硬膜外疗法治疗非分娩女性的重度先兆子痫
摘要
研究背景
先兆子痫是一种妊娠特异性多器官紊乱疾病,以高血压和多系统器官受累为特征,具有显著的母婴发病率和死亡率。胎盘血管重塑失败和子宫胎盘血流减少是先兆子痫的病因学基础。先兆子痫有几种既定的治疗方法,包括抗高血压药和抗惊厥药。这些疗法中的大多数治疗旨在控制血压或预防高血压升高的并发症,或两者兼有。硬膜外治疗的目的是阻断动脉的血管舒缩张力,从而增加子宫胎盘血流量。本系统综述旨在评价有关硬膜外疗法在治疗重度先兆子痫中可能的获益和风险的现有证据,以确定该治疗的现有证据水平,并确定需要哪些(如果有的话)进一步的证据。
研究目的
评价延长使用硬膜外疗法治疗非分娩妇女的重度先兆子痫的有效性、安全性和成本。本综述旨在比较延长硬膜外治疗与其他方法的使用情况,其他方法包括静脉注射硫酸镁、除硫酸镁以外的抗惊厥药、在治疗重度先兆子痫时联合使用或不联合使用降压药和辅助药物。
本系统综述仅考虑了硬膜外麻醉在产前重度先兆子痫的治疗中的应用,而不考虑在分娩时用于缓解疼痛。
检索策略
我们检索了Cochrane妊娠和分娩试验注册库(Cochrane Pregnancy and Childbirth’s Trials Register)、美国临床试验注册平台(ClinicalTrials.gov)和世界卫生组织(World Health Organization, WHO)国际临床试验注册平台(International Clinical Trials Registry Platform,ICTRP)(2017年7月13日),并检索了所获研究的参考文献列表。
纳入排除标准
比较硬膜外治疗与传统疗法(抗高血压药、抗惊厥药、硫酸镁、低剂量多巴胺、皮质类固醇或这些药物的组合形式)治疗先兆子痫的随机对照试验(randomised controlled reials, RCTs)或半随机对照试验符合纳入标准。使用整群设计的试验和仅以摘要形式发表的研究也符合本系统综述的纳入标准。交叉试验不符合本系统综述的纳入标准。
资料收集与分析
两名综述作者独立评价纳入的试验和试验质量。没有可提取的相关资料。
主要结果
我们纳入了一项小型研究(共24名女性)。该研究是在墨西哥进行的一项单中心随机试验。这项研究对接受抗高血压治疗、抗惊厥治疗、血浆扩张剂、皮质类固醇和双嘧达莫的对照组与接受硬膜外阻滞而不是抗高血压药物以及所有其他四种药物的干预组进行比较。使用10mg/次以及5mg/时的0.25%布比卡因并持续硬膜外输注6小时进行腰椎硬膜外阻滞。该研究在三个领域的偏倚风险低,但由于缺乏分配方案隐藏和对女性和工作人员的盲法,以及随机序列生成和结局评价者盲法的不明确,因此在两个领域被评价为高偏倚风险。
纳入的研究没有报告本系统综述的任何重要结局。无法进行meta分析。
对母亲而言,这些结局为:孕产妇死亡(妊娠期间或妊娠结束后42天内死亡,或妊娠结束后42天后死亡);发生子痫或癫痫复发;中风;任何严重的疾病:定义为中风、肾功能衰竭、肝功能衰竭、HELLP综合征(溶血、肝酶升高和血小板减少)、弥散性血管内凝血、肺水肿中的至少一种。
对婴儿而言,这些结局是:死亡:死产(妊娠20周或之后在宫内死亡)、围产期死亡(死产加上出生后第一周死亡)、出院前死亡、新生儿死亡(出生后28天内死亡),出生28天后死亡;早产(定义为妊娠不足37周就分娩);以及干预的副作用。
报告的结局
纳入的研究仅报告了本系统综述关注的一项单一次要结局:婴儿出生时和出生五分钟后的Apgar评分,干预组和对照组之间无明显差异。
纳入的研究还报告了产妇动脉舒张压的降低。然而,两组之间在本试验的其他报告结局即产妇平均动脉压和动脉收缩压的变化方面无显著差异。
作者结论
目前,尚无足够的随机对照试验证据来评价硬膜外疗法治疗非分娩女性的重度先兆子痫的有效性、安全性或成本。
需要高质量的随机对照试验来评价硬膜外药物治疗重度先兆子痫的使用情况。使用硬膜外麻醉的理由是有充分根据的。然而,没有足够的随机对照试验证据表明硬膜外麻醉的效果转化为改善的母婴结局。因此,需要进行更大规模、设计良好的研究,以得出循证结论,即通过硬膜外治疗降低血管舒缩张力是否可以改善母婴结局以及可以维持多长时间。另一个需要回答的重要问题是延长硬膜外麻醉应用多久才能确保任何潜在的临床获益,以及可能相关的副作用和成本是什么。与其他治疗方式的相互作用和女性满意度可以代表其他研究途径。
PICOs
简语概要
硬膜外疗法治疗重度妊娠高血压综合征以降低母婴的发病率和死亡率
研究问题是什么?
