Aripiprazole for autism spectrum disorders (ASD)
Information
- DOI:
- https://doi.org/10.1002/14651858.CD009043.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 26 June 2016see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Developmental, Psychosocial and Learning Problems Group
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
For the original Cochrane review, Heidi Ching drafted the protocol, selected trials for inclusion, extracted data from trials, entered data into RevMan, drafted the final review and carried out the analysis. For this version only, Lauren Hirsch selected trials for inclusion, extracted data from trials, entered data into RevMan and drafted and edited the final review. For both the original Cochrane review (Ching 2012) and this version, Tamara Pringsheim edited the protocol, selected trials for inclusion, extracted data from trials, interpreted the analysis and drafted the final review. Tamara has overall responsibility for the review and will keep the review up‐to‐date.
Sources of support
Internal sources
-
University of Calgary, Department of Clinical Neurosciences, Canada.
Salary support for Dr. Pringsheim
External sources
-
None, Other.
Declarations of interest
Lauren Hirsch has no conflicts of interest to declare.
Tamara Pringsheim (TP) has received direct financial gain from:
-
Teva Canada Innovation, on one occasion in 2014, for consulting. TP declares that Teva does not make or market any products relevant to this review.
TP has received indirect financial gain from:
-
Shire Canada, in 2015. TP received an unrestricted educational grant to create an educational curriculum on assessment and treatment of aggression in youth. TP declares that Shire does not make or market any products relevant to this review.
TP declares that the entities listed below are funding agencies that do not make treatments for ASD or any other disease.
-
Canadian Institutes of Health Research, for research funding to create a patient decision aid in 2014 and 2015.
-
Alberta Mental Health Strategic Clinical Network, for research funding on off‐label prescribing of antipsychotics in 2015.
-
Mathison Centre for Mental Health Research and Education, for research funding for the Canadian Schizophrenia Guidelines in 2015.
Acknowledgements
The review authors wish to acknowledge the Departments of Clinical Neurosciences and Pediatrics, at the University of Calgary, and the Cochrane Developmental, Psychosocial and Learning Problems Group.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jun 26 | Aripiprazole for autism spectrum disorders (ASD) | Review | Lauren E Hirsch, Tamara Pringsheim | |
| 2012 May 16 | Aripiprazole for autism spectrum disorders (ASD) | Review | Heidi Ching, Tamara Pringsheim | |
| 2011 Mar 16 | Aripiprazole for Autism Spectrum Disorders (ASD) | Protocol | Heidi Ching, Tamara Pringsheim | |
Differences between protocol and review
-
We choose not to exclude participants receiving non‐pharmacological therapy (i.e. behaviour therapy), provided it was equally accessible to all study participants, stable before trial entry and consistent throughout the study. This was not specified in the protocol (Ching 2011) and therefore reflects a post hoc decision.
-
We have specified in the Types of outcome measures section that we use the term 'side effects' to describe any harms, adverse effects or adverse drug reactions associated with aripiprazole.
-
We added Conference Proceedings Citation Index ‐ Science and WorldCat to the electronic sources listed in our protocol (Ching 2011) to increase the chance of finding conference papers and theses. We also added Autism Data to search the holdings of the National Autistic Society Information Centre Library. For this update, we also searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effects (DARE) to check the reference lists of previous reviews related to our topic.
-
In the Assessment of risk of bias in included studies, we reported separately methods for assessing the risk of performance bias due to inadequate blinding of participants and personnel, and methods for assessing the risk of detection bias due to inadequate blinding of outcome assessment.
-
We analysed time‐to‐event data using hazard ratios and their associated 95% CIs. This type of data analysis was not listed in the protocol (Ching 2011) and therefore reflects a post hoc decision.
-
We reported tau² ‐ an estimate of between‐study variance ‐ when reporting results from the random‐effects model. This was not specified in the protocol (Ching 2011) and therefore reflects a post hoc decision.
-
We added a section on 'Summary of findings', beneath the section on 'Data synthesis'.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adolescent; Child; Female; Humans; Male;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes.
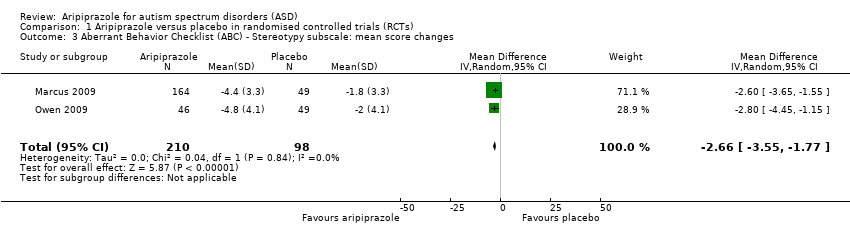
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores.
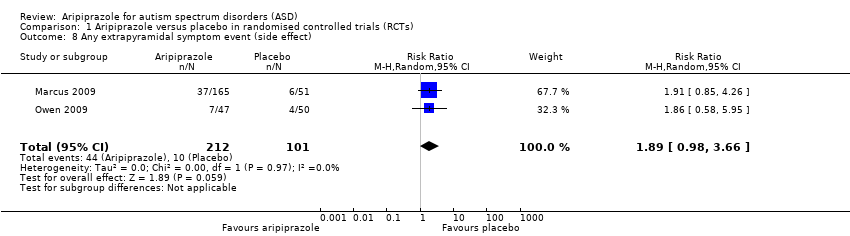
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 8 Any extrapyramidal symptom event (side effect).
