Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009069.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 09 May 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Conception of the systematic review: M. Arbyn, L Markowitz, P. Martin‐Hirsch.
Study design: M. Arbyn.
Writing of the protocol: M. Arbyn, A. Bryant, C. Simoens, L Markowitz.
Writing of the full review: M. Arbyn, L Xu, C Simeons.
Retrieval of references: M. Arbyn, L. Xu, C. Simoens.
Checking eligibility of references: M. Arbyn, L. Xu, C. Simoens.
Extraction of data: M. Arbyn, C. Simoens, L. Xu.
Statistical analysis: M. Arbyn, Lan Xu.
Critical review of the manuscript: P. Martin‐Hirsch, C. Simoens, L. Markowitz.
Sources of support
Internal sources
-
Scientific Institute of Public Health (Brussels), Belgium.
Bibliographic support to obtain literature references, secretarial and logistic support in organising contacts and meetings with co‐authors and to store and sort bibliographic references. references
External sources
-
National Institute for Health Research, UK.
NHS Cochrane Programme Grant Scheme CPG‐506 funding to the Gynaecological, Neuro‐oncology and Orphan Cancer Group.
-
European Cancer Network and the European Co‐operation on development and implementation of Cancer screening and prevention Guidelines (ECCG), via the International Agency for Research on Cancer, Lyon), France.
Financial support received from the European Commission (DG SANCO, Luxembourg) for the production of guidelines for cervical cancer screening and HPV vaccination.
-
Belgian Foundation Against Cancer (Brussels), Belgium.
Financial support to conduct methodological research on evaluation of emerging screening methods and to continue systematic reviews on cervical cancer prevention methods.
-
IWT (Institute for the Promotion of Innovation by Science and Technology in Flanders, Brussels, project number 060081), Belgium.
Financial support to collect data for mathematical modelling of HPV infection (natural history, cost‐effectiveness of HPV screening and vaccination).
-
CoheaHr Network (Comparing Health Services Interventions for the Prevention of HPV‐Related Cancer) (grant number 603019) funded by the 7th Framework Programme of DG Reasearch and Innovation, European Commission (Brussels), Belgium.
Financial support to update the Cochrane review (2015‐18)
Declarations of interest
MA: has received travel grants from MSD‐Sanofi‐Pasteur and GSK, (ceased in 2008).
PM‐H: travel grants received from GSK and MSD‐Sanofi‐Pasteur (ceased in 2008).
LX: no conflict of interest.
CS received travel grant from GSK (2007).
Authors of this review were assessed by the Cochrance Funding Arbiter Committee after Cochrane received correspondence and feedback on the published protocol. Current authors were approved by this committee based on stringent Cochrane conflict of interest guidelines. A unrestricted grant was provided by Sanofi‐Pasteur‐MSD to the University of Ghent who co‐ordinated the SEHIB study (Surveillance of Effects of HPV Immunisation in Belgium). The grant was given in the framework of the EMA (European Medicine Agency) request to set up post‐marketing surveillance of HPV vaccination effects in non‐Nordic member states of the European Union. The Sciensano (employer of MA and LX, former name “Scientific Institute of Public Health”) collaborated with the University of Ghent to conduct the SEHIB study.
Acknowledgements
We would like to acknowledge the input of the following individuals in the development of the protocol for this review: L Markowitz, A Bryant, J Dillner, E. Paraskevaidis, P. Beutels, M Steben, A Schneider, A Kauffman, Z‐H Zhao, Y‐L. Qiao, and A Hildesheim. We thank Jo Morrison and Tess Lawrie for clinical advice, Jo Platt for designing the search strategy and Toby Lasserson, Gail Quinn and Clare Jess for their contribution to the editorial process.
We acknowledge Lauri Markowitz for her invaluable advice and contributions by reviewing the results and discussion sections.
This project was supported by the National Institute for Health Research, via Cochrane Programme Grant Funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Koen de Visscher (KODEVIS, Lokeren, Belgium) is acknowledged for the production of high‐quality graphical png files
Version history
| Published | Title | Stage | Authors | Version |
| 2018 May 09 | Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors | Review | Marc Arbyn, Lan Xu, Cindy Simoens, Pierre PL Martin‐Hirsch | |
| 2013 Dec 30 | Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors | Protocol | Marc Arbyn, Andrew Bryant, Pierre PL Martin‐Hirsch, Lan Xu, Cindy Simoens, Lauri Markowitz | |
| 2011 Apr 13 | Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors | Protocol | Marc Arbyn, Andrew Bryant, Philippe Beutels, Pierre PL Martin‐Hirsch, Evangelos Paraskevaidis, Elke Van Hoof, Marc Steben, Youlin Qiao, Fang‐Hui Zhao, Achim Schneider, Andreas Kaufmann, Joakim Dillner, Lauri Markowitz, Allan Hildesheim | |
Differences between protocol and review
The three following items, foreseen in the original protocol, were not addressed in the current version of the review and the reasons why are explained in the Discussion.
-
Immunogenicity of the vaccines
-
Request for non‐published available data
-
Protection against high‐grade cervical intra‐epithelial neoplasia (CIN2 or worse) attributed to non‐vaccine HPV types.
We were not able to conduct the latter analysis but the latter outcome was included indirectly in the outcome CIN2+ irrespective of HPV types.
The three points not assessed in the current review will be integrated in future updates of the review.
In the Cochrane protocol (developed when several trials were still ongoing), it was foreseen that websites of regulatory agencies like the US Food & Drug Administration (FDA) and the European Medicine Agency (EMEA) would be consulted to obtain data on safety and efficacy effects. However, currently, nearly all end‐of‐study reports have been published in the peer‐reviewed literature. We therefore did not need to consult these additional sources any more. For serious adverse events, death after vaccination and pregnancy outcomes, we consulted data posted on www.clinicaltrials.gov and http://www.gsk‐clinicalstudyregister.com/ to obtain additional data on critical safety issues not available from the peer‐reviewed sources. This has been incorporated into a sensitivity analysis (see Sensitivity analysis).
Assessment of the variation of vaccine efficacy by age group in more detail than the broad distinction younger or older than 25 years could not be done for most studies by lack of reported age‐specific data. However, for the bivalent vaccine, an analysis by five‐year age group could be performed.
Methods described in the protocol to handle continuous data were not used since immunogenicity was dropped from the review as an objective. Time‐to‐event data methods were not applied either, because of the abundance of dichotomous data reported at repeated time points and because of the rarity of presentation of results in longitudinal formats. Specific statistical methods to assess cluster‐randomised trials were not required since all trials randomised enrolled participants at individual level.
In this Cochrane review, treatment effects were expressed as risk ratios (RR) and not as "vaccine efficacy" since the latter is not supported by Cochrane software.
Sensitivity analyses excluding studies at moderate or high risk of bias were foreseen in the protocol. However, given the low risk of bias of all the trials reporting efficacy outcomes and the detailed subgroup analyses and meta‐regression analyses assessing the impact of each separate item of the Cochrane tool for assessment of risk of bias, these sensitivity analyses were considered as superfluous.
We planned to distinguish adverse effects occurring in the period between zero to four weeks and more than four weeks after administration of vaccines. However, since this timing of observation of adverse events was not documented uniformly in the trials reports, this distinction could not be implemented in the review. No sensitivity analysis based on risk of bias was performed as described in the original protocol, as the studies were assessed to be at low risk of bias. Impact of influential factors, such as involvement of the vaccine manufacturers, were addressed sufficiently by meta‐regression.
Notes
Following the publication of a critical commentary of this review in July 2018 (https://ebm.bmj.com/content/23/5/165), its findings were subject to an investigation overseen by the then Editor in Chief of the Cochrane Library, Dr David Tovey. The outcome of this investigation was published online in September 2018 and can be found here: https://www.cochrane.org/news/cochranes‐editor‐chief‐responds‐bmj‐ebm‐article‐criticizing‐hpv‐review. Since this time, a systematic review by the team of authors who wrote this commentary was published in March 2020: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643‐019‐0983‐y, with a related methods article (https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643‐020‐01300‐1), and an accompanying commentary (https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643‐020‐01299‐5).
In 2018 Cochrane made a public commitment to incorporate the findings of this assessment as an amendment of this review. In order to ensure that its numerical findings match with those presented in the original investigation of the review, this work is now being commissioned. Furthermore, in view of the continued importance of this vaccine, there is now an opportunity to look at the comparative effects of these vaccines and to incorporate evidence from multiple sources of data that are now available for these trials. This will be investigated as a separate Cochrane Review
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Human papillomavirus 16;
- Human papillomavirus 18;
- Papillomavirus Infections [complications, mortality, *prevention & control];
- Papillomavirus Vaccines [*administration & dosage, adverse effects];
- Precancerous Conditions [mortality, *prevention & control, virology];
- Pregnancy Outcome;
- Randomized Controlled Trials as Topic;
- Uterine Cervical Dysplasia [mortality, *prevention & control, virology];
- Uterine Cervical Neoplasms [mortality, *prevention & control, virology];
- Vaccination;
Medical Subject Headings Check Words
Adolescent; Adult; Female; Humans; Middle Aged; Pregnancy; Young Adult;
PICOs

Flow diagram summarising the retrieval, inclusion and exclusion of relevant reports of randomised trials assessing the safety and effects of prophylactic HPV vaccines.
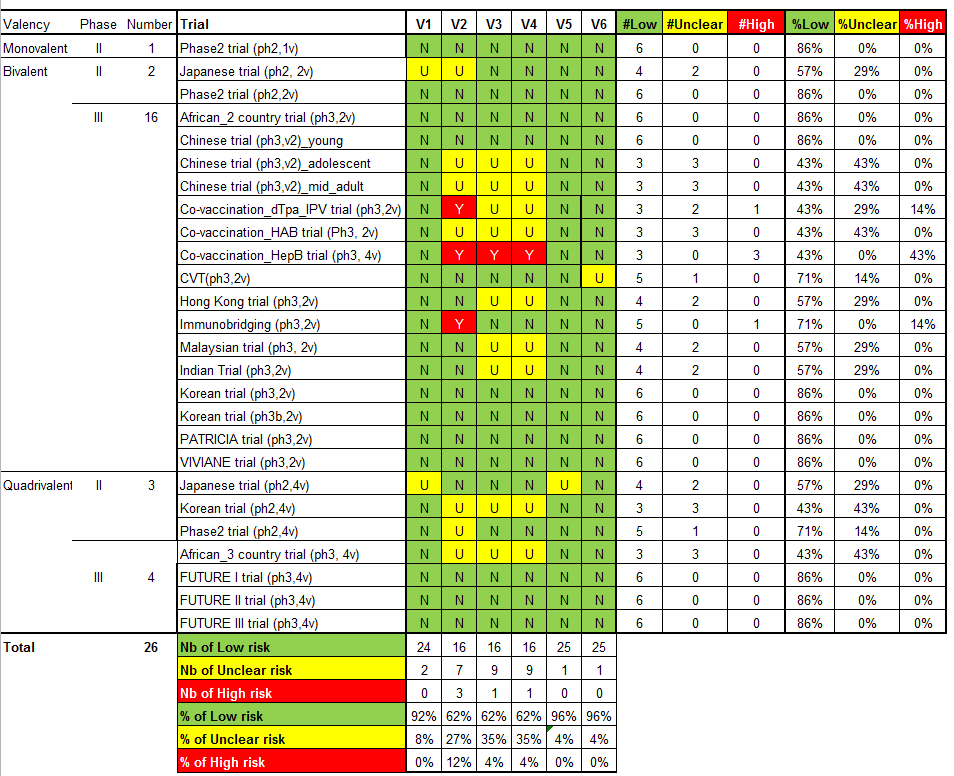
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
V1 = Random sequence generation; V2 = Allocation concealment; V3 = Blinding participants & personnel; V4 = Blinding of outcome assessment; V5 = Incomplete outcomes; V6 = Selective reporting.
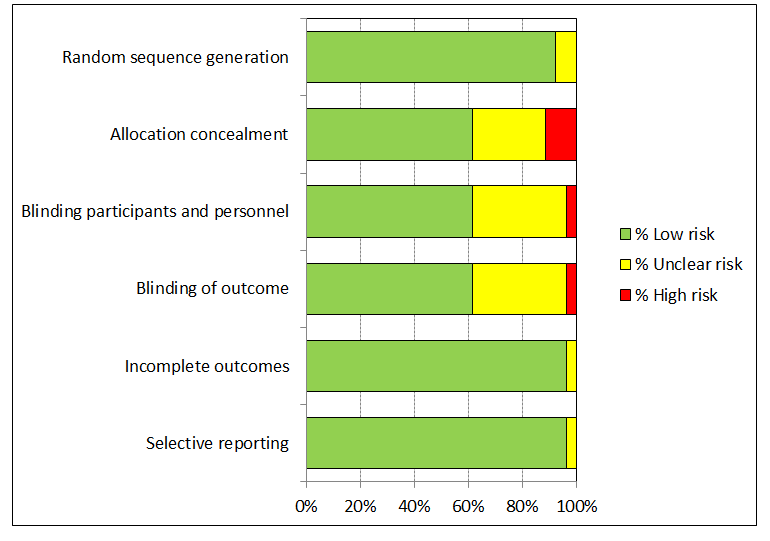
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Protection against CIN2+ irrespective of presence of HPV types in women, aged 15‐26 years, regardless of their HPV DNA status at baseline, who received at least one dose.
![Summary of vaccine efficacy estimates, by age group, outcome and HPV DNA status at enrolment (for women who received at least one dose). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][](/cdsr/doi/10.1002/14651858.CD009069.pub3/media/CDSR/CD009069/image_n/nCD009069-AFig-FIG06.png)
Summary of vaccine efficacy estimates, by age group, outcome and HPV DNA status at enrolment (for women who received at least one dose). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][
![Summary of vaccine efficacy estimates by age group, outcome and number of received doses (for women who were HPV16/18 DNA negative at enrolment). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][](/cdsr/doi/10.1002/14651858.CD009069.pub3/media/CDSR/CD009069/image_n/nCD009069-AFig-FIG07.png)
Summary of vaccine efficacy estimates by age group, outcome and number of received doses (for women who were HPV16/18 DNA negative at enrolment). [REFS BETWEEN SQUARE BRACKETS MUST BE ADAPTED][
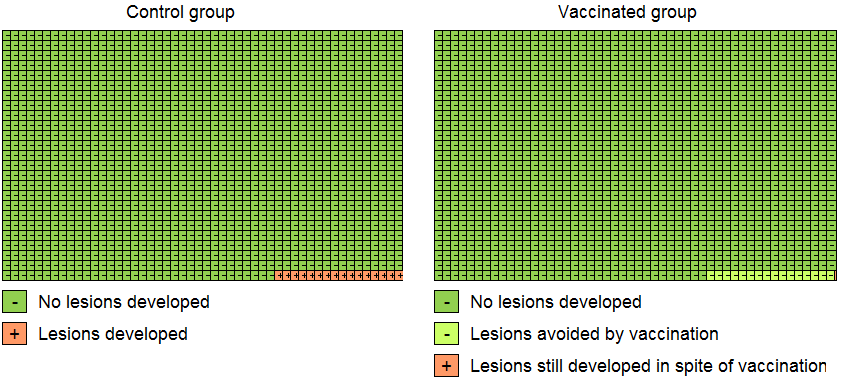
Modified Cates plot: Number of cases of CIN2+ associated with HPV16/18 occurring in women who were all hrHPV DNA negative at baseline. 16 out of 1000 non‐vaccinated women developed the lesion (left) whereas fewer than one (0.2) out 1000 vaccinated women developed the lesion (right). Relative risk= 0.01 (95% CI: 0.01 to 0.05).

Modified Cates plot: Number of cases of CIN2+ irrespective of HPV types occurring in women who were all hrHPV DNA negative at baseline. 28 out of 1000 non‐vaccinated women developed the lesion (left) whereas 11 out 1000 vaccinated women developed the lesion (right). Relative risk= 0.37 (95% CI: 0.25 to 0.55).
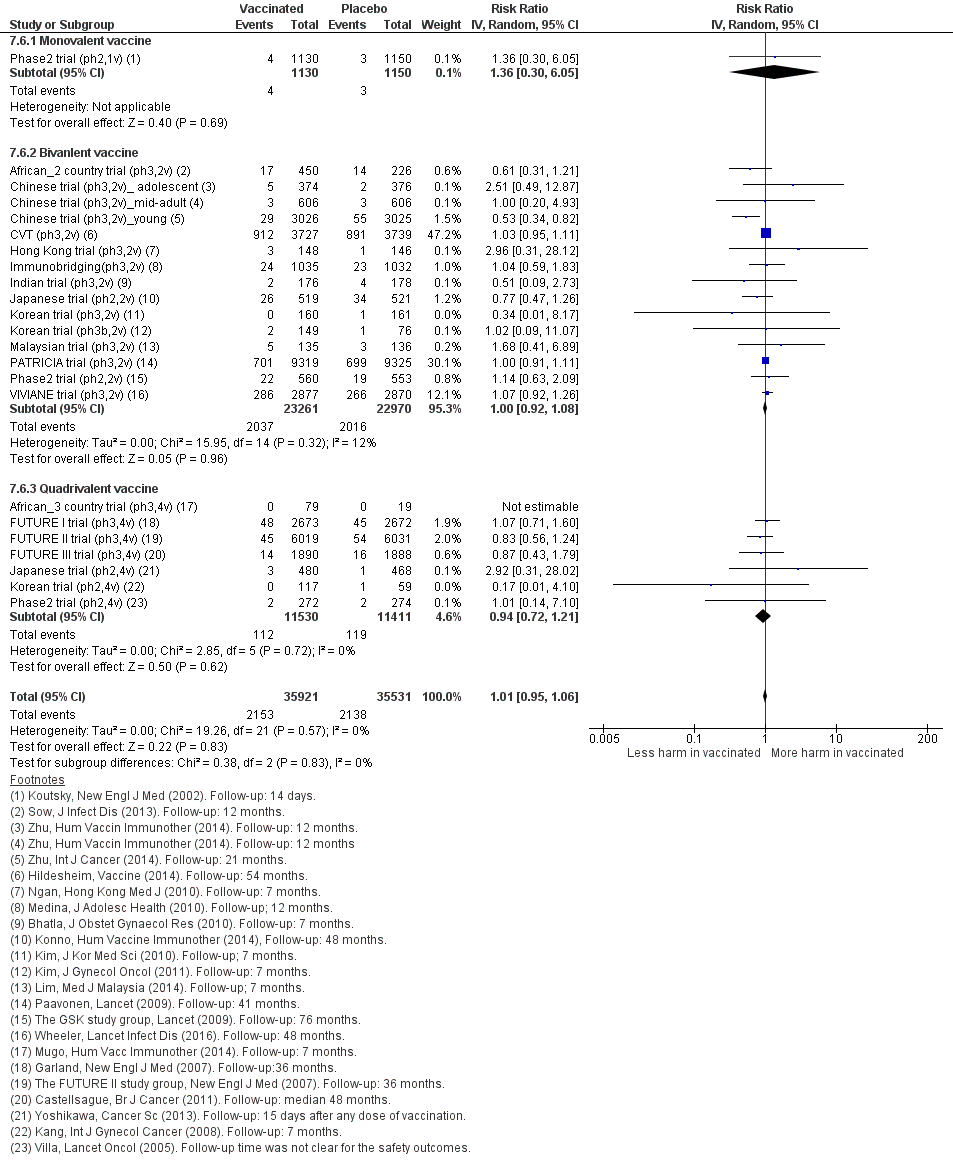
Sensitivity analysis of Analysis 7.6 on severe adverse effects restricting to data extracted from publications in peer‐reviewed journals.

Sensitivity analysis of Analysis 7.7 on deaths restricting to data extracted from publications in peer‐reviewed journals.

Protection against CIN2+ associated with HPV16/18 in women, aged 15‐26 years, who were HPV DNA 16/18 negative at baseline, by number of doses.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose.
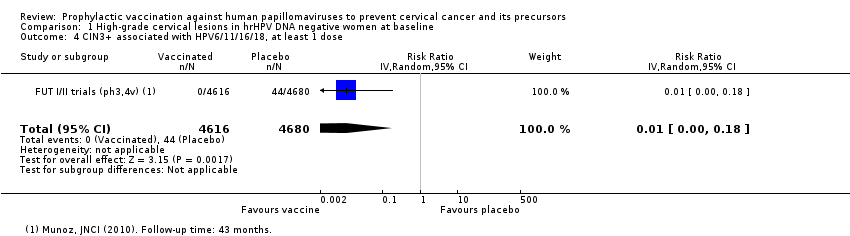
Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 5 AIS associated with HPV16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 8 Any CIN3+ irrespective of HPV types, at least 1 dose.

Comparison 1 High‐grade cervical lesions in hrHPV DNA negative women at baseline, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 1 CIN2+ associated with HPV16/(18), 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 2 CIN2+ associated with HPV16/(18), at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 4 CIN2+ associated with HPV6/11/16/18, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis).

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 13 Any CIN2+ irrespective of HPV types, 3 doses.

Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 14 Any CIN2+ irrespective of HPV types, at least 1 dose.
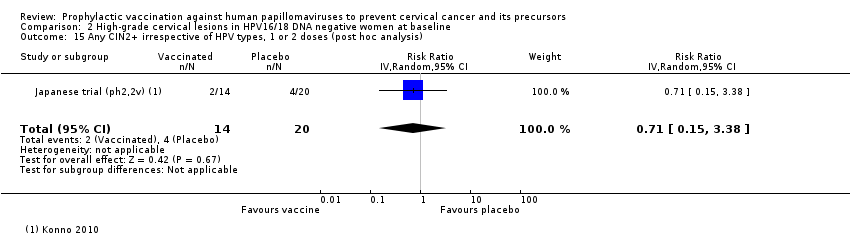
Comparison 2 High‐grade cervical lesions in HPV16/18 DNA negative women at baseline, Outcome 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis).

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 1 CIN2+ associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 3 CIN3+ associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 5 AIS associated with HPV16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 6 AIS associated with HPV6/11/16/18, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 7 Any CIN2+ irrespective of HPV types, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 8 Any CIN3+ HPV type, at least 1 dose.

Comparison 3 High‐grade cervical lesions in women regardless of baseline HPV DNA status, Outcome 9 Any AIS irrespective of HPV types, at least 1 dose.
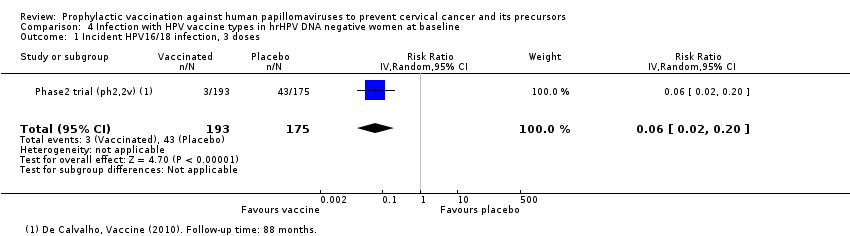
Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 2 Persistent HPV16/18 infection (6M), 3 doses.
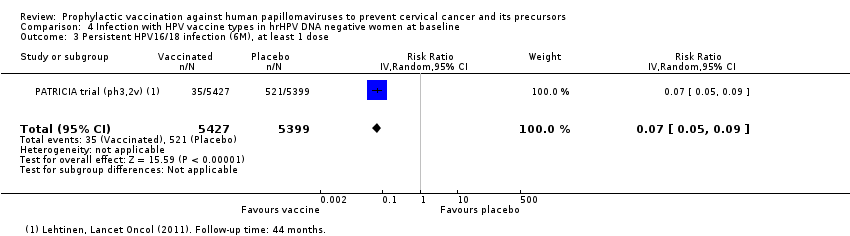
Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 3 Persistent HPV16/18 infection (6M), at least 1 dose.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection(12M), 3 doses.

Comparison 4 Infection with HPV vaccine types in hrHPV DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 1 Incident HPV16/18 infection, 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 2 Incident HPV16/18 infection, at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis).

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 4 Persistent HPV16/18 infection (6M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 5 Persistent HPV16/18 infection (6M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis).

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 7 Persistent HPV6/11/16/18 infection (6M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose.
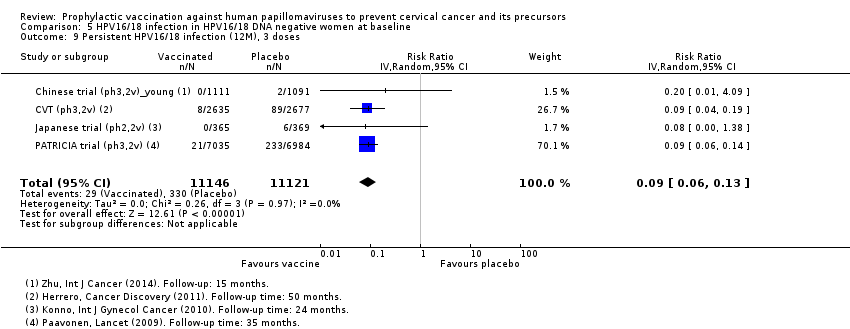
Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 9 Persistent HPV16/18 infection (12M), 3 doses.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 10 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 5 HPV16/18 infection in HPV16/18 DNA negative women at baseline, Outcome 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis).
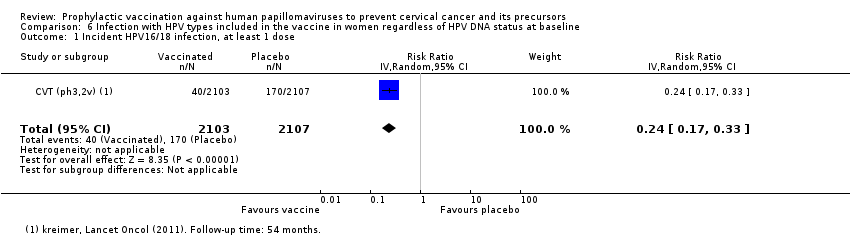
Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 1 Incident HPV16/18 infection, at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 2 Persistent HPV16/18 infection (6M), at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose.
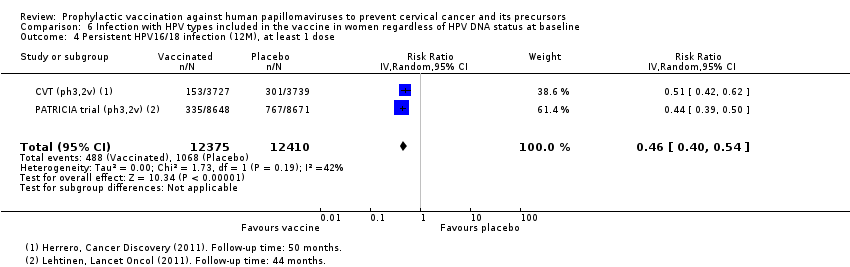
Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 4 Persistent HPV16/18 infection (12M), at least 1 dose.

Comparison 6 Infection with HPV types included in the vaccine in women regardless of HPV DNA status at baseline, Outcome 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis).
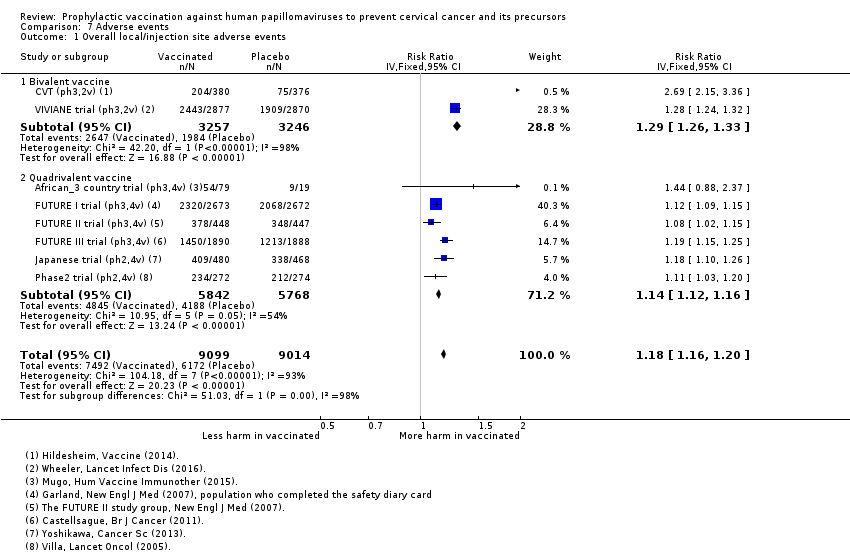
Comparison 7 Adverse events, Outcome 1 Overall local/injection site adverse events.

Comparison 7 Adverse events, Outcome 2 Pain at injection site.
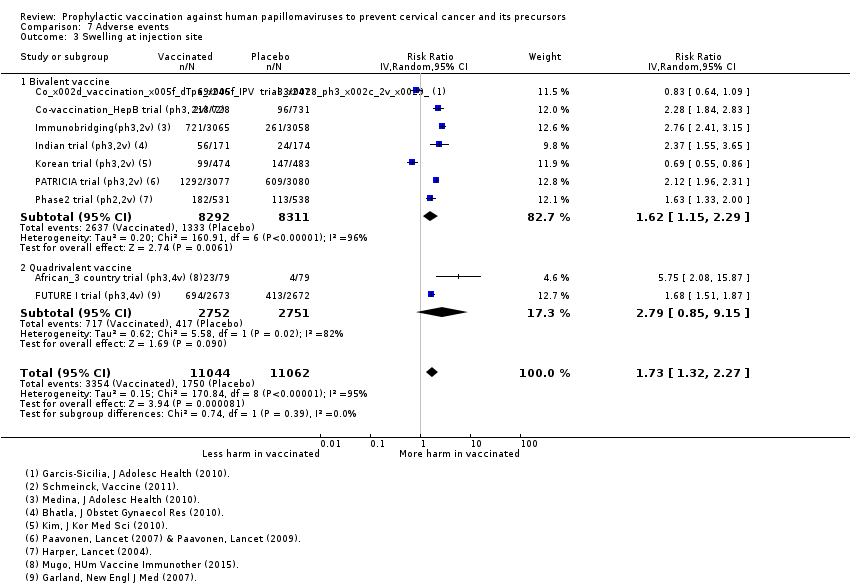
Comparison 7 Adverse events, Outcome 3 Swelling at injection site.

Comparison 7 Adverse events, Outcome 4 Redness at injection site.

Comparison 7 Adverse events, Outcome 5 Overall systemic event and general symptoms.

Comparison 7 Adverse events, Outcome 6 Serious adverse events.

Comparison 7 Adverse events, Outcome 7 Deaths.

Comparison 8 Pregnancy outcomes, Outcome 1 Normal infant.

Comparison 8 Pregnancy outcomes, Outcome 2 Spontaneous abortion/miscarriage.

Comparison 8 Pregnancy outcomes, Outcome 3 Elective termination/induced abortion.

Comparison 8 Pregnancy outcomes, Outcome 4 Stillbirth.

Comparison 8 Pregnancy outcomes, Outcome 5 Abnormal infant.
| HPV vaccine effects on cervical lesions in adolescent girls and women who are hrHPV DNA negative at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 26 years who are hrHPV negative before vaccination Setting: Europe, Asia Pacific countries, South & North America Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18. Follow‐up: 3 to 5 years | 164 per 10,000 | 2 per 10,000 | RR 0.01 | 23,676 | ⊕⊕⊕⊕ | |
| CIN3+ associated with HPV16/18 Follow‐up: 3 to 5 years | 70 per 10,000 | 0 per 10,000 | RR 0.01 | 20,214 | ⊕⊕⊕⊕ | Continuity correction |
| AIS associated with HPV16/18 Follow‐up: 3 to 5 years | 9 per 10,000 | 0 per 10,000 | RR 0.10 | 20,214 | ⊕⊕⊕⊝ | Continuity correction |
| Any CIN2+ irrespective of HPV type, bivalent or quadrivalent vaccine Follow‐up: 2 to 6 years | 287 per 10,000 | 106 per 10,000 | RR 0.37 | 25,180 | ⊕⊕⊕⊕ | Substantial subgroup heterogeneity was observed (I2= 84.3%) for bi‐ and quadrivalent vaccines. So results are reported separately for the 2 vaccines (see next 2 rows). |
| Any CIN2+ irrespective of HPV type Follow‐up (bivalent): 3.5 to 6 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.33 (0.25 to 0.43) | 15,884 (4 RCTs) | ⊕⊕⊕⊕ | ||
| 285 per 10,000 | 94 per 10,000 (71 to 122) | |||||
| Quadrivalent vaccine | RR 0.57 (0.44 to 0.76) | 9296 (1 RCT) | ⊕⊕⊕⊝ | |||
| 291 per 10,000 | 166 per 10,000 (128 to 221) | |||||
| Any CIN3+ irrespective of HPV type, bivalent or quadrivalent vaccine Follow‐up: 3.5 to 4 years | 109 per 10,000 | 23 per 10,000 | RR 0.21 | 20,719 | ⊕⊕⊕⊝ | Substantial subgroup heterogeneity was observed (I2 = 84.3%) for bi‐ and quadrivalent vaccines. So results are reported separately for the 2 vaccines (see next 2 rows). |
| Any CIN3+ irrespective of HPV type Follow‐up (bivalent): 4 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.08 (0.03 to 0.23) | 11,423 (2 RCTs) | ⊕⊕⊕⊕ | ||
| 81 per 10,000 | 6 per 10,000 (3 to 19) | |||||
| Quadrivalent vaccine | RR 0.54 (0.36 to 0.82) | 9296 (1 RCT) | ⊕⊕⊕⊝ | |||
| 143 per 10,000 | 77 per 10,000 (51 to 117 ) | |||||
| Any AIS irrespective of HPV type | 10 per 10,000 | 0 per 10,000 | RR 0.10 | 20,214 | ⊕⊕⊕⊝ | Continuity correction |
| 1The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). When risk in vaccine group is zero, the 95% CI is computed using an exact binomial method. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates. 2 Downgraded due to serious imprecision in effect estimate (width 95% CI around RR > 0.6). 3 Downgraded one level due to serious imprecision. Few events observed in the two studies (9 in placebo arms and 0 in vaccination arms for the outcome of AIS HPV16/18 and 7 in placebo arms and 0 in vaccination arms for outcome of AIS of any type). | ||||||
| HPV vaccine effects on cervical lesions in adolescent girls and women negative for HPV16/18 DNA at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years who were HPV16/18 negative before vaccination | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18 Follow‐up (age 15 to 26 years): 1 to 8.5 years Follow‐up (age 24 to 45 years): 4 to 6 years | 15 to 26 years | RR 0.05 | 34,478 | ⊕⊕⊕⊕ | ||
| 113 per 10,000 | 6 per 10,000 | |||||
| 24 to 45 years | RR 0.30 (0.11 to 0.81) | 7552 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |||
| 45 per 10,000 | 14 per 10,000 (5 to 37) | |||||
| CIN3+ associated with HPV16/18 (age 15 to 26 years) Follow‐up: 3 years | 57 per 10,000 | 3 per 10,000 (1 to 8) | RR 0.05 (0.02 to 0.14) | 33,199 (3 studies) | ⊕⊕⊕⊕ | |
| AIS associated with HPV16/18 or 6/11/16/18 (age 15 to 26 years) Follow‐up: 3 years | 12 per 10,000 | 0 per 10,000 | RR 0.09 | 17,079 | ⊕⊕⊕⊝ MODERATE 2 | Continuity correction |
| Any CIN2+ irrespective of HPV type (age 15 to 26 years) Follow‐up: 2 to 6.5 years | 231 per 10,000 | 95 per 10,000 | RR 0.41 | 19,143 | ⊕⊕⊕⊕ | |
| Any CIN3+ irrespective of HPV type ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Any AIS irrespective of HPV type ‐ not measured | ‐ | ‐ | ||||
| 1The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Exception: when risk in vaccine group is zero, the 95% CI is computed using an exact binomial method.. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates. 2 Downgraded due to serious imprecision in effect estimate (width 95% CI around RR > 0.6). | ||||||
| HPV vaccine effects on cervical lesions in adolescent girls and women unselected for HPV DNA status at baseline | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years regardless of HPV DNA status at baseline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccination1 | |||||
| Cervical cancer ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| CIN2+ associated with HPV16/18 Follow‐up (age 15 to 26 years): 3.5 to 8.5 years Follow‐up (age 24 to 45 years): 3.5 years | 15 to 26 years | RR 0.46 (0.37 to 0.57 | 34,852 | ⊕⊕⊕⊕ | ||
| 341 per 10,000 | 157 per 10,000 | |||||
| 24 to 45 years | RR 0.74 (0.52 to 1.05) | 9200 (2 studies) | ⊕⊕⊕⊝ | |||
| 145 per 10,000 | 107 per 10,000 (76 to 152) | |||||
| CIN3+ associated with HPV16/18 Follow‐up: 3.5 years | 165 per 10,000 | 91 per 10,000 (74 to 127) | RR 0.55 (0.45 to 0.67) | 34,562 (2 RCTs) | ⊕⊕⊕⊕ | |
| Adeno carcinoma in situ (AIS) associated with HPV16/18 Follow‐up: 3.5 years | 14 per 10,000 | 5 per 10,000 | RR 0.36 | 34,562 | ⊕⊕⊕⊕ | |
| Any CIN2+ irrespective of HPV type Follow‐up (age 15 to 26 years): 3.5 to 8.5 years Follow‐up (age 24 to 45 years): 3.5 to 6 years | 15 to 26 years | RR 0.70 | 35,779 | ⊕⊕⊕⊕ | ||
| 559 per 10,000 | 391 per 10,000 | |||||
| 24 to 45 years | RR 1.04 | 9287 | ⊕⊕⊕⊝ | |||
| 343 per 10,000 | 356 per 10,000 | |||||
| Any CIN3+ irrespective of HPV type (age 15 to 26 years) Follow‐up: 3.5 to 4 years | 266 per 10,000 | 178 per 10,000 (231 to 247) | RR 0.67 (0.49 to 0.93) | 35,489 (3 RCTs) | ⊕⊕⊕⊝ | Substantial subgroup heterogeneity was observed (I2 = 84.3%) for bivalent and quadrivalent vaccines. So results are reported separately for two vaccines. |
| Any CIN3+ irrespective of HPV type (age 15 to 26 years), Follow‐up (bivalent): 3.5 to 4 years Follow‐up (quadrivalent): 3.5 years | Bivalent vaccine | RR 0.55 (0.43 to 0.71) | 18,329 (2 RCTs) | ⊕⊕⊕⊕ | ||
| 188 per 10,000 | 104 per 10,000 (81 to 134) | |||||
| Quadrivalent vaccine | 0.81 (0.69 to 0.96) | 17,160 (1 RCT) | ⊕⊕⊕⊝ | |||
| 349 per 10,000 | 283 per 10,000 (241 to 335) | |||||
| Any AIS irrespective of HPV type (age 15 to 26 years) Follow‐up: 3.5 years | 17 per 10,000 | 5 per 10,000 | RR 0.32 | 34,562 | ⊕⊕⊕⊕ | |
| Serious adverse events Follow‐up: 6 months to 7 years | 669 per 10,000 | 656 per 10,000 | RR 0.98 | 71,597 | ⊕⊕⊕⊕ | |
| Deaths Follow‐up: 7 months to 10 years. Most of the information in the analysis comes from studies with follow‐up ranging from 5‐10 years. | 11 per 10,000 | 14 per 10,000 | RR 1.29 | 71,176 | ⊕⊕⊝⊝ | Older women had higher fatality rate (RR 2.36, 95% CI 1.10 to 5.03). Assessment of the deaths in the studies has not been able to identify a pattern in the cause or timing of death. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Assumed risk calculated from the sum of control group event rates for all outcomes unless otherwise stated. 2 Downgraded due to serious imprecision. Confidence interval is wide and includes large decrease and small increase in lesions with vaccination group in the older age group. 3 Downgraded one level due to serious inconsistency. Reduction in lesions was greater in younger women than in older women (RR 0.46 in 15 to 26 years versus RR 0.74 in 24 to 45 years; P = 0.02 for interaction). 4 Downgraded one level due to serious imprecision. Confidence interval includes potentially meaningful increase in risk of mortality. 5 Downgraded one level due to serious inconsistency. Despite limited evidence of statistical variation, sub grouping studies by age showed higher fatality rate with vaccines in older age group. There is no clear pattern in causes or timing of deaths. | ||||||
| HPV vaccine adverse pregnancy outcomes (regardless of DNA status and age) | ||||||
| Patient or population: adolescent girls and women aged 15 to 45 years who became pregnant during the study | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with HPV vaccines | |||||
| Spontaneous abortion/miscarriage Follow‐up: 1 to 7 years | Study population | RR 0.88 | 8618 | ⊕⊕⊕⊕ | ||
| 1618 per 10,000 | 1,424 per 10,000 | |||||
| Elective termination/induced abortion Follow‐up: 1 to 7 years | Study population | RR 0.90 | 10,909 | ⊕⊕⊕⊕ | ||
| 931 per 10,000 | 838 per 10,000 | |||||
| Stillbirth Follow‐up: 1 to 3.5 years | Study population | RR 1.12 | 8754 | ⊕⊕⊕⊝ | ||
| 70 per 10,000 | 78 per 10,000 | |||||
| Babies born with congenital malformations Follow‐up: 3 to 7 years | Study population | RR 1.22 | 9252 | ⊕⊕⊕⊝ | ||
| 205 per 10,000 | 250 per 10,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Confidence interval rules out an increased risk of termination so there is no downgrade for imprecision. 2 Downgraded one level due to serious imprecision. Confidence intervals for both outcomes include meaningful increase and reduction in risk of stillbirth or abnormal infants following vaccination. | ||||||
| Valency | Phase | Number of trials | Appelation | N | Outcomes | Main References |
| Monovalent | II | 1 | 2392 | Efficacy, safety | ||
| Bivalent | II | 2 | 1040 | Efficacy, safety | ||
| 1113 | Efficacy, safety | |||||
| III | 16 | 676 | Safety | |||
| 6051 | Efficacy, safety | |||||
| 750 | Safety | |||||
| 1212 | Safety | |||||
| 494 | Safety | |||||
| 494 | Safety | |||||
| 541 | Safety | |||||
| 7466 | Efficacy, safety | |||||
| 294 | Safety | |||||
| 2067 | Safety | |||||
| 354 | Safety | |||||
| 208 | Safety | |||||
| 321 | Safety | |||||
| 271 | Safety | |||||
| 18,644 | Efficacy, safety | |||||
| 5752 | Efficay, safety, | |||||
| Quadrivalent | II | 3 | 1021 | Safety | ||
| 176 | Safety | |||||
| 552 | Efficacy, safety | |||||
| III | 4 | 98 | Safety | |||
| 5455 | Efficacy, safety | |||||
| 12,167 | Efficacy, safety | |||||
| 3819 | Efficacy, safety | |||||
| Total | 26 | 73,428 |
| Outcomes and exposure subgroups | Absolute risk / per 10,000 | Relative risk | Vaccine efficacy (95% CI) | Risk difference/ per 10,000 (95% CI) | No of Participants | Certainty of evidence | |
| Placebo | Vaccinated | ||||||
| 1. High‐grade cervical lesions in women who were hrHPV DNA negative at baseline | |||||||
| Analysis 1.1 CIN2+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 164 | 2 | 0.01 (0.00 to 0.05) | 99% (95% to 100%) | 162 (157 to 164) | 23,676 | ⊕⊕⊕⊕ high |
| Analysis 1.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 197 | 2 | 0.01 (0.00 to 0.09) | 99% (91% to 100%) | 195 (179 to 197) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.3 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 70 | 0* | 0.01 (0.00 to 0.10) | 99% (90% to 100%) | 70 (63 to 70) | 20,214 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 1.4 CIN3+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 94 | 0* | 0.01 (0.00 to 0.18) | 99% (82% to 100%) | 94 (77 to 94) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.5 AIS associated with HPV16/18, at least 1 dose, age 15‐26 years | 9 | 0* | 0.10 (0.01 to 0.82) | 90% (18% to 99%) | 9 (2 to 9) | 20,214 | ⊕⊕⊕⊝ |
| Analysis 1.6 AIS associated with HPV6/11/16/18m at least 1 dose, age 15‐26 years | 6 | 0* | 0.14 (0.01 to 2.8) | 86% (‐180% to 99%) | 6 (‐12 to 6) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.7.1 Any CIN2+ irrespective of HPV types, at least 1 dose of the bivalent vaccine, age 15‐26 years | 285 | 94 | 0.33 (0.25 to 0.43) | 67% (57% to 75%) | 191 (163 to 214) | 15,884 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 1.7.2 Any CIN2+ irrespective of HPV types, at least 1 dose of the quadrivalent vaccine, age 15‐26 years | 291 | 166 | 0.57 (0.44 to 0.76) | 43% (24 to 56%) | 125 (70 to 163) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.8.1 Any CIN3+ irrespective of HPV types, at least 1 dose of the bivalent vaccine, age 15‐26 years | 81 | 6 | 0.08 (0.03 to 0.23) | 92% (77% to 97%) | 74 (62 to 78) | 11,423 (2 studies) | ⊕⊕⊕⊕ |
| Analysis 1.8.2 Any CIN3+ irrespective of HPV types, at least 1 dose of the quadrivalent vaccine, age 15‐26 years | 143 | 77 | 0.54 (0.36 to 0.82) | 46% (17% to 64%) | 66 (26 to 92) | 9296 (1 study) | ⊕⊕⊕⊝ |
| Analysis 1.9 Any AIS irrespective of HPV types, at least 1 dose | 10 | 0* | 0.10 (0.01 to 0.76) | 90% (24% to 99%) | 10 (2 to 10) | 20,214 | ⊕⊕⊕⊝ |
| 2. High‐grade cervical lesions in women who were HPV16/18 negative at baseline | |||||||