先兆子痫是一种严重的、与妊娠有关的多器官疾病,通常在妊娠晚期影响母亲和婴儿。高血压和尿蛋白是先兆子痫的早期征兆。如果情况严重,妇女会出现头痛、视觉障碍、胃部或上腹部疼痛、恶心及呕吐。他们面临癫痫发作、溶血(红细胞分解)肝酶升高和血小板减少(haemolysis elevated liver enzymes and low platelets,HELLP)综合征、肺水肿、广泛激活凝血、视力丧失、肾或肝功能衰竭以及胎盘早剥(即胎盘从子宫中分离出来)的高风险。液体从血管渗漏到周围组织中,导致肿胀,循环血量减少和流向重要器官的血流量减少。婴儿在出生时或出生后不久面临生长受限、死产、早产或死亡的风险。常见原因大概是流向子宫和胎盘的血流量减少。
本研究为什么重要?
患有重度先兆子痫的妇女接受使用降血压的药物、硫酸镁或其他抗惊厥药以预防癫痫(子痫)发作,以及接受控制血液凝固的药物进行治疗。
延长硬膜外麻醉可能有助于降低重度先兆子痫患者发生中风或脑出血、肾功能衰竭和肝功能衰竭的风险。这可以为最佳的分娩计划提供时间,从而改善母亲和婴儿的结局。流向子宫和胎盘的血流量可能会增加,从而改善分娩结局。延长硬膜外镇痛也已被报告其耐受性可达一周。本系统综述旨在评价硬膜外治疗作为重度先兆子痫的一种治疗方式的应用情况,并将这种治疗与其他已有的治疗进行比较。
我们发现了什么证据?
我们在2017年7月检索了证据,并确定了一项小型随机对照研究(共24名女性)纳入本系统综述。这些妇女怀孕30周或更长时间,被诊断出患有重度先兆子痫,正在重症监护室接受治疗。她们被随机分配到硬膜外阻滞加其他药物治疗组或治疗高血压的药物加其他药物治疗组。经过六个小时的治疗,她们都接受了剖宫产手术。
纳入的研究未报告本系统综述中关注的任何重要结局,如母亲死亡、婴儿死亡(出生前或出生后)、母亲或婴儿所患的严重疾病、母亲患子痫或癫痫,或干预的副作用。
综述作者确实报告了两组在婴儿Apgar评分方面的差异。综述作者还报告了与另一组相比,硬膜外组的舒张压明显降低。两组女性的收缩压和平均血压相似。然而,该研究没有报告本系统综述中关注的其它母亲或婴儿结局。
这些证据意味着什么?
没有足够的随机对照试验证据来评价硬膜外治疗用于重度先兆子痫以改善母亲或婴儿的结局。需要高质量的试验来评价硬膜外治疗用于重度先兆子痫的有效性、安全性以及成本。未来的研究可以报告如本系统综述中所列的重要结局。
Authors' conclusions
Background
The protocol for this review (Dutta 2012) was based on the published generic protocol by Duley 2009 for interventions for treating pre‐eclampsia, and their consequences.
Description of the condition
Pre‐eclampsia is a pregnancy‐specific multi‐system disorder with unpredictable, variable and widespread manifestations. The classic signs of pre‐eclampsia are hypertension and proteinuria, usually after 20 weeks of gestation, although their absence does not exclude the diagnosis.
Pre‐eclampsia occurs in 2% to 8% of pregnancies (WHO 1988; WHO 2005) after 20 weeks' gestation. It causes significant maternal and neonatal morbidity, both in developed and developing countries. Pre‐eclampsia has been found to be the second most common cause of direct maternal death in the 2003 to 2005 CEMACH report (CEMACH 2007), which is a robust and effective system for maternal mortality audit in the UK. Perinatal mortality is also increased with this condition. Manifestations of pre‐eclampsia may present in the antepartum, intrapartum or postpartum periods.
Definitions and classification
Hypertension in pregnancy is usually defined as a systolic blood pressure of at least 140 mmHg, a diastolic blood pressure of at least 90 mmHg, or both on two consecutive blood pressure readings, at least four to six hours apart, after the 20th week of gestation (Cunningham 2014; Gabbe 2013). Proteinuria during pregnancy is defined as 300 mg protein or more in 24 hours (Brown 2000). In a single midstream urine sample, this usually correlates with 30 mg/dL, 1+ or more on a dipstick, or a spot urine protein/creatinine ratio of at least 30 mg/mmol (Duley 2009).
The four main categories of hypertensive disorders of pregnancy are:
-
gestational hypertension or pregnancy‐induced hypertension;
-
pre‐eclampsia/eclampsia (this review concerns this group);
-
chronic hypertension or essential hypertension (pre‐existing hypertension);
-
pre‐eclampsia superimposed on chronic hypertension.
Pre‐eclampsia is a heterogenous multi‐system disorder which causes widespread effects beyond hypertension and proteinuria. Pre‐eclampsia affects up to 10% of nulliparous women, whereas the incidence of severe pre‐eclampsia is about 1%. Although hypertension and proteinuria are the most common manifestations of pre‐eclampsia, the wider spectrum of the disorder should always be considered. Women may present with headache, visual disturbance, epigastric or right upper quadrant pain, nausea or vomiting. Several possible crises may develop. They are eclampsia, haemolysis elevated liver enzymes and low platelets (HELP) syndrome, pulmonary oedema, placental abruption, cerebral blindness, cortical blindness, disseminated intravascular coagulation, renal failure and hepatic failure (Nelson Piercy 2007).
Pre‐eclampsia
Hypertension is regarded as a systolic blood pressure greater than 140 mmHg and a diastolic blood pressure more than 90 mmHg along with significant proteinuria (1+ or more on dipstick on more than one occasion or more than 300 mg in 24 hours on 24‐hour urine collection); otherwise asymptomatic.
Severe pre‐eclampsia
The following features are regarded as signs and symptoms of severe disease: severe hypertension (systolic blood pressure at least 160 mmHg, or diastolic blood pressure of 110 mmHg), severe proteinuria (at least 3 g within range 2 g to 5 g) protein in 24 hours, or 3+ on dipstick), reduced urinary volume (less than 500 mL in 24 hours), neurological disturbances such as headache, visual disturbances, and exaggerated tendon reflexes, epigastric pain, pulmonary oedema, high serum creatinine, low platelet count (less than 100 x 106/L), impaired liver function tests (alanine aminotransferase (ALT) or aspartate aminotransferase (AST) rising to above 70 IU/L), intrauterine growth restriction or reduced liquor volume (Brown 2000; NHBPEP 2000).
Aetiology of pre‐eclampsia
Several risk factors have been associated with the development of pre‐eclampsia. These are: age greater than 40 years, body mass index (BMI) greater than 30, first‐degree relatives, for example, mothers and sisters with a history of pre‐eclampsia, primiparity, multiple pregnancy, long birth interval greater than 10 years, previous pre‐eclampsia, molar pregnancy, pre‐existing hypertension, renal diseases, diabetes, antiphospholipid antibodies, connective tissue diseases and inherited thrombophilia. Placentation and trophoblast invasion is abnormal in pre‐eclampsia, which leads to uteroplacental ischaemia. The placental bed does not undergo the normal vascular remodelling. The spiral arteries fail to become high capacitance, low‐resistance vessels. An Ischaemic placenta leads to endothelial cell damage, development of free radicals, decreased prostaglandin production, and increased thromboxane production, which in turn lead to increased capillary permeability (Nelson Piercy 2007). Endothelial dysfunction results in widespread vasoconstriction and activation of platelets and the coagulation system. Injured endothelial cells allow leakage of fluid out of the blood vessels and into the surrounding tissues, causing oedema and a reduction in the circulating blood volume. There is then inadequate blood flow to many of the woman's organs, especially the kidneys, liver and brain. It is the vasoconstriction, micro clots and reduced circulating blood volume that result in the clinical manifestations of pre‐eclampsia.
Besides endothelial dysfunction, pro‐angiogenic and anti ant‐angiogenic factors have brought newer understanding to the aetiology of pre‐eclampsia in recent years. Pro‐angiogenic factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), are highly expressed by invasive cytotrophoblasts in normal placental development, and their expression is decreased in pre‐eclampsia (Ahmed 2004; Rana 2007). Anti angiogenic factors such as soluble endoglin (sEng) and soluble fms‐like tyrosine kinase‐1 (sFlt1), which act by antagonising VEGF and PIGT have been found to have greater expression in pre‐eclampsia (Maynard 2003) These data linking angiogenic factors to pre‐eclampsia seem to indicate that an imbalance between the anti‐ and pro‐angiogenic factors may have an important role to play in the aetiology of pre‐eclampsia and have exciting clinical implications (Maynard 2011; Rana 2007).
Prevention and treatment of pre‐eclampsia
-
Antenatal surveillance, which includes ambulatory rather than conventional assessment of blood pressure (Bergel 2002), patterns of antenatal care (Dowswell 2015) and admission to day care units (Dowswell 2009).
-
Modification of lifestyle, which includes exercise for prevention of pre‐eclampsia (Meher 2006a), rest for normotensive women (Meher 2006d), protein and energy intake (Ota 2015a) and whether to alter salt intake (Duley 2005).
