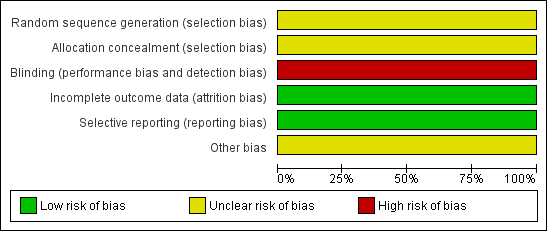
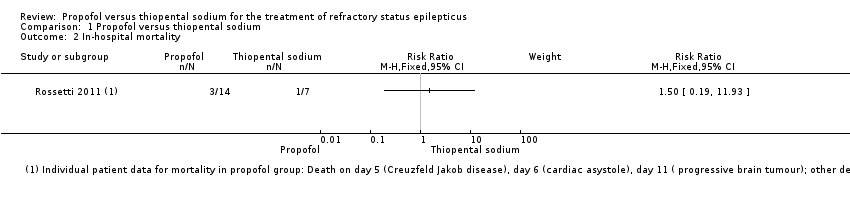
Related content
Related reviews and protocols
Manya Prasad, Pudukode R Krishnan, Reginald Sequeira, Khaldoon Al‐Roomi | 10 September 2014
Amy McTague, Timothy Martland, Richard Appleton | 10 January 2018
Graham Powell, Matthew Saunders, Alexandra Rigby, Anthony G Marson | 9 December 2016
Francesco Brigo, Stanley C Igwe, Nicola Luigi Bragazzi | 18 May 2017
JinSong Geng, JianCheng Dong, Youping Li, HengJian Ni, Kui Jiang, Li Li Shi, GuoHua Wang | 2 December 2019
Lin Song, Fang Liu, Yao Liu, Ruoqi Zhang, Huanhuan Ji, Yuntao Jia | 20 April 2020
John Paul Leach, Anthony G Marson, Jane L Hutton | 21 October 2002
Jia Liu, Lu-Ning Wang | 11 May 2021
Mariangela Panebianco, Sarah Al-Bachari, Jane L Hutton, Anthony G Marson | 12 January 2021
Francesco Brigo, Stanley C Igwe, Alessandra Del Felice | 11 August 2016