| Analysis 2.1.1 CIN2+ associated with HPV16/18, 3 doses, age 15‐26 years | 74 | 5 | 0.07 (0.03 to 0.15) | 93% (85% to 97%) | 69 (63 to 72) | 36,579 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.1.2 CIN2+ associated with HPV16/18, 3 doses, 24‐45 years | 36 | 6 | 0.16 (0.04 to 0.74) | 84% (26% to 96%) | 30 (9 to 34) | 6797 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.2.1 CIN2+ associated with HPV16/18, at least 1 dose, 15‐26 years | 113 | 6 | 0.05 (0.03 to 0.10) | 95% (90% to 97%) | 107 (102 to 110) | 34,478 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.2.2 CIN2+ associated with HPV16/18, at least 1 dose, age 24‐45 years | 45 | 14 | 0.30 (0.11 to 0.81) | 70% (19% to 89%) | 32 (9 to 40) | 7552 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.3.1 CIN2+ associated with HPV16/18, 1 or 2 doses, 15‐26 years*** | 436 | 44 | 0.10 (0.04 to 0.26) | 90% (74% to 96%) | 392 (323 to 418) | 2958 (5 studies) | ⊕⊕⊝⊝ low1$ |
| Analysis 2.3.2 CIN2+ associated with HPV16/18, 1 or 2 doses, age 24‐45 years*** | 134 | 82 | 0.61 (0.14 to 2.67) | 39% (‐167% to 86%) | 52 (‐2245 to 115) | 755 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.4 CIN2+ associated with HPV6/11/16/18, 3 doses, age 15‐45 years | 99 | 6 | 0.06 (0.01 to 0.61) | 94% (39% to 99%) | 93 (39 to 98) | 7664 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.4.1 CIN2+ associated with HPV6/11/16/18, 3 doses, age 15‐26 years | 142 | 0* | 0.02 (0.00 to 0.25) | 98% (75% to 100%) | 142 (93 to 190) | 4499 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.4.2 CIN2+ associated with HPV6/11/16/18, 3 doses, age 24‐45 years | 38 | 6 | 0.17 (0.02 to 1.39) | 83% (‐39% to 98%) | 32 (‐1 to 32) | 3165 (1 study) | ⊕⊕⊝⊝ |
| Analysis 2.5.1 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 160 | 0* | 0.01 (0.00 to 0.19) | 99% (81% to 100%) | 160 (130 to 159) | 5351 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.5.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 24‐45 years | 44 | 16 | 0.37 (0.10 to 1.41) | 63% (‐41% to 90%) | 28 (‐18 to 40) | 3629 (1 study) | ⊕⊕⊕⊝ |
| Analysis 2.6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 15‐45 years*** | 199 | 48 | 0.24 (0.01 to 5) | 76% (‐400% to 99%) | 151 (‐795 to 197) | 1316 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.6.1 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 15‐26 years*** | 258 | 0* | 0.04 (0.00 to 0.74) | 96% (26% to 100%)) | 258 (108 to 409) | 852 (1 study) | ⊕⊝⊝⊝ |
| Analysis 2.6.2 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses, age 24‐45 years*** | 88 | 85 | 0.97 (0.14 to 6.80) | 3% (‐580% to 86%) | 3 (‐165 to 171) | 464 (1 study) | ⊕⊝⊝⊝ |
| Analysis 2.7 CIN3+ associated with HPV16/18, 3 doses, age 15‐26 years | 40 | 3 | 0.07 (0.02 to 0.29) | 93% (71% to 98%) | 37 (28 to 39) | 29,720 (3 studies) | ⊕⊕⊕⊕ |
| Analysis 2.8 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 57 | 3 | 0.05 (0.02 to 0.14) | 95% (86% to 98%) | 54 (49 to 56) | 33,199 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.9 CIN3+ associated with HPV16/18, 1 or 2 doses, age 15‐26 years*** | 200 | 12 | 0.06 (0.01 to 0.24) | 94% (26% to 100%) | 188 (152 to 198) | 3479 (3 studies) | ⊕⊕⊝⊝ low1$ |
| Analysis 2.10 AIS+ associated with HPV16/18, 3 doses, age 15‐26 years | 8 | 0* | 0.12 (0.02 to 0.70) | 88% (36% to 99%) | 8 (2 to 8) | 29,707 (3 studies) | ⊕⊕⊕⊝ |
| Analysis 2.11 AIS+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 12 | 0* | 0.09 (0.01 to 0.72) | 81% (28% to 99%) | 12 (3 to 12) | 17,079 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 2.12 AIS+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses, age 15‐26 years*** | 29 | 0* | 0.15 (0.01 to 2.97) | 85% (‐197% to 99%) | 29 (‐57 to 29) | 2015 (2 studies) | ⊕⊝⊝⊝ |
| Analysis 2.13 CIN2+ irrespective of HPV types, 3 doses, age 15‐26 years | 166 | 66 | 0.40 (0.25 to 0.64) | 60% (36% to 75%) | 99 (60 to 124) | 7320 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.14 CIN2+ irrespective of HPV types, at least 1 dose, age 15‐26 years | 231 | 95 | 0.41 (0.32 to 0.52) | 58% (46% to 67%) | 136 (111 to 157) | 19,143 (3 studies) | ⊕⊕⊕⊕ high |
| Analysis 2.15 CIN2+ irrespective of HPV types, 1 or 2 doses, age 20‐25 years*** | 1000 | 710 | 0.71 (0.15 to 3.38) | 29% (‐238% to 85%) | 290 (‐2,380 to 850) | 34 (1 study) | ⊕⊝⊝⊝ |
| 3. High‐grade cervical lesions in all women regardless of HPV DNA status at baseline** | |||||||
| Analysis 3.1.1 CIN2+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 341 | 157 | 0.46 (0.37 to 0.57) | 54% (43% to 63%) | 184 (147 to 215) | 34,852 | ⊕⊕⊕⊕ high |
| Analysis 3.1.2 CIN2+ associated with HPV16/18, at least 1 dose, age 24‐45 years | 157 | 116 | 0.74 (0.52 to 1.05) | 26% (‐5% to 48%) | 41 (‐8 to 75) | 9200 (2 studies) | ⊕⊕⊕⊝ moderate4 |
| Analysis 3.2.1 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 15‐26 years | 436 | 217 | 0.50 (0.42 to 0.59) | 50% (41% to 58%) | 219 (166 to 272) | 17,160 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.2.2 CIN2+ associated with HPV6/11/16/18, at least 1 dose, age 24‐45 years | 145 | 113 | 0.78 (0.44 to 1.37) | 22% (‐37% to 56%) | 143 (72 to 204 | 3723 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.3 CIN3+ associated with HPV16/18, at least 1 dose, age 15‐26 years | 165 | 91 | 0.55 (0.43 to 0.68) | 74% (55% to 91%) | 74 (55 to 91) | 34,562 | ⊕⊕⊕⊕ high |
| Analysis 3.4 CIN3+ associated with HPV16/18, 1 or 2 doses, age 15‐26 years*** | 230 | 124 | 0.54 (0.43 to 0.68) | 46% (32% to 57%) | 106 (74 to 131) | 17,160 (1 study) | ⊕⊕⊝⊝ |
| Analysis 3.5 AIS associated with HPV16/18, at least 1 dose, age 15‐26 years | 14 | 5 | 0.36 (0.17 to 0.78) | 64% (22% to 83%) | 9 (3 to 12) | 34,562 | ⊕⊕⊕⊕ high |
| Analysis 3.6 AIS associated with HPV6/11/16/18, at least 1 dose, age 15‐45 years | 15 | 6 | 0.40 (0.16 to 0.98) | 60% (2% to 84%) | 9 (0 to 13) | 20,830 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.7.1 Any CIN2+ irrespective of HPV types, at least 1 dose, age 15‐26 years | 559 | 391 | 0.70 (0.58 to 0.85) | 30% (15% to 42%) | 168 (84 to 235) | 35,779 | ⊕⊕⊕⊕ high |
| Analysis 3.7 2 Any CIN2+ irrespective of HPV types, at least 1 dose, age 24‐45 years | 342 | 356 | 1.04 (0.83 to 1.30) | ‐4% (‐30% to 17%) | ‐14 (‐103 to 58) | 9287 (2 studies) | ⊕⊕⊝⊝ |
| Analysis 3.8 Any CIN3+ irrespective of HPV types, at least 1 dose, age 18‐26 years, bivalent vaccine | 188 | 103 | 0.55 (0.43 to 0.71) | 45% (29% to 57%) | 84 (54 to 1107) | 18,329 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 3.8 Any CIN3+ irrespective of HPV types, at least 1 dose, age 15‐26 years, quadrivalent vaccine | 349 | 283 | 0.81 (0.69 to 0.96) | 19% (4% to 31%) | 66 (14 to 108) | 17,160 (1 study) | ⊕⊕⊕⊝ |
| Analysis 3.9 Any AIS irrespective of HPV types, at least 1 dose, age 15‐26 years | 17 | 5 | 0.32 (0.15 to 0.67) | 68% (33% to 0.85%) | 11 (6 to 14) | 34,562 | ⊕⊕⊕⊕ high |
| 4. HPV16/18 infection in women who were hrHPV DNA negative at baseline | |||||||
| Analysis 4.1 Incident HPV16/18 infection, 3 doses, age 18‐26 years | 2,457 | 147 | 0.06 (0.02 to 0.20) | 94% (80% to 98%) | 2,310 (1,966 to 2,408) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.2 Persistent HPV16/18 infection(6M), 3 doses, age 15‐26 years | 971 | 29 | 0.02 (0.00 to 0.35) | 97% (57% to 100%) | 942 (554 to 971) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.3 Persistent HPV16/18 infection(6M), at least 1 dose, age 18‐25 years | 96 | 7 | 0.07 (0.05 to 0.09) | 93% (81% to 95%) | 90 (88 to 91) | 10,826 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.4 Persistent HPV16/18 infection(12M), 3 doses, age 15‐26 years | 571 | 23 | 0.04 (0.00 to 0.73) | 96% (27% to 100%) | 549 (154 to 571) | 368 (1 study) | ⊕⊕⊕⊝ |
| Analysis 4.5 Persistent HPV16/18 infection(12M), at least 1 dose, age 15‐26 years | 462 | 37 | 0.08 (0.05 to 0.12) | 92% (88% to 95%) | 425 (406 to 439) | 14,153 ( 2 studies) | ⊕⊕⊕⊕ high |
| 5. HPV16/18 infection in women who were HPV16/18 negative at baseline | |||||||
| Analysis 5.1 Incident HPV16/18 infection, 3 doses, age 15‐26 years | 474 | 81 | 0.17 (0.10 to 0.31) | 87% (78% to 92%) | 412 (369 to 436) | 8,034 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.2 Incident HPV16/18 infection, at least 1 dose, age 15‐26 years | 1,326 | 305 | 0.23 (0.14 to 0.37) | 81% (71% to 88%) | 1,074 (941 to 1,167) | 23,872 (5 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.3 Incident HPV16/18 infection, 1 or 2 dose, age 15‐26 years*** | 2,568 | 1207 | 0.47 (0.26 to 0.84) | 74% (31% to 90%) | 1,901 (796 to 2,311) | 331 (3 studies) | ⊕⊕⊕⊝ moderate1 |
| Analysis 5.4.1 Persistent HPV16/18 infection (6M), 3 doses, age 15‐26 years | 581 | 35 | 0.06 (0.05 to 0.08) | 94% (91% to 95%) | 546 (534 to 552) | 27,385 (6 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.4.2 Persistent HPV16/18 infection (6M), 3 doses, age 24‐45 years | 350 | 38 | 0.11 (0.06 to 0.20) | 89% (80% to 94%) | 311 (280 to 329) | 6728 (2 studies) | ⊕⊕⊕⊝ |
| Analysis 5.5.1 Persistent HPV16/18 infection (6M), at least 1 dose, age 15‐26 years | 657 | 66 | 0.10 (0.08 to 0.13) | 90% (87% to 92%) | 591 (572 to 605) | 22,803 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.5.2 Persistent HPV16/18 infection (6M), at least 1 dose, age 24‐45 years | 441 | 75 | 0.17 (0.10 to 0.29) | 83% (71% to 90%) | 366 (313 to 397) | 7520 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.6.1 Persistent HPV16/18 infection (6M), 1 or 2 doses, age 15‐26 years*** | 996 | 119 | 0.12 (0.03 to 0.42) | 88% (58% to 97%) | 876 (577 to 966) | 437 (2 studies) | ⊕⊕⊝⊝ |
| Analysis 5.6.2 Persistent HPV16/18 infection (6M), 1 or 2 doses, age 24‐45 years*** | 1,221 | 379 | 0.31 (0.18 to 0.54) | 69% (46% to 82%) | 843 (562 to 1002) | 792 (2 studies) | ⊕⊕⊕⊝ moderate1 |
| Analysis 5.7 Persistent HPV6/11/16/18 infection (6M), 3 doses | 518 | 62 | 0.12 (0.06 to 0.21) | 88% (79% to 94%) | 456 (409 to 487) | 4008 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose | 907 | 118 | 0.13 (0.05 to 0.37) | 87% (63% to 95%) | 789 (571 to 862) | 4129 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.9 Persistent HPV16/18 infection (12M), 3 doses | 297 | 27 | 0.09 (0.06 to 0.13) | 91% (87% to 94%) | 270 (258 to 279) | 22,267 (4 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.10 Persistent HPV16/18 infection (12M), at least 1 dose | 365 | 58 | 0.16 (0.01 to 0.13) | 84% (87% to 99%) | 306 (292 to 361) | 29,464 (5 studies) | ⊕⊕⊕⊕ high |
| Analysis 5.11 Persistent HPV16/18 infection (12M), 1 or 2 doses*** | 205 | 27 | 0.13 (0.06 to 0.33) | 87% (67% to 94%) | 178 (137 to 193) | 3912 (3 studies) | ⊕⊕⊕⊝ moderate1 |
| 6. HPV16/18 infection regardless of HPV DNA status at baseline** | |||||||
| Analysis 6.1 Incident HPV16/18 infection, at least 1 dose, age 15‐26 years | 807 | 194 | 0.24 (0.17 to 0.33) | 76% (67% to 83%) | 613 (541 to 670) | 4210 (1 study) | ⊕⊕⊕⊝ |
| Analysis 6.2.1 Persistent HPV16/18 infection (6M), at least 1 dose, age 15‐26 years | 1,359 | 598 | 0.44 (0.38 to 0.51) | 56% (49% to 62%) | 761 (666 to 842) | 25,199 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 6.2.2 Persistent HPV16/18 infection (6M), at least 1 dose, age 24‐45 years | 642 | 366 | 0.57 (0.47 to 0.69) | 43% (31% to 53%) | 276 (199 to 341) | 8648 (2 studies) | ⊕⊕⊕⊕ high |
| Analysis 6.3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose, age 24‐45 years | 1,136 | 591 | 0.52 (0.42 to 0.65) | 48% (35% to 58%) | 545 (398 to 659) | 3713 (1 study) | ⊕⊕⊕⊝ |
| Analysis 6.4 Persistent HPV16/18 infection (12M), at least 1 dose, age 15‐26 years | 861 | 396 | 0.46 (0.40 to 0.54) | 54% (46% to 60%) | 465 (396 to 516) | 24,785 (2 studies) | ⊕⊕⊕⊕ high |
| CI: Confidence interval; RR: Risk Ratio; | |||||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||||
| 1In case of study flaws as assessed by the Cochrane Collaboration's tool for assessing risk of bias in randomised trials (Higgins 2011b), not observed but calculated outcome; 2 Substantial heterogeneity defined as I2 >30%, when multiple studies were available for the considered outcome; 3When only one study was retrieved for the outcome; 4Imprecision, when the width of the 95% confidence interval around RR >0.60. 0* When zero events occurred in the vaccine group a continuity correction was applied to compute the RR and its confidence interval. Nevertheless, in this case the absolute risks in the vaccine arms in Table 2 were computed considering an exact binomal distribution. ** Relative and absolute effects in women regardless of HPV DNA status at baseline (headings 3 and 6) must be interpreted with care since influenced by the prevalence of HPV infection at enrolment in the respective trials. *** Post hoc analysis for women who received <3 doses. $ For the precancer endpoints (CIN2/3 and AIS),a higher risk in the placebo arms was observed if <3 doses were received compared to those who received 3 doses Therefore the quality of evidence was downgraded to low or very low. | |||||||