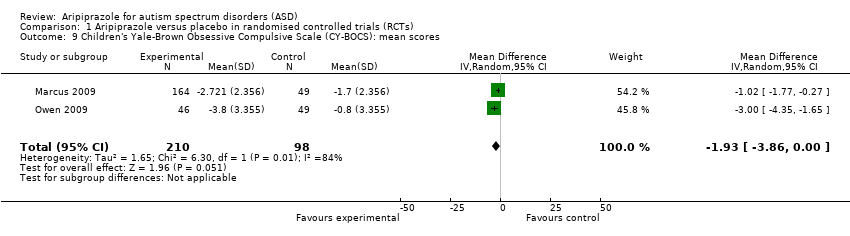
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores.

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 10 Clinically relevant weight gain (side effect).
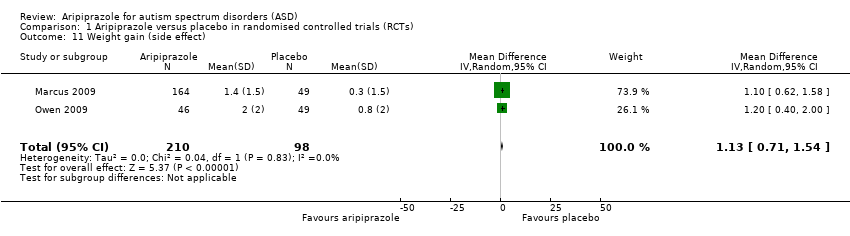
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 11 Weight gain (side effect).
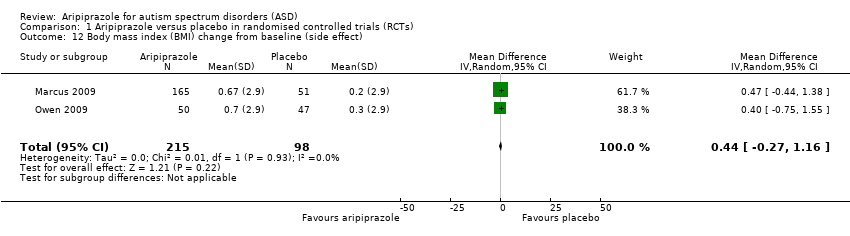
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 12 Body mass index (BMI) change from baseline (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 13 Elevated fasting triglycerides at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 14 Elevated low‐density lipoprotein at endpoint (side effect).
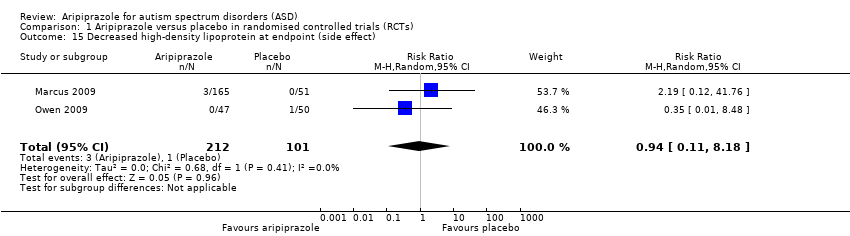
Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 15 Decreased high‐density lipoprotein at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 16 Elevated fasting blood glucose at endpoint (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 17 Sedation (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 18 Drooling (side effect).

Comparison 1 Aripiprazole versus placebo in randomised controlled trials (RCTs), Outcome 19 Tremor (side effect).
| Aripiprazole compared with placebo for autism spectrum disorders (ASD)* | ||||
| Patient or population: children/youth with ASD Settings: ambulatory care Intervention: aripiprazole Comparison: placebo | ||||
| Outcomes | Effect (95% confidence interval) | Number of participants (studies) | Quality of the evidence | Comments |
| ABC ‐ Irritability subscale Mean score changes 8 weeks of treatment | MD ‐6.17 (‐9.07 to ‐3.26) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Hyperactivity subscale Mean score changes 8 weeks of treatment | MD ‐7.93 (‐10.98 to ‐4.88) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| ABC ‐ Stereotypy subscale Mean score changes 8 weeks of treatment | MD ‐2.66 (‐3.55 to ‐1.77) points relative to placebo, in favour of aripiprazole | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Weight gain 8 weeks of treatment | MD 1.13 (0.71 to 1.54) points relative to placebo | 308 (2) | ⊕⊕⊕⊝ | ‐ |
| Sedation 8 weeks of treatment | RR 4.28 (1.58 to 11.60) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Tremor 8 weeks of treatment | RR 10.26 (1.37 to 76.63) | 313 (2) | ⊕⊕⊕⊝ | ‐ |
| Relapse rate 16 weeks of treatment | HR 0.57 (0.28 to 1.12) | 85 (1) | Lowb | ‐ |
| ABC: Aberrant Behavior Checklist; GRADE: Grades of Recommendation, Assessment, Development and Evaluation; HR: hazard ratio;MD: mean difference; RR: risk ratio | ||||
| GRADE Working Group grades of evidence | ||||
| aQuality was rated as moderate because of unclear risk of bias for some domains in Marcus 2009 and Owen 2009, and because of the overall small number of studies conducted using aripiprazole in ASD. *A small number of studies have evaluated the use of aripiprazole for ASD. Only one study examined aripiprazole at a duration longer than eight weeks. Future trials of aripiprazole in children/adolescents with ASD are likely to impact the estimates found in this review. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aberrant Behavior Checklist (ABC) ‐ Irritability subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐9.07, ