporting of study methods, high attrition in one study, and high risk of other bias in both studies cImprecision of results (wide confidence intervals and/or few events), downgraded one level for serious imprecision dDowngraded one level for serious inconsistency | ||||||
| Gestrinone versus leuprolin for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: leuprolin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Leuprolin | Gestrinone | |||||
| Dysmenorrhoea, verbal rating scale Follow‐up: 6 months | In control group, mean score for dysmenorrhoea on verbal rating scale was 0.04 points | Mean score in gestrinone group was 0.35 points higher (0.12 to 0.58 higher) | 55 | ⊕⊕⊝⊝ | Verbal rating scale defines dysmenorrhoea according to limitation of ability to work (mild = 1, moderate = 2, incapacitated = 3) | |
| Dyspareunia, verbal rating scale Follow‐up: 6 months | In control group, mean score for dyspareunia on verbal rating scale was 0.43 points | Mean score in gestrinone group was 0.33 points lower (0.62 to 0.04 lower) | 52 | ⊕⊕⊝⊝ | Verbal rating scale defines dyspareunia according to limitation of sexual activity (discomfort tolerated = 1; pain interrupts intercourse = 2, intercourse avoided owing to pain = 3) | |
| Amenorrhoea Follow‐up: 6 months | 962 per 1000 | 500 per 1000 | OR 0.04 (0.01 to 0.38) | 49 | ⊕⊕⊝⊝ | Only 55 events overall |
| Spotting or bleeding Follow‐up: 6 months | 38 per 1000 | 475 per 1000 | OR 22.92 (2.64 to 198.66) | 49 | ⊕⊕⊝⊝ | Only 12 events overall |
| Side effects: hot flushes Follow‐up: 6 months | 679 per 1000 | 297 per 1000 (112 to 571) | OR 0.20 (0.06 to 0.63) | 55 | ⊕⊕⊝⊝ | Only 27 events overall |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for very serious imprecision: confidence intervals were compatible with no clinically meaningful difference between groups, or with small benefit in one group bDowngraded two levels for very serious imprecision: small overall sample size (n = 55) and low event rates | ||||||
| Outcome | Study | Comparison | Measure | Int group | Control group | P value | |
| Combined non‐pelvic pain, dysmenorrhoea, and dyspareunia | Asoprisnil: 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32) Placebo (n = 34) | Mean reduction at 3 months on 0‐4 pain scale | 0.5 points for each dose | < 0.1 points | < 0.05 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dysmenorrhoea at 3 months Show forest plot | 1 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.17] |
| 1.1 Mifepristone 2.5 mg | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.64] |
| 1.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.16] |
| 1.3 Mifepristone 10 mg | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.17] |
| 2 Dyspareunia at 3 months Show forest plot | 1 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.51] |
| 2.1 Mifepristone 2.5 mg | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.18, 2.13] |
| 2.2 Mifepristone 5 mg | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.40] |
| 2.3 Mifepristone 10 mg | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Amenorrhoea at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 686.16 [92.29, 5101.33] |
| 2 Amenorrhoea at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 207.67 [27.50, 1568.36] |
| 2.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 2.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 3 Hot flushes at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.79 [3.93, 210.73] |
| 4 Hot flushes at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.24 [2.49, 148.54] |
| 4.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.82 [3.11, 182.24] |
| 4.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 46.76 [6.21, 351.92] |
| 5 Nausea at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.20, 15.03] |
| 5.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 5.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.12, 48.60] |
| 6 Vomiting at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.01] |
| 6.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 6.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 7 Fatigue/Tiredness at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.48 [0.71, 42.27] |
| 7.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.21, 73.08] |
| 7.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.40, 125.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of dysmenorrhoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 2.5 mg vs 5 mg | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.22, 3.29] |
| 1.2 5 mg vs 10 mg | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.32, 4.71] |
| 2 Dysmenorrhoea score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prevalence of dyspareunia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2.5 mg vs 5 mg | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.37 [0.74, 54.81] |
| 3.2 5 mg vs 10 mg | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.90] |
| 4 Dyspareunia score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Prevalence of pelvic pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 2.5 mg vs 5 mg | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.63, 5.17] |
| 5.2 5 mg vs 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.79, 19.97] |
| 6 Prevalence of amenorrhoea Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 6.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.56] |
| 6.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.08, 4.41] |
| 7 Prevalence of hot flushes Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.89] |
| 7.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.58] |
| 7.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 8 Prevalence of nausea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.71] |
| 8.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.24] |
| 9 Prevalence of vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.46] |
| 9.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
| 10 Prevalence of fatigue/tiredness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.92 [0.77, 250.94] |
| 10.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.54] |
| 11 Prevalence of endometrial thickness > 20 mm Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 None or mild pelvic pain Show forest plot | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.56] |
| 2 None or mild dysmenorrhoea Show forest plot | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.33] |
| 3 None or mild dyspareunia Show forest plot | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| 4 Adverse effects Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Acne | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.33] |
| 4.2 Seborrhoea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.67, 4.46] |
| 4.3 Hirsutism | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.60, 4.32] |
| 4.4 Voice problems | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.43] |
| 4.5 Swelling hands/feet | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.38] |
| 4.6 Hot flushes | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.26] |
| 4.7 Decreased breast size | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.98] |
| 4.8 Leg or muscle cramps | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 4.9 Headache | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.21] |
| 4.10 Nausea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.19] |
| 4.11 Vomiting | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.43] |
| 4.12 Loss of appetite | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.72, 2.37] |
| 4.13 Hunger | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.97] |
| 4.14 Dizziness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.75, 2.05] |
| 4.15 Tiredness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.84, 2.45] |
| 4.16 Faintness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.54, 2.76] |
| 4.17 Skin rash | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.20] |
| 4.18 Weight gain | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.27] |
| 4.19 Vaginal dryness | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| 4.20 Raised liver transaminases | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painful periods, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.15, 1.49] |
| 2 Painful periods, verbal rating scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.12, 0.58] |
| 3 Pain on intercourse, visual analogue scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐2.08, ‐0.24] |
| 4 Pain on intercourse, verbal rating scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.04] |
| 5 Non‐menstrual pain, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.76, 0.94] |
| 6 Side effects Show forest plot | 1 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 6.1 Seborrhoea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.2 Swelling hands/feet | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.26, 121.96] |
| 6.3 Hot flushes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.4 Leg or muscle cramps | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.55] |
| 6.5 Headache | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.6 Nausea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.7 Dizziness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.8 Skin rash | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.9 Vaginal dryness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.21] |
| 6.10 Mood changes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.10, 4.34] |
| 6.11 Joint pain | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.12 Drowsiness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.13 Tachycardia | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.14 Amenorrhoea | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.38] |
| 6.15 Spotting or bleeding | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.92 [2.64, 198.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 improvement in pain Show forest plot | 1 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 8.46] |
| 2 Side effect Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Noted any side effect | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 1.12] |
| 2.2 Discontinued because of headaches | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 8.42] |
| 2.3 Irregular bleeding | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.16, 38.60] |