-
Nutritional supplementation which includes calcium supplementation (Hofmeyr 2014), marine oils (Makrides 2006), antioxidants such as vitamins C and E and selenium (Rumbold 2008), garlic (Meher 2006b), zinc supplements (Ota 2015b), magnesium supplements (Makrides 2014) and pharmacological therapy, which includes diuretic drugs (Churchill 2007), antiplatelet agents (Duley 2010a), anticoagulants (Walker 2003), antihypertensive drugs for women with gestational or chronic hypertension (Abalos 2014), nitric oxide (Meher 2007) and progesterone (Meher 2006c).
All these have been used to prevent pre‐eclampsia and their efficacy is reviewed in Cochrane publications on this subject. There is now evidence that for women with pre‐eclampsia, particularly those with severe pre‐eclampsia, magnesium sulphate reduces the risk of eclampsia and probably reduces the risk of maternal death (Duley 2010b). Magnesium sulphate is currently the anticonvulsant of choice for treating women with eclampsia, with substantial reductions in the risk of further seizures compared with diazepam (Duley 2010e), phenytoin (Duley 2010d), and lytic cocktail (Duley 2010c). Magnesium sulphate is indicated for primary and secondary prophylaxis to women with severe pre‐eclampsia to prevent eclamptic seizures (Magpie 2002).
Once severe pre‐eclampsia is established, the principles of therapy are to control the blood pressure (normally with antihypertensive drugs, although epidural analgesia may also have a role for women in labour), prevent eclampsia (e.g. with magnesium sulphate) and to determine the optimal timing of delivery for the mother and baby (Duley 2009).
Management of severe pre‐eclampsia
Women with severe pre‐eclampsia should be managed in a high‐dependency unit environment according to a set protocol of the unit. Severe pre‐eclampsia is often an indication for delivery. Expectant management is possible only if the woman is stable on the antihypertensive medications with stable laboratory values and reassuring fetal tests. Strict control of hypertension is the single most important manoeuvre. For more than 40 years, dihydralazine has been the drug of choice for this indication. The first choice of the antihypertensive agent for acute control varies, but is usually hydralazine (intermittent intravenous bolus), labetalol (orally or continuous intravenous infusion), and/or nifedipine orally. All of them are equally effective. Labetolol is generally well‐tolerated and no significant maternal toxicity is noted (Cruickshank 1992). The goal is to maintain the diastolic blood pressure between 90 and 105 mmHg or the mean arterial pressure between 100 and 125 mmHg, when on oral doses, as a treatment for mild to moderate cases. Continuous fetal monitoring is necessary, and along with it, renal function and fluid balance of the mother must be monitored carefully. The platelet count and liver function tests should also be monitored. Some women may also have very low urine output in severe pre‐eclampsia, which causes further complications. The drugs which help increase the urine output may possibly help with this problem. Low‐dose dopamine has been used to be one such drug (Steyn 2007). The drug of choice for primary and secondary seizure prophylaxis in severe pre‐eclampsia to prevent eclampsia is intravenous magnesium sulphate (loading dose, 4 g to 6 g infusion, 1 g to 4 g/hour; continued for 24 hours postpartum). Indications for urgent delivery in severe pre‐eclampsia are severe refractory hypertension for more than 24 hours; worsening thrombocytopenia; worsening hepatic or renal disease; eclampsia or premonitory signs of eclampsia (e.g. neurological changes), evidence of fetal growth restriction, or some or all of these. The definitive treatment is always delivery of the fetus and placenta; if the fetus is less than 34 weeks of gestation and delivery can be deferred, corticosteroids should be given, although after 24 hours the benefits of the conservative management should be reassessed (RCOG 2006). Magnesium sulphate more than halves the risk of eclampsia, and probably reduces the risk of maternal death (Kanayama 1999). It does not improve the outcome for the baby in the short term. A quarter of women experience side effects, particularly flushing with this drug (Duley 2010b). Regional anaesthesia is appropriate in most of the women without coagulopathy. This helps control of hypertension by reduction of the pre‐ and afterload and by providing adequate analgesia.
Description of the intervention
Epidural analgesia is one of the most effective methods of pain relief for labour and delivery. This effective technique with an indwelling catheter provides a reliable form of analgesia, that can be titrated as the situation evolves. During labour and delivery, adequate sensory analgesia with minimal motor blockade is the goal (Simmons 2012). Bupivacaine, with a high degree of sensory to motor block, is the most commonly used agent during labour for epidural analgesia worldwide. Concentrations of 0.0625% to 0.125% are commonly used to initiate and maintain the block. The extended use of antepartum epidural therapy induces segmental vasodilatation (Leighton 2002).
Current standard teaching supports the view that epidurals should not be seen as treatment of hypertension nor for any other role than reduction of the catecholamine‐driven consequences of pain (Leighton 2002; Simmons 2012).
How the intervention might work
Pre‐eclampsia is associated with reduced utero‐placental blood flow (UPBF) and fetal growth restriction (Gabbe 2013). As no therapy has been identified to reliably improve UPBF in pre‐eclampsia, obstetric management remote from term frequently involves early delivery, which may impose the additional burden of prematurity on an already compromised fetus. In pre‐eclampsia, impaired trophoblast invasion causes uterine spiral arteries to retain their muscular walls, making them abnormally responsive to sympathetic tone (Cunningham 2014). Increased activity of the sympatho‐adrenal system has been implicated in the genesis and maintenance of elevated blood pressure and vasoconstriction in various vessels such as the uterine artery, and has been observed in pre‐eclampsia and eclampsia. The sympathetic blockade achieved with epidural anaesthesia has been observed to cause vasodilatation in the lower body (Leighton 2002; Sharrrock 1996; Simmons 2012). Epidural anaesthesia could significantly improve placental blood flow in pregnancies complicated by pre‐eclampsia and it has been recommended as the method of choice in labours complicated by pre‐eclampsia.
Two small studies have assessed the effects of antenatal (pre‐labour) extended epidural local anaesthetics on UPBF in women with pre‐eclampsia and fetal growth restriction (Ginosar 2009; Kanayama 1999). These two studies, which varied in the dose of epidural local anaesthetic used, reported reduced umbilical and uterine artery vascular resistance as assessed by Doppler study in some women following treatment (Ginosar 2009; Kanayama 1999). Extended epidural anaesthesia may thus have the potential to improve pre‐eclampsia and the associated adverse outcomes.
Extended epidural analgesia has also been reported to be well‐tolerated for up to one week (Malvasi 2009).
Why it is important to do this review
There is a significant mortality and morbidity associated with severe pre‐eclampsia, and additional therapeutic options would be welcome. The aim of this review is to evaluate the available evidence about the possible benefits and risks of epidural therapy in the management of severe pre‐eclampsia, to define the current evidence level of this therapy, and to determine what (if any) further evidence is required.
Objectives
To assess the effectiveness, safety and cost of the extended use of epidural therapy for treating severe pre‐eclampsia in non‐labouring women. This review aims to compare the use of extended epidural therapy with other methods, which include intravenous magnesium sulphate, anticonvulsants other than magnesium sulphate, with or without use of the antihypertensive drugs and adjuncts in the treatment of severe pre‐eclampsia.
This review only considered the use of epidural anaesthesia in the management of severe pre‐eclampsia in the antepartum period and not as pain relief in labour.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials and quasi‐randomised trials comparing extended epidural therapy with either expectant management or alternative therapies.
Trials using a cluster‐randomised controlled trial design would have been eligible for inclusion in this review but none were identified.
Studies published in abstract form only would have been considered for inclusion, but none were identified. In future updates, studies published in abstract form will only be included providing sufficient information is available. Where there is insufficient information, we will add the study to Studies awaiting classification until the full text is available from the authors. If we do not receive the information needed, we will exclude the study. Cross‐over trials are not eligible for inclusion in this review as the study design is not appropriate for the topic under review.
Types of participants
Pregnant women presenting with signs and symptoms suggestive of severe pre‐eclampsia, eclampsia or any of its complications, such as severe hypertension, stroke, kidney failure with singleton or twin pregnancy and between 20 and 42 weeks of gestation. We did not include women with severe pre‐eclampsia in the postpartum period.
Types of interventions
We had aimed to consider the following comparisons.
-
Extended antepartum epidural therapy versus expectant management
-
Extended antepartum epidural therapy versus combined epidural spinal
-
Extended antepartum epidural therapy versus intravenous magnesium sulphate (all regimens)
-
Extended antepartum epidural therapy versus antihypertensives
-
Extended antepartum epidural therapy versus other anticonvulsants
-
Extended antepartum epidural therapy versus corticosteroids
-
Extended antepartum epidural therapy versus low‐dose dopamine
-
Extended antepartum epidural therapy versus a combination of the above therapies
-
Extended antepartum epidural therapy + standard care versus standard care alone
We considered only the use of epidural in the management of severe pre‐eclampsia in non‐labouring women and not as pain relief in labour.
Types of outcome measures
Primary outcomes
For the woman
-
Death: during pregnancy or up to 42 days after the end of the pregnancy, or death more than 42 days after the end of the pregnancy
-
Development of eclampsia or recurrence of seizures
-
Stroke
-
Any serious morbidity: defined as at least one of stroke, kidney failure, liver failure, HELLP syndrome (haemolysis, elevated liver enzymes and low platelets), disseminated intravascular coagulation, pulmonary oedema
For the child
-
Death: stillbirths (death in utero at or after 20 weeks' gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from the hospital, neonatal deaths (death within the first 28 days after birth), deaths after the first 28 days
-
Preterm birth, defined as the birth before 37 completed weeks' gestation
Secondary outcomes
For the woman
-
Kidney failure
-
Liver failure
-
HELLP syndrome
-
Disseminated intravascular coagulation
-
Pulmonary oedema (fluid in the lungs)
-
Progression to severe hypertension: defined where possible as systolic blood pressure 160 mmHg or more, or diastolic blood pressure 110 mmHg or more
-
Use of antihypertensive drugs or need for additional antihypertensive drug
-
Abruption of the placenta or antepartum haemorrhage
-
Elective delivery: induction of labour or caesarean section
-
Caesarean section: emergency and elective
-
Postpartum haemorrhage: defined as blood loss of 500 mL or more
-
Side effects or adverse events: any side effects or adverse events related to the intervention (e.g. epidural haematoma, abscess, or other epidural‐related complications); intervention stopped due to side effects
-
Breastfeeding, at discharge and up to one year after the birth
-
Women's experiences and views of the interventions: childbirth experience, physical and psychological trauma, mother‐infant interaction and attachment
-
Coagulopathy
-
Infection (epidural abscess)
-
Neurological complications (spinal cord injury leading to transient paraesthesia or paraplegia)
For the child
-
Severity of preterm birth: very preterm birth (before 32 to 34 completed weeks) and extremely preterm birth (before 26 to 28 completed weeks)
-
Death before discharge from hospital or in a special care nursery for more than seven days
-
Respiratory distress syndrome
-
Infection
-
Necrotising enterocolitis
-
Retinopathy of prematurity
-
Intraventricular haemorrhage
-
Fetal growth restriction:defined as growth below the third centile, or lowest centile reported
-
Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported
-
Use of hospital resources: admission to special care nursery, length of stay, endotracheal intubation, use of mechanical ventilation
-
Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy
-
Side effects associated with the intervention
Economic outcomes
-
Costs to health service resources: short term and long term for both mother and baby
-
Costs to the woman, her family, and society
Post dural puncture headache is also an outcome of interest but this can be addressed only for the group of women who receive epidural.
Search methods for identification of studies
The search methods for identification of studies for this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (13 July 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (13 July 2017) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1.
Searching other resources
We searched bibliographies of retrieved primary articles.
We did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth Group.
Selection of studies
Both review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. There were no disagreements between the two assessors. In future updates, any disagreements will be resolved through discussion or, if required, by consulting a third person.
Data extraction and management
We designed a form to extract data and both review authors independently extracted data(as listed below) for the one included trial. In future updates of this review if more eligible studies are identified we plan to extract data under the same headings and contact authors of the original reports to provide further details if needed, after which we will enter the data into Review Manager software (RevMan 2014) and check for accuracy.
Study characteristics
-
Baseline characteristics (randomised controlled study, single or multi‐centric, etc)
-
Method of sequence generation
-
Method of allocation concealment
-
Blinding (of participants, personnel and outcome assessors)
-
Number of women randomised, excluded or lost to follow‐up
-
Whether an intention‐to‐treat (ITT) analysis was done
-
Whether a power calculation was done
-
Duration, timing and location of the study
-
Source of funding
-
Trialists' declaration of interest
Participant characteristics
-
Age
-
Period of gestation
-
Symptoms and signs that indicate severe pre‐eclampsia
-
Time of onset of disease (we will not include postpartum pre‐eclampsia)
-
Inclusion criteria
-
Exclusion criteria
Interventions
-
Details of epidural therapy including the dose,duration, and combination with other medical interventions
-
Type of control group (what interventions were used)
Outcomes
-
The review authors extracted, where possible, data for the outcomes listed in the Types of outcome measures
Funding
-
The authors extracted where possible data for funding sources
Declarations of interest
-
The authors also noted whether there was any declarations of interest by the trialists in full text of the included study
The review authors aimed to compare interventions individually and in combination. In the one included study the epidural therapy was used in combination with other interventions. If in future updates the review authors are able to compare epidural therapy independently or in combination with other interventions in randomised controlled studies, they will include them for meta‐analysis.
The review had aimed to compare outcomes as specified in the section Types of outcome measures. Except for the neonatal Apgar score, the included study did not have any other outcomes specified in this section. If, in future versions of the review such outcomes are reported, we will extract the data. Also, we will extract outcome data at short‐ and long‐term follow‐up, (short‐term and long‐term epidural therapy).
Assessment of risk of bias in included studies
Both review authors (SR and AR) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
For the one included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include any missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included study any important concerns we have about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We only included a single study in this review. For future updates, we will assess the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons. The following outcomes will be assessed using GRADE.
For the woman
-
Death: during pregnancy or up to 42 days after the end of the pregnancy, or death more than 42 days after the end of the pregnancy
-
Development of eclampsia or recurrence of seizures
-
Stroke
-
Any serious morbidity: defined as at least one of stroke, kidney failure, liver failure, HELLP syndrome (haemolysis, elevated liver enzymes and low platelets), disseminated intravascular coagulation, pulmonary oedema
For the child
-
Death: stillbirths (death in utero at or after 20 weeks' gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from the hospital, neonatal deaths (death within the first 28 days after birth), deaths after the first 28 days
-
Preterm birth, defined as the birth before 37 completed weeks' gestation
-
Side effects of the intervention.
In future updates, we will use the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we planned to present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. However, if we identify any in future updates, we will include them in our analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Studies using a cross‐over design are not eligible for inclusion in this review ‐ we consider this design to be inappropriate for the topic under review.
Dealing with missing data
In future updates, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
There are no meta‐analysis in this review. In future updates, we will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We did not carry out data synthesis in this review due to insufficient data. In future updates, we will carry out statistical analysis using RevMan 5 software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: that is, where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I² statistic.
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
-
according to the anaesthetic drug used; opioid analgesics versus opioid analgesics and local anaesthetics.
We will restrict subgroup analyses to the review's main outcomes.
We will assess the subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. We will also perform a sensitivity analysis to examine the robustness of the combined results between the study designs. If we combine cluster‐RCTS along with individually‐randomised RCTs we will carry out sensitivity analysis to investigate the effect of the randomisation unit.
We will restrict sensitivity analysis to the primary outcomes of the review.
Results
Description of studies
Results of the search
See: Figure 1 (study flow diagram).

Study flow diagram.
The search retrieved four reports relating to three studies (Ginosar 2009; Pardo Morales 2004; Pyregov 2010). Three reports were described as randomised controlled trials wherein epidural therapy was used in non‐labouring women with pre‐eclampsia. The fourth report was a protocol for one of the three trials (Ginosar 2009). One study was included (Pardo Morales 2004) and two were excluded (Ginosar 2009; Pyregov 2010).
Included studies
We included one study in this review (Pardo Morales 2004) involving 24 women.
Design and settings
Pardo Morales 2004 was a single‐centre randomised trial conducted at the Pre‐eclampsia‐Eclampsia Research Unit, Mother & Child Institute of the State of Mexico, Toluca. A total of 24 women were included in the study with 12 participants in each group.
Participants
Participants in the included study were women diagnosed with severe pre‐eclampsia according to international criteria (hypertension and albuminuria), 30 weeks of gestational or more, inpatients in the intensive care unit, with a platelet count higher than 70.000/mm3, and a clotting time higher than 70% without contraindications to epidural block and after written consent.
Interventions and comparisons
The control group in the Pardo Morales 2004 study received antihypertensive therapy, anticonvulsant therapy, plasma expanders, corticosteroids and dypyridamole. The intervention group received epidural block instead of antihypertensives, as well as the same additional four drugs as the control group (i.e. anticonvulsants, plasma expanders, corticosteroids and dipyridamole. Lumbar epidural block was given using 0.25% bupivacaine, 10 mg bolus and 5 mg each hour on continuous epidural infusion for six hours. Subsequently, all 24 women underwent caesarean section.
Outcomes
The one included study (Pardo Morales 2004), reported only one outcome of interest to the review: the Apgar score of the infant at birth and five minutes, which did not show any difference between the two groups. There were no numerical data given by the trialists for individual participants in either group. No other outcome of interest to the review was reported. Maternal outcomes of mean arterial pressure, systolic arterial pressure and diastolic arterial pressure were reported, of which only the diastolic pressures showed significant reduction in the intervention group.
Declaration of interest
The trialists did not report any declarations about conflict of interests.
Funding
The trialists did not report any funding from any source for their study.
Excluded studies
We excluded two studies (Ginosar 2009; Pyregov 2010). Ginosar 2009 was a cross‐over design and therefore, is not eligible for inclusion. Pyregov 2010 was only available in abstract form. It was not clear from the abstract whether there were two comparison groups or the intervention had been used in the same group with results assessed before and after. We attempted to contact the trial author to clarify this issue but did not receive a reply. Consequently, we decided to exclude this study.
Risk of bias in included studies
The risk of bias has been summarised in the Figure 2.
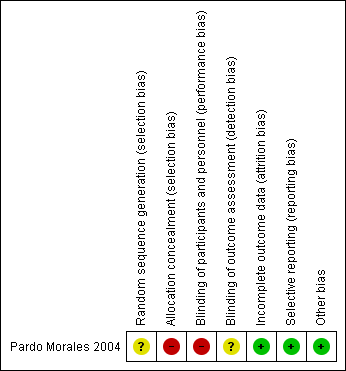
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
The included (Pardo Morales 2004) study was described as 'randomised' but the way in which the sequence was generated was not specified. Therefore, we have stated unclear risk of bias for sequence generation.
Allocation concealment
The included study (Pardo Morales 2004) stated that it was an open study, probably because allocation could not have been concealed, so we have assessed this study as high risk of bias for this domain.
Blinding
Performance bias
The study was an open trial (for participants and personnel) so we have assessed the study as being at a high risk of performance bias.
Detection bias
It was not clear whether outcome assessors were blinded and so this domain was assessed to be at unclear risk of detection bias. However, as the outcome measures were mostly objective (mean arterial blood pressure, diastolic blood pressure, systolic blood pressure), this is unlikely to affect the recording of these outcomes.
Incomplete outcome data
The Pardo Morales 2004 study had a total of 24 participants with 12 in each arm. All of the participants completed the study and all results were reported and the study was stated as low risk of bias for this domain.
Selective reporting
All the stated outcomes have been reported in the included study so we assessed the study as being at a low risk of reporting bias.
Other potential sources of bias
No other sources of bias were identified (low risk of bias).
Effects of interventions
There was only one included study (Pardo Morales 2004). Consequently, there are no data or meta‐analysis in this review.
Below we provide a brief narrative summary of the included study for information only.
Epidural versus antihypertensives
One small study (Pardo Morales 2004), with a total of 24 women, included a comparison of epidural versus antihypertensives (both groups of women also received plasma expanders, anticonvulsants and corticosteroids). Pardo Morales 2004 examined mean arterial blood pressure, diastolic blood pressure, systolic blood pressure and infant Apgar score at birth. The trial authors concluded that diastolic blood pressure showed a statistically significant difference in favour of epidural but there were no between‐group differences detected for the other outcomes measured and no adverse events were reported. The infant Apgar scores at birth and at five minutes did not show any significant difference between the two groups.
Discussion
Summary of main results
Although epidural anaesthesia is extensively used for pain relief in labour, there are limited data available on the use of epidural as treatment for severe pre‐eclampsia (Leighton 2002; Simmons 2012.) The rationale behind use of epidural as a therapy is its ability to cause sympathetic blockade, which causes vasodilatation and which in turn increases the placental blood flow (Ginosar 2009; Kanayama 1999). Although pre‐eclampsia has several causative elements, the final common pathway is the failure of the trophoblastic tissue to invade the spiral arteries (Cunningham 2014; Gabbe 2013), thus limiting placental blood flow. Inducing vasodilatation may improve blood flow, as well as potentially lowering the blood pressure and preventing several complications of pre‐eclampsia both to the mother and the fetus or neonate (Ginosar 2009; Kanayama 1999).
We identified one small study (Pardo Morales 2004) for inclusion in this review, The study reported only one outcome of interest to the review namely the infant Apgar score at birth and at five minutes. This did not show any difference between the two groups. For this outcome, the trialists did not provide any numerical data for individual participants. It also reported a favourable response to epidural therapy by way of lowering the diastolic pressure. The study (Pardo Morales 2004) had two separate groups: one received antihypertensives with other standard therapies while the other group received epidural therapy together with the same standard therapies as the antihypertensive group. There were no significant differences between the two groups in terms of systolic and mean arterial pressure. The study authors reported that diastolic pressure showed a significant drop (P < 0.05) in the epidural group. Since there was only one study meta‐analysis was not possible.
Overall completeness and applicability of evidence
The issue of effectiveness and safety of epidural therapy as a treatment for severe pre‐eclampsia remains unknown. The rationale behind use of epidural therapy is theoretically sound. The mechanism of action of the epidural (vasodilatation) also answers the issue of lowering of the blood pressure and the ability of the trophoblast to invade the spiral arteries, which is the final common pathway for development of pre‐eclampsia. However, there is no evidence from randomised controlled trials to show that this action translates to better maternal and fetal outcomes.
Quality of the evidence
We were unable to carry out an evaluation of the quality of the body of evidence using GRADE because there only one study was included, which did not contribute any data for our main outcomes. The study that we included in this review was at a high risk of selection and performance bias as it was an open trial. It was unclear what method of random sequence generation was used, and what effect lack of blinding of outcome assessors would have on the outcomes.
Potential biases in the review process
Searching for, and selection of studies for the review was thorough. We carried out and extensive search for studies that encompassed both published and unpublished data and the search was iterative. The potential for missing any relevant or eligible study seems unlikely.
Agreements and disagreements with other studies or reviews
The definitive treatment of pre‐eclampsia is the birth of the baby (Cunningham 2014; Duley 2009; Gabbe 2013). The many therapies that are used in women with severe pre‐eclampsia aim at prolonging the pregnancy to term without complications to mother and child. There are several reviews including Cochrane reviews that have generated robust and sound evidence as regards the efficacy of various methods for prevention and treatment of severe pre‐eclampsia (Churchill 2007; Duley 2009; Ota 2015b; Meher 2006c). Adequate evidence has also been generated in terms of anticonvulsants (Duley 2010b), and antihypertensives (Abalos 2014), which are usually used as adjuvant therapy in severe pre‐eclampsia. There is no study or review that looks at the efficacy of extended epidural therapy in terms of clinical outcomes for the mother and infant.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