| Outcome | Initial HPV status at enrolment | |
| hrHPV negative | Regardless of HPV status | |
| Lesions associated with HPV16/18 | NNV (95% CI) | NNV (95% CI) |
| CIN2+ | 62 (61 to 64) | 54 (46 to 68) |
| CIN3+ | 204 (149 to 333) | 135 (110 to 263) |
| AIS+ | 1111 (714 to 5000) | 1111 (625 to 3333) |
| Lesions irrespective of HPV types | NNV (95% CI) | NNV (95% CI) |
| CIN2+ | 60 (50 to 76) | 68 (52 to 97) |
| CIN3+ | 141 (106 to 208) | 133 (94 to 227) |
| AIS+ | 1000 (556 to 10,000) | 833 (526 to 2000) |
| AIS: adenocarcinoma in situ, CIN: cervical intraepithelial neoplasia, CIN2+: CIN of degree II or worse, CIN3+: CIN of degree 3 or worse, hrHPV: high‐risk human papillomavirus types, NNV: number needed to vaccinate. | ||
| Outcomes | Absolute risk/ per 10,000 | Relative effect | No of Participants | Quality of the evidence | |
| placebo | vaccinated | ||||
| Analysis 7.1Overall local/injection site adverse events | 6847 | 8080 | 1.18 (1.16 to 1.20) | 18,113 | ⊕⊕⊕⊝ |
| Analysis 7.2Pain at injection site | 6505 | 8782 | 1.35 (1.23 to 1.49) | 25,691 | ⊕⊕⊕⊝ |
| Analysis 7.3Swelling at injection site | 1582 | 2737 | 1.73 (1.32 to 2.27) | 22,106 | ⊕⊕⊕⊝ |
| Analysis 7.4Redness at injection site | 1938 | 3333 | 1.72 (1.50 to 1.97) | 19,996 | ⊕⊕⊕⊝ |
| Analysis 7.5Overall systematic event and general symptoms | 6102 | 6224 | 1.02 (0.98 to 1.07) | 18,191 | ⊕⊕⊕⊝ |
| Analysis 7.6Serious adverse events | 605 | 611 | 1.01 (0.95 to 1.07) | 6978 | ⊕⊕⊕⊕ |
| Analysis 7.7Deaths | 11 | 13 | 1.25 (0.81 to 1.93) | 71,452 (23 studies) | ⊕⊕⊝⊝ |
| Analysis 8.1Normal infant | 7171 | 7171 | 1.00 (0.97 to 1.02) | 8782 | ⊕⊕⊕⊕ |
| Analysis 8.2Spontaneous abortion/miscarriage | 1618 | 1424 | 0.88 (0.68 to 1.14) | 8618 | ⊕⊕⊕⊕ |
| Analysis 8.3Elective termination/induced abortion | 931 | 838 | 0.90 (0.80 to 1.02) | 10.909 | ⊕⊕⊕⊕ |
| Analysis 8.4Stillbirth | 70 | 78 | 1.12 (0.68 to 1.83) | 8754 | ⊕⊕⊕⊝4 |
| Analysis 8.5Abnormal infant | 205 | 250 | 1.22 (0.88 to 1.69) | 9252 | ⊕⊕⊕⊝4 |
| CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |||||
| 1In case of study flaws as assessed by Cochrane's tool for assessing risk of bias in randomised trials (Higgins 2011b), not observed but calculated outcome 2 Substantial heterogeneity defined as I2 > 30%, when multiple studies were available for the considered outcome 3When only one study was retrieved for the outcome 4Imprecision, when the width of the 95% confidence interval around RR > 0.60 † inter‐age group heterogeneity, absence of pattern in causes of deaths | |||||
| ID | Group | Death causes |
| 1 | C | Pulmonary thromboembolism with background of acute lymphoblastic leukaemia |
| 2 | V | Breast cancer |
| 3 | V | Pulmonary tuberculosis |
| 4 | V | Thyrotoxicosis |
| 5 | V | Cerebral haemorrhage subsequent to hypertension |
| 6 | V | Pericarditis on a background of lupus erythematosus |
| 7 | V | Nasopharyngeal cancer with metastases to brain |
| 8 | V | Pulmonary embolism after intervention for uterine myoma |
| RR of deaths in vaccine vs placebo arm (7 over 1,890 vs 1 over 1888): RR = 6.99 (95% CI 0.86 to 56.78), 2‐sided pexact=0.070. The age at death varied between 29 and 45 years, seven of the deaths occurred in the Philippines and one in Columbia. All participants received three doses of HPV vaccine or placebo except one who received only two doses of vaccine. The time interval between last dose and date at death ranged between 6 and 37 months. | ||
| Group:V = vaccinated against HPV, C = control group. Source: end‐of‐study analysis after a median follow‐up of four years (Castellsagué 2011) and personal communication with Alfred Saah (MSD, 6/05/2016). | ||
| Patient | Cause of death | Group | Age | Country | Source |
| 1 | Breast cancer metastatic | V | 47 | Canada | 1 |
| 2 | Suicide | V | 47 | Mexico | 1 |
| 3 | Lower respiratory tract infection and sepsis* | C | 55 | Mexico | 1 |
| 4 | Cervix cancer metastatic** | V | 45 | Mexico | 1 |
| 5 | Interstitial lung disease | V | 41 | Mexico | 1 |
| 6 | Breast cancer | *** | 32 | Mexico | 1*** |
| 7 | Suicide | V | 41 | Mexico | 1 |
| 8 | Cardiac valve disease and liver disorder* | C | 38 | Mexico | 1 |
| 9 | Drug hypersensitivity and acute renal failure* | V | 46 | Peru | 1 |
| 10 | Cardiorespiratory arrest | C | 44 | Phillipines | 1 |
| 11 | Acute myocardial infarction | V | 31 | Phillipines | 1 |
| 12 | Multiple myeloma and pulmonary embolism* | V | 50 | Phillipines | 1 |
| 13 | Homicide | V | 32 | Phillipines | 1 |
| 14 | Bronchopneumonia | V | 40 | Singapore | 1 |
| 15 | Lung neoplasm malignant | V | 41 | Thailand | 1 |
| 16 | Suicide | V | 28 | USA | 1 |
| 17 | Glioblastoma multiforme | V | 45 | USA | 1 |
| 18 | Anaplastic astrocytoma | C | 43 | **** | 2 |
| 19 | Nasopharyngeal cancer | C | 41 | **** | 2 |
| Remarks | |||||
| * | Multiple death causes | ||||
| ** | This woman had normal cytology but was HPV‐18 DNA‐positive at study entry (May 2006). At the next scheduled cytology testing at Month 12 (April 2007), the cytology finding was atypical squamous cells cannot exclude high‐grade squamous intraepithelial lesion. She was diagnosed with metastatic cervical cancer in May 2007 (approximately 7 months after receiving the third dose of vaccine or control) and died in July 2008 | ||||
| *** | One case of death due to breast cancer reported in the 48 month report (Skinner 2014) had to be excluded from the analysis (Wheeler 2016). | ||||
| **** | Two additional cases of death occurring in the control arm were reported in the 84‐month report (Wheeler 2016). The country for these two cases was not reported. | ||||
| Source: 1) interim analysis after 48 months of follow‐up (Skinner 2014); 2) report at 84 months of follow‐up (Wheeler 2016) The 84‐month follow‐up report revealed 13 deaths in the HPV arm (N = 2877) versus 5 (N = 2870), with death causes allocated to the trial arms (vaccine versus placebo arm) the RR was 2.59 (95% CI 0.93 to 7.27), 2‐sided pexact=0.0957. No pattern was noticed which could indicate a causal role attributed to HPV vaccination. | |||||
| Trial | Target age group | Age category | Reported age sub‐groups |
| 16‐23 | younger | none | |
| 15‐25 | younger | none | |
| 16‐23 | younger | none | |
| 20‐25 | younger | none | |
| 15‐25 | younger | 15‐17, 18‐20, 21‐25 | |
| 18‐25 | younger | 18‐19, 20‐21, 22‐23, 24‐25 | |
| 26+ | older | 26‐35, 36‐45, 46+ | |
| 16‐24 | younger | none | |
| 15‐26 | younger | none | |
| 25‐45 | older | none |
| Outcome | Age | Event/N Vaccine | Event/N Placebo | Relative risk (95% CI) | Vaccine efficacy % (95% CI) | P value for linear effect of age |
| In women with hrHPV DNA negative status at baseline | ||||||
| CIN2+ associated with HPV16/18 | 15‐17 | 1/1997 | 53/2022 | 0.02 (0.00 to 0.14) | 98% (86 to 100%) | 0.995 |
| 18‐20 | 0/1096 | 27/1144 | 0.02 (0.00 to 0.32) | 98% (68 to 100%) | ||
| 21‐25 | 0/2363 | 17/2281 | 0.03 (0.00 to 0.47) | 97% (53 to 100%) | ||
| CIN2+ irrespective of HPV types | 15‐17 | 34/1997 | 101/2022 | 0.34 (0.23 to 0.50) | 66% (50 to 77%) | 0.355 |
| 18‐20 | 10/1096 | 38/1144 | 0.27 (0.14 to 0.55) | 73% (45 to 86%) | ||
| 21‐25 | 17/2363 | 33/2281 | 0.50 (0.28 to 0.89) | 50% (11 to 72%) | ||
| CIN3+ associated with HPV16/18 | 15‐17 | 0/1997 | 14/2022 | 0.04 (0.00 to 0.61) | 96% (39 to100%) | 1.000 |
| 18‐20 | 0/1096 | 8/1144 | 0.07 (0.00 to 1.13) | 93% (‐13 to 100%) | ||
| 21‐25 | 0/2363 | 5/2281 | 0.10 (0.00 to 1.74) | 90%(‐74 to 100%) | ||
| CIN3+ irrespective of HPV types | 15‐17 | 2/1997 | 24/2022 | 0.08 (0.02 to 0.36) | 92% (64 to 98%) | 0.488 |
| 18‐20 | 1/1096 | 11/1144 | 0.09 (0.01 to 0.73) | 91% (27 to 99%) | ||
| 21‐25 | 0/2363 | 9/2281 | 0.05 (0.00 to 0.92) | 95% (8 to 100%) | ||
| Persistent HPV16/18 infection (6M) | 15‐17 | 14/1989 | 303/2020 | 0.05 (0.03 to 0.08) | 95% (92 to 97%) | 0..042 |
| 18‐20 | 9/1090 | 110/1125 | 0.08 (0.04 to 0.17) | 92%(83 to 96%) | ||
| 21‐25 | 12/2338 | 108/2249 | 0.11 (0.06 to 0.19) | 89% (81 to 94%) | ||
| Regardless of women’s baseline HPV DNA status | ||||||
| CIN2+ associated with HPV16/18 | 15‐17 | 21/2882 | 100/2892 | 0.21 (0.13 to 0.24) | 79% (66 to 87%) | 0.000 |
| 18‐20 | 23/1871 | 66/1908 | 0.36 (0.22 to 0.57) | 64% (43 to 78%) | ||
| 21‐25 | 46/3929 | 62/3898 | 0.74 (0.50 to 1.08) | 26% (‐8 to 50%) | ||
| CIN2+ irrespective of HPV types | 15‐17 | 112/2882 | 200/2892 | 0.56 (0.45 to 0.70) | 44% (30 to 55%) | 0.006 |
| 18‐20 | 62/1871 | 105/1908 | 0.60 (0.44 to 0.82) | 40% (18 to 56%) | ||
| 21‐25 | 113/3929 | 123/3898 | 0.91 (0.09 to 1.17) | 9% (‐17 to 29%) | ||
| CIN3+ associated with HPV16/18 | 15‐17 | 7/2882 | 36/2892 | 0.20 (0.09 to 0.44) | 80% (56 to 91%) | 0.000 |
| 18‐20 | 13/1871 | 30/1908 | 0.44 (0.23 to 0.84) | 56% (16 to 77%) | ||
| 21‐25 | 31/3929 | 28/3898 | 1.10 (0.66 to 1.83) | ‐10% (‐83 to 34%) | ||
| CIN3+ irrespective of HPV types | 15‐17 | 21/2882 | 61/2892 | 0.35 (0.21 to 0.57) | 65% (43 to 79%) | 0.008 |
| 18‐20 | 22/1871 | 44/1908 | 0.51 (0.31 to 0.85) | 49% (15 to 69%) | ||
| 21‐25 | 43/3929 | 53/3898 | 0.80 (0.54 to 1.20) | 20% (‐20 to 46%) | ||
| Persistent HPV16/18 infection (6M) | 15‐17 | 167/2916 | 588/2920 | 0.28 (0.24 to 0.34) | 72% (66 to 76%) | 0.000 |
| 18‐20 | 143/1925 | 283/1961 | 0.51 (0.43 to 0.62) | 49% (38 to 57%) | ||
| 21‐25 | 194/4009 | 356/3979 | 0.54 (0.46 to 0.64) | 46% (36 to 54% ) | ||
| Source: Lehtinen 2012. CIN: cervical intraepithelial neoplasia, CIN2+: CIN of degree II or worse, CIN3+: CIN of degree 3 or worse, HPV: human papillomavirus types.. | ||||||
| Outcome | Age | Vaccine | Placebo | Relative risk (95% CI) | Vaccine efficacy (95% CI) | P value for linear effect of age |
| In women with HPV16/18 DNA negative status at baseline cohort | ||||||
| Persistent HPV16/18 infection (6M) | 18‐19 | 1/825 | 51/870 | 0.02 (0.00 to 0.10) | 98% (90% to 100%) | 0.145 |
| 20‐21 | 3/659 | 36/649 | 0.08 (0.02 to 0.24) | 92% (76% to 98%) | ||
| 22‐23 | 2/588 | 36/625 | 0.06 (0.00 to 0.20) | 94% (80% to 100%) | ||
| 24‐25 | 3/563 | 20/533 | 0.14 (0.03 to 0.44) | 86% (56% to 97%) | ||
| Regardless if women’s baseline HPV DNA status | ||||||
| Persistent HPV16/18 infection (6M) | 18‐19 | 47/1193 | 165/1,244 | 0.30 (0.21 to 0.41) | 70% (59% to 79%) | 0.000 |
| 20‐21 | 64/946 | 134/905 | 0.46 (0.34 to 0.61) | 54% (39% to 66%) | ||
| 22‐23 | 59/818 | 112/848 | 0.55 (0.40 to 0.75) | 45% (25% to 60%) | ||
| 24‐25 | 61/770 | 75/742 | 0.78 (0.56 to 1.99) | 22 %(‐9.9 to 44%) | ||
| Source: Herrero 2011. | ||||||
| Outcome | Age | Event/NVaccine | Event/NPlacebo | Relative risk (95% CI) | Vaccine efficacy (95% CI) | P value for linear effect of age |
| In women with HPV16/18 DNA negative status at baseline cohort | ||||||
| Persistent HPV16/18 infection (6M) | 26‐35 | 3/834 | 22/800 | 0.13 (0.04 to 0.44) | 87% (56% to 96%) | 0.532 |
| 36‐45 | 3/816 | 12/809 | 0.25 (0.07 to 0.88) | 75%(12% to 93%) | ||
| 46+ | 0/219 | 0/213 | N.A. | N.A. | ||
| Regardless if women’s baseline HPV DNA status | ||||||
| Persistent HPV16/18 infection (6M) | 26‐35 | 48/1221 | 78/1242 | 0.63 (0.44 to 0.89) | 37% (11% to 56%) | 0.177 |
| 36‐45 | 19/1244 | 43/1228 | 0.44 (0.26 to 0.74) | 56% (26% to 74%) | ||
| 46+ | 4/300 | 11/306 | 0.37 (0.12 to 1.15) | 63% (‐15% to 88%) | ||
| Source: Skinner 2014. | ||||||
| Initial HPV DNA/ status | Serology status | Vaccine | Placebo | Relative Risk (95% CI) | Relative Risk ratio |
| DNA(‐) | Sero‐ | 0/2,241 | 32/2258 | 0.00 (0.02 to 0.26) | 15.93 |
| Sero+ | 0/377 | 2/379 | 0.25 (0.01 to 5.20) | ||
| DNA(+) | Sero‐ | 27/232 | 31/213 | 0.80 (0.49 to 1.29) | 1.50 |
| Sero+ | 41/156 | 30/137 | 1.20 (0.80 to 1.81) | ||
| DNA(‐) | Sero‐ | 0/5,305 | 28/5260 | 0.02(0.00 to 0.14) | 7.41 |
| Sero+ | 0/498 | 4/524 | 0.13 (0.01 to 2.43) | ||
| DNA(+) | Sero‐ | 33/423 | 35/402 | 0.90 (0.57 to 1.41) | 1.12 |
| Sero+ | 47/298 | 52/332 | 1.01 (0.70 to 1.45 | ||
| DNA(‐) | Sero‐ | 5/8709 | 92/8112 | 0.05 (0.02 to 0.12) | 6.16 |
| Sero+ | 3/1710 | 10/1777 | 0.31 (0.09 to 1.13) | ||
| DNA(+) | Sero‐ | 20/309 | 29/293 | 0.65 (0.38 to 1.13) | 1.70 |
| Sero+ | 53/333 | 44/307 | 1.11 (0.77 to 1.61) | ||
| Pooled results for CIN2+ associated with HPV16/18 | |||||
| DNA(‐) | Sero‐ | 5/14,014 | 120/13,372 | 0.03 (0.02 to 0.09) | 5.85 (0.53 to 65.10) |
| Sero+ | 3/2205 | 14/2301 | 0.19 (0.09 t0 o.77) | ||
| DNA(+) | Sero‐ | 53/679 | 64/695 | 0.79 (0.60 to 1.05 | 1.37 (0.97 to 1.93) |
| Sero+ | 100/531 | 96/639 | 1.10 (0.88 to 1.36) | ||
| *RR against HPV 6/11/16/18 related cervical lesions ** RR against HPV16/18 related CIN2+ *** Pooled only for FUTURE II and PATRIACIA, since, in the FUTURE I trial, the endpoints were cervical lesions and not CIN2+ associated with HPV16/18 | |||||
| Outcome | P value | ||||||
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | |
| Persistent HPV16/18 infection (6M), in women being baseline HPV16/18 negative 3 doses | 0.70 | 0.60 | np | np | 0.90 | np | 0.42 |
| Persistent HPV16/18 infection (6M), in women being baseline HPV16/18 negative at least 1 dose | 0.56 | 0.56 | np | np | np | np | np |
| Persistent HPV16/18 infection (12M), in women being baseline HPV16/18 negative 3 doses | 0.94 | 0.94 | np | np | np | np | 0.73 |
| Persistent HPV16/18 infection (12M), in women being baseline HPV16/18 negative at least 1 dose | 0.67 | 0.67 | np | np | np | np | np |
| Influence of study quality (items V1‐V6) and independence of the research team towards the vaccine manufacturer (V7) on protection against persistent HPV16/18 infection assessed by meta‐regression. The P values correspond with the statistical significance of the incorporation of each item in the meta‐regression. V1: Random sequence generation; V2: Allocation concealment; V3: Blinding participants and personnel; V4: Blinding of outcome; V5: Incomplete outcomes; V6: Selective reporting; V7: Involvement of manufacturer, np: meta‐regression not possible because of collinearity. | |||||||
| Outcome | No. of doses | Vaccine arm | Placebo arm | Relative Risk (95%CI) | P value for linear dose‐effect relation |
| 12‐month persistent HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 84/11,104 | 627/11,203 | 0.135 (0.108 to 0.169) | 0.303 |
| 2 | 3/611 | 26/574 | 0.108 (0.033 to 0.356) | ||
| 1 | 1/292 | 17/249 | 0.050 (0.007 to 0.374) | ||
| 6‐month persistent HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 114/11,104 | 1000/11,209 | 0.115 (0.095 to 0.139) | 0.269 |
| 2 | 4/611 | 35/574 | 0.107 (0.038 to 0.300) | ||
| 1 | 1/292 | 24/250 | 0.036 (0.005 to 0.261) | ||
| Incident HPV16/18 infection in women being HPV16/18 negative at baseline | 3 | 529/11,110 | 2172/11,217 | 0.246 (0.224 to 0.269) | 0.337 |
| 2 | 22/611 | 82/574 | 0.252 (0.160 to 0.398) | ||
| 1 | 8/292 | 45/251 | 0.153 (0.073 to 0.318) | ||
| 12‐month persistent HPV16/18 infection in women being hrHPV negative at baseline | 3 | 27/6634 | 351/6656 | 0.077 (0.052 to 0.114) | 0.996 |
| 2 | 2/273 | 12/276 | 0.168 (0.038 to 0.746) | ||
| 1 | 0/138 | 5/99 | 0.071 (0.004 to 1.289) | ||
| 6‐month persistent HPV16/18 infection in women being hrHPV negative at baseline | 3 | 38/6634 | 567/6660 | 0.067 (0.049 to 0.093) | 0.809 |
| 2 | 2/273 | 16/276 | 0.126 (0.029 to 0.544) | ||
| 1 | 0/138 | 8/100 | 0.045 (0.003 to 0.774) | ||
| Incident HPV16/18 infection in women being hrHPV negative at baseline | 3 | 38/6634 | 567/6660 | 0.067 (0.049 to 0.093) | 0.809 |
| 2 | 2/273 | 16/276 | 0.126 (0.029 to 0.544) | ||
| 1 | 0/138 | 8/100 | 0.045 (0.003 to 0.774) | ||
| Source: PATRICIA & CVT (ph3,2v) (Kreimer 2015). | |||||
| Outcome | No. of doses | n events | N vaccinated | % (95%CI) | P* for difference with 3 doses |
| Cumulative incidence HPV16/18 infections | 3 | 88 | 2023 | 4.3 (3.5 to 5.3) | ‐ |
| 2 (at months 0 & 6) | 3 | 78 | 3.8 (1.0 to 10.1) | 1.00 | |
| 2 (at months 0 & 1) | 7 | 192 | 3.6 (1.6 to 7.1) | 0.85 | |
| 1 | 2 | 133 | 1.5 (0.3 to 4.9) | 0.17 | |
| Source: Safaeian 2018. * two‐sided exact test for difference between proportions. | |||||
| Outcomes | Age Group (years) | Studies | RR if 3 doses (95% CI) | RR if 1‐2 doses (95% CI) |
| CIN2+ due to HPV16/18 | 15‐26 | 5 (FUTURE II trial (ph3,4v); Japanese trial (ph2,2v); PATRICIA trial (ph3,2v); Phase2 trial (ph2,1v); Chinese trial (ph3,2v)_young) | 0.07 (0.03 to 0.14)* | 0.10 (0.04 to 0.26)* |
| 24‐45 | 0.14 (0.03 to 0.79)* | 0.98 (0.20 to 4.83) | ||
| CIN3+ due to HPV16/18 | 15‐26 | 0.20 (0.04 to 0.91)* | 0.04 (0.01 to 0.74)* | |
| Incident HPV16/18 infection | 15‐26 | 3 (Japanese trial (ph2,2v); Phase2 trial (ph2,1v);Chinese trial (ph3,2v)_young) | 0.20 (0.10 to 0.41)* | 0.47 (0.26 to 0.84)* |
| 6‐month persistent HPV16/18 infection | 15‐26 | 0.05 (0.01 to 0.27)* | 0.12 (0.03 to 0.42)* | |
| 24‐45 | 0.15 (0.09 to 0.27)* | 0.34 (0.19 to 0.61)* | ||
| 12‐month persistent HPV16/18 infection | 15‐26 | 3 (Japanese trial (ph2,2v);CVT (ph3,2v); Chinese trial (ph3,2v)_young) | 0.09 (0.05 to 0.19)* | 0.13 (0.06 to 0.33)* |
| *Vaccine efficacy in women being HPV16/18 DNA negative at enrolment and having received all three or less than three doses (computed from trials where per‐protocol [all doses administered] and intention‐to‐treat analyses [at least one dose administered] are reported). | ||||
| Outcomes | Study | Report (duration of follow‐up) | Vaccine | Placebo | Relative Risk (95%CI) | P value for linear difference of follow‐up time effect |
| CIN2+ associated with HPV16/18 in women being HPV negative at baseline | PATRICIA | Paavonen 2007 14.8 moths | 2/7788 | 21/7838 | 0.096 (0.007 to 0.466) | 0.512 |
| Paavonen 2009 34.9 months | 5/8040 | 91/8080 | 0.054 (0.016 to 0.137) | |||
| Szarewski 2011 39.4 months | 5/8079 | 92/8112 | 0.054 (0.016 to 0.137) | |||
| Lehtinen 2011 43.7 months | 5/7338 | 97/7305 | 0.051 (0.016 to 0.123) | |||
| FUTURE | The FUTURE II study group 2007 36 months | 3/5865 | 87/5836 | 0.039 (0.011 to 0.109) | 0.994 | |
| Munoz 2010* 43 months | 0/4616 | 89/4680 | 0.006 (0.000 to 0.092) | |||
| CIN2+ irrespective of HPV types regardless of women’s initial HPV DNA status | PATRICIA | Paavonen 2009 34.9 months | 224/8667 | 322/8682 | 0.696 (0.579 to 0.8369) | 0.750 |
| Lehtinen 2011 43.7 months | 287/8694 | 428/8708 | 0.669 (0.574 to 0.778) | |||
| FUTURE | The FUTURE II study group 2007 36 months | 281/6087 | 361/6080 | 0.780 (0.668 to 0.905) | 0.665 | |
| Munoz 2010 43 months | 421/8562 | 520/8598 | 0.807 (0.690 to 0.943) | |||
| Assessment of the influence of duration of follow‐up on study outcomes using meta‐regression. p‐values correspond with the statistical significance of incorporating average follow‐up time as a continuous variable. | ||||||
| Number of sex partners | Vaccine | Placebo | Relative Risk (95% CI) | P value of number of sexual partners effect |
| In women being HPV16/18 DNA negative at baseline cohort | ||||
| Virgin | 1/566 | 17/615 | 0.064 (0.003 to 0.352) | 0.7448 |
| 1 partner | 3/904 | 27/915 | 0.112 (0.007 to 0.335) | |
| 2 partners | 1/544 | 17/519 | 0.056 (0.003 to 0.309) | |
| 3+ partners | 3/621 | 28/628 | 0.108 (0.026 to 0.321) | |
| Regardless of women’s baseline HPV DNA status | ||||
| Virgin | 4/733 | 21/819 | 0.202 (0.059 to 0.551) | < 0.0001 |
| 1 partner | 40/1237 | 83/1256 | 0.489 (0.333 to 0.711) | |
| 2 partners | 38/777 | 81/753 | 0.455 (0.307 to 0.665) | |
| 3+ partners | 71/940 | 116/911 | 0.593 (0.440 to 0.796) | |
| The influence of the number of lifetime sexual partners on vaccine efficacy was assessed by Poisson regression. The P value corresponds with the likelihood ratio test comparing a Poisson model with and without inclusion of the sexual history with 3 possible categories. Source: CVT (ph3,2v) (Herrero 2011). | ||||
| Outcomes | Study | Number of participants | Study size | Vaccine | Placebo | Relative Risk (95%CI) | P value |
| CIN2+ associated with HPV16/18 in women being HPV16/18 negative at baseline | Phase2 trial (V1) | 2392 | S | 0/126 | 8/127 | 0.062* (0.004 to 1.071) | 0.598 |
| Phase2 trial (V2) | 1113 | S | 0/219 | 3/212 | 0.161* (0.008 to 3.091) | ||
| Japanese trial (ph2,2v) | 1040 | S | 0/422 | 2/427 | 0.252* (0.012 to 5.241) | ||
| PATRICIA trial (ph3,2v) | 18,644 | L | 5/8040 | 91/8080 | 0.055 (0.022 to 0.136) | ||
| FUTURE II trial (ph3, 4v) | 12,167 | L | 3/5865 | 87/5863 | 0.034 (0.011 to 0.109) | ||
| Chinese trial (ph3,2V) | 6051 | L | 0/2543 | 4/2554 | 0.125 (0.001 to 8.681) | ||
| CIN2+ irrespective of HPV types and regardless of women’s initial HPV DNA status | FUTURE I/II trial (ph3,4v) | 17,622 | L | 421/8562 | 520/8598 | 0.813 (0.718 to 0.921) | 0.703 |
| PATRICIA trial (ph3,2v) | 18,644 | L | 287/8694 | 428/8708 | 0.672 (0.582 to 0.778) | ||
| Phase2 trial (v1) | 2392 | S | 8/148 | 12/142 | 0.640 (0.269 to 1.568) | ||
| Assesment of the influence of the study size on the protection against CIN2+ associated with HPV16/18 according to study size (S = small, < 3000 participants, L = large >= 3000 participants) in women aged 15‐26 years and received at least 1 dose. * P values correspond with the statistical significance of a meta‐regression with vs without study size category. | |||||||
| Analysis | Endpoint | Initial HPV status | Doses | Relative Risk |
| Monovalent vaccine (Rowhani‐Rahbar, 2009): 102 months of follow‐up | ||||
| 3.1 | CIN2+ associated with HPV16 | HPV16‐ | 3 | 0.00 |
| 3.2 | CIN2+ associated with HPV16 | HPV16‐ | >= 1 | 0.00 |
| 3.3 | CIN2+ associated with HPV16 | HPV16‐ | 1‐2 | 0.00 |
| 4.1 | Incident HPV16 infection | HPV16‐ | 3 | 0.05 |
| 4.3 | Incident HPV16 infection | HPV16‐ | >= 1 | 0.11 |
| 4.3 | Incident HPV16 infection | HPV16‐ | 1‐2 | 0.25 |
| 5.1 | CIN2+ associated with HPV16 | regardless of HPV infection | >= 1 | 0.36 |
| 5.3 | CIN2+ irrespective of HPV types | regardless of HPV infection | >= 1 | 0.64 |
| Bivalent vaccine (De Calvaho, 2012): 88 months of follow‐up | ||||
| 2.2 | 6M persistent HPV16/18 infection | hrHPV‐ | 3 | 0.00 |
| 2.4 | 12M persistent HPV16/18 infection | hrHPV‐ | 3 | 0.00 |
| 3.2 | CIN2+ associated with HPV16/18 | HPV16/18‐ | >= 1 | 0.00 |
| Quadrivalent vaccine (Villa, 2006): 60 months of follow‐up | ||||
| 4.8 | Persistent HPV6/11/16/18 infection | HPV16/18‐ | >= 1 | 0.07 |
| Trials | Ref | Endpoint | Relative Risk (95% CI) | P value for difference in VE | |
| Bivalent | Quadrivalent | ||||
| 6‐month persistent HPV31 infection | 0.229 (0.156 to 0.228) | 0.538 (0.336 to 0.847) | 0.003 | ||
| 6‐month persistent HPV45 infection | 0.210 (0.106 to 0.387) | 0.922 (0.507 to 1.670) | 0.0003 | ||
| CIN2+ associated with HPV33 | 0.177 (0.053 to 0.466) | 0.760 (0.328 to 1.712) | 0.02 | ||
| CIN2+ associated with HPV45 | 0.000 (0.000 to 0.583) | 0.481 (0.174 to 1.177) | 0.04 | ||
| CIN2+ associated with other hrHPV | 0.401 (0.192 to 0.793) | ||||
| 6‐month persistent HPV31 infection | 0.209 (0.041 to 0.724) | ||||
| 6‐month persistent HPV45 infection | 0.221 (0.044 to 0.914) | ||||
| Adverse effect | Relative risk | Relative risk ratio | p value | |
| Quadrivalent vs placebo | Bivalent/Quadrivalent | |||
| 1 | Overall adverse effects at injection site | 1.19 (0.89 to 1.59) | 1.69 (0.96 to 2.96) | 0.061 |
| 2 | Pain at injection site | 1.20 (0.78 to 1.85) | 1.19 (0.67 to 2.12) | 0.501 |
| 3 | Swelling at injection site | 2.72 (0.77to 9.61) | 0.62 (0.16 to 2.41) | 0.427 |
| 4 | Redness at injection site | 1.46 (1.23 to 1.74) | 1.08 (0.88 to 1.32) | 0.307 |
| 5 | Overall systemic events | 0.99 (0.91 to 1.07) | 1.06 (0.95 to 1.19) | 0.210 |
| 6 | Serious adverse events | 0.94 (0.70 to 1.26) | 1.08 (0.80 to 1.45) | 0.583 |
| 7 | Deaths | 1.18 (0.25 to 5.62) | 0.84 (0.14 to 4.91) | 0.775 |
| Relative risks of the quadrivalent vaccine versus placebo and the relative risk ratios were computed by meta‐regression including vaccine, age group and type of product injected in the control group (aluminium adjuvants alone or other vaccine such as Hepatitis A vaccine) as covariate. The relative risk ratio reflects how much more an adverse effect is observed after vaccination with the bivalent versus the quadrivalent vaccine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 3 | 23676 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.05] |
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.09] |
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.10] |
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.82] |
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.80] |
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 5 | 25180 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.25, 0.55] |
| 7.1 Bivalent vaccine | 4 | 15884 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.25, 0.43] |
| 7.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.44, 0.76] |
| 8 Any CIN3+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 20719 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.04, 1.10] |
| 8.1 Bivalent vaccine | 2 | 11423 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.03, 0.23] |
| 8.2 Quadrivalent vaccine | 1 | 9296 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.36, 0.82] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 20214 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.01, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/(18), 3 doses Show forest plot | 8 | 43376 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.04, 0.16] |
| 1.1 Age group 15‐26 years | 6 | 36579 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.03, 0.15] |
| 1.2 Age group 24‐45 years | 2 | 6797 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.04, 0.74] |
| 2 CIN2+ associated with HPV16/(18), at least 1 dose Show forest plot | 8 | 42030 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.05, 0.20] |
| 2.1 Age group 15‐26 years | 6 | 34478 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.03, 0.10] |
| 2.2 Age group 24‐45 years | 2 | 7552 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.11, 0.81] |
| 3 CIN2+ associated with HPV16/(18), 1 or 2 doses (post hoc analysis) Show forest plot | 7 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.19 [0.07, 0.51] |
| 3.1 women age 15‐26 years | 5 | 2958 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.04, 0.26] |
| 3.2 women age 24‐45 years | 2 | 755 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.14, 2.67] |
| 4 CIN2+ associated with HPV6/11/16/18, 3 doses Show forest plot | 2 | 7664 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.61] |
| 4.1 Age group 15‐26 years | 1 | 4499 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.25] |
| 4.2 Age group 24‐45 years | 1 | 3165 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.02, 1.39] |
| 5 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 8980 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.00, 2.41] |
| 5.1 Age group 15‐26 years | 1 | 5351 | Risk Ratio (IV, Random, 95% CI) | 0.01 [0.00, 0.19] |
| 5.2 Age group 24‐45 years | 1 | 3629 | Risk Ratio (IV, Random, 95% CI) | 0.37 [0.10, 1.41] |
| 6 CIN2+ associated with HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 1316 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.01, 5.00] |
| 6.1 Age group 15‐26 years | 1 | 852 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.74] |
| 6.2 Age group 24‐45 years | 1 | 464 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.14, 6.80] |
| 7 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29720 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.02, 0.29] |
| 8 CIN3+ associated with HPV 16/18 or HPV6/11/16/18, at least 1 dose Show forest plot | 3 | 33199 | Risk Ratio (IV, Random, 95% CI) | 0.05 [0.02, 0.14] |
| 9 CIN3+ associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3479 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.01, 0.24] |
| 10 AIS associated with HPV16/18 or HPV6/11/16/18, 3 doses Show forest plot | 3 | 29707 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.02, 0.70] |
| 11 AIS associated with HPV16/18 or 6/11/16/18, at least 1 dose Show forest plot | 2 | 17079 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.01, 0.72] |
| 12 AIS associated with HPV16/18 or HPV6/11/16/18, 1 or 2 doses (post hoc analysis) Show forest plot | 2 | 2015 | Risk Ratio (IV, Random, 95% CI) | 0.15 [0.01, 2.97] |
| 13 Any CIN2+ irrespective of HPV types, 3 doses Show forest plot | 3 | 7320 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.25, 0.64] |
| 14 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 3 | 19143 | Risk Ratio (IV, Random, 95% CI) | 0.41 [0.32, 0.52] |
| 15 Any CIN2+ irrespective of HPV types, 1 or 2 doses (post hoc analysis) Show forest plot | 1 | 34 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.15, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CIN2+ associated with HPV16/18, at least 1 dose Show forest plot | 5 | 44052 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.41, 0.67] |
| 1.1 Age group 15‐26 years | 3 | 34852 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.37, 0.57] |
| 1.2 Age group 24‐45 years | 2 | 9200 | Risk Ratio (IV, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 CIN2+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20883 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 2.1 Age group 15‐26 years | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.42, 0.59] |
| 2.2 Age group 24‐45 years | 1 | 3723 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.44, 1.37] |
| 3 CIN3+ associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.45, 0.67] |
| 4 CIN3+ associated with HPV6/11/16/18, at least 1 dose Show forest plot | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.43, 0.68] |
| 5 AIS associated with HPV16/18, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.17, 0.78] |
| 6 AIS associated with HPV6/11/16/18, at least 1 dose Show forest plot | 2 | 20830 | Risk Ratio (IV, Random, 95% CI) | 0.40 [0.16, 0.98] |
| 7 Any CIN2+ irrespective of HPV types, at least 1 dose Show forest plot | 6 | 45066 | Risk Ratio (IV, Random, 95% CI) | 0.79 [0.65, 0.97] |
| 7.1 Age group 15‐26 years | 4 | 35779 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.58, 0.85] |
| 7.2 Age group 24‐45 years | 2 | 9287 | Risk Ratio (IV, Random, 95% CI) | 1.04 [0.83, 1.30] |
| 8 Any CIN3+ HPV type, at least 1 dose Show forest plot | 3 | 35489 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.49, 0.93] |
| 8.1 Bivalent vaccine | 2 | 18329 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 8.2 Quadrivalent vaccine | 1 | 17160 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.69, 0.96] |
| 9 Any AIS irrespective of HPV types, at least 1 dose Show forest plot | 2 | 34562 | Risk Ratio (IV, Random, 95% CI) | 0.32 [0.15, 0.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.02, 0.20] |
| 2 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.02 [0.00, 0.35] |
| 3 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 1 | 10826 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.05, 0.09] |
| 4 Persistent HPV16/18 infection(12M), 3 doses Show forest plot | 1 | 368 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.00, 0.73] |
| 5 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 14153 | Risk Ratio (IV, Random, 95% CI) | 0.08 [0.05, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, 3 doses Show forest plot | 4 | 8034 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.31] |
| 2 Incident HPV16/18 infection, at least 1 dose Show forest plot | 5 | 23872 | Risk Ratio (IV, Random, 95% CI) | 0.23 [0.14, 0.37] |
| 3 Incident HPV16/18 infection, 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 331 | Risk Ratio (IV, Random, 95% CI) | 0.47 [0.26, 0.84] |
| 4 Persistent HPV16/18 infection (6M), 3 doses Show forest plot | 8 | 34113 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.06, 0.09] |
| 4.1 Age group 15‐26 years | 6 | 27385 | Risk Ratio (IV, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 4.2 Age group 24‐45 years | 2 | 6728 | Risk Ratio (IV, Random, 95% CI) | 0.11 [0.06, 0.20] |
| 5 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 6 | 30323 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.08, 0.17] |
| 5.1 Age group 15‐26 years | 4 | 22803 | Risk Ratio (IV, Random, 95% CI) | 0.10 [0.08, 0.12] |
| 5.2 Age group 24‐45 years | 2 | 7520 | Risk Ratio (IV, Random, 95% CI) | 0.17 [0.10, 0.29] |
| 6 Persistent HPV16/18 infection (6M), 1 or 2 doses (post hoc analysis) Show forest plot | 4 | 1229 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.16, 0.44] |
| 6.1 Age group 15‐26 years | 2 | 437 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.03, 0.42] |
| 6.2 Age group 24‐45 years | 2 | 792 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.18, 0.54] |
| 7 Persistent HPV6/11/16/18 infection (6M), 3 doses Show forest plot | 2 | 4008 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.06, 0.21] |
| 8 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 2 | 4129 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.05, 0.37] |
| 9 Persistent HPV16/18 infection (12M), 3 doses Show forest plot | 4 | 22267 | Risk Ratio (IV, Random, 95% CI) | 0.09 [0.06, 0.13] |
| 10 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 5 | 29464 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.12, 0.20] |
| 11 Persistent HPV16/18 infection (12M), 1 or 2 doses (post hoc analysis) Show forest plot | 3 | 3912 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.06, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incident HPV16/18 infection, at least 1 dose Show forest plot | 1 | 4210 | Risk Ratio (IV, Random, 95% CI) | 0.24 [0.17, 0.33] |
| 2 Persistent HPV16/18 infection (6M), at least 1 dose Show forest plot | 4 | 33847 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.41, 0.57] |
| 2.1 Age group 15‐26 years | 2 | 25199 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.38, 0.51] |
| 2.2 Age group 24‐45 years | 2 | 8648 | Risk Ratio (IV, Random, 95% CI) | 0.57 [0.47, 0.69] |
| 3 Persistent HPV6/11/16/18 infection (6M), at least 1 dose Show forest plot | 1 | 3713 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.42, 0.65] |
| 4 Persistent HPV16/18 infection (12M), at least 1 dose Show forest plot | 2 | 24785 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.40, 0.54] |
| 5 Persistent HPV16/18 infection (12M) by dose (post hoc analysis) Show forest plot | 1 | 7153 | Risk Ratio (IV, Random, 95% CI) | 0.18 [0.12, 0.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall local/injection site adverse events Show forest plot | 8 | 18113 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [1.16, 1.20] |
| 1.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Fixed, 95% CI) | 1.29 [1.26, 1.33] |
| 1.2 Quadrivalent vaccine | 6 | 11610 | Risk Ratio (IV, Fixed, 95% CI) | 1.14 [1.12, 1.16] |
| 2 Pain at injection site Show forest plot | 13 | 25691 | Risk Ratio (IV, Random, 95% CI) | 1.35 [1.23, 1.49] |
| 2.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 1.05 [1.01, 1.09] |
| 2.2 Bivalent vaccine | 8 | 16897 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.26, 1.75] |
| 2.3 Quadrivalent vaccine | 4 | 6514 | Risk Ratio (IV, Random, 95% CI) | 1.13 [1.07, 1.19] |
| 3 Swelling at injection site Show forest plot | 9 | 22106 | Risk Ratio (IV, Random, 95% CI) | 1.73 [1.32, 2.27] |
| 3.1 Bivalent vaccine | 7 | 16603 | Risk Ratio (IV, Random, 95% CI) | 1.62 [1.15, 2.29] |
| 3.2 Quadrivalent vaccine | 2 | 5503 | Risk Ratio (IV, Random, 95% CI) | 2.79 [0.85, 9.15] |
| 4 Redness at injection site Show forest plot | 6 | 19996 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.50, 1.97] |
| 4.1 Quadrivalent vaccine | 1 | 5345 | Risk Ratio (IV, Random, 95% CI) | 1.46 [1.32, 1.63] |
| 4.2 Bivalent vaccine | 5 | 14651 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.53, 2.11] |
| 5 Overall systemic event and general symptoms Show forest plot | 8 | 18191 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.98, 1.07] |
| 5.1 Bivalent vaccine | 2 | 6503 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.97, 1.19] |
| 5.2 Quadrivalent vaccine | 6 | 11688 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.98, 1.04] |
| 6 Serious adverse events Show forest plot | 23 | 71597 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 6.1 Monovalent vaccine | 1 | 2387 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.51, 1.78] |
| 6.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.96, 1.07] |
| 6.3 Quadrivalent vaccine | 7 | 22979 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.65, 1.02] |
| 7 Deaths Show forest plot | 23 | 71176 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.85, 1.98] |
| 7.1 Monovalent vaccine | 1 | 2280 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Bivalent vaccine | 15 | 46231 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.66, 2.22] |
| 7.3 Quadrivalent vaccine | 7 | 22665 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.73, 3.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Normal infant Show forest plot | 8 | 8782 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 2 Spontaneous abortion/miscarriage Show forest plot | 9 | 8618 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.68, 1.14] |
| 3 Elective termination/induced abortion Show forest plot | 9 | 10909 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.80, 1.02] |
| 4 Stillbirth Show forest plot | 6 | 8754 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.68, 1.83] |
| 5 Abnormal infant Show forest plot | 5 | 9252 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.88, 1.69] |

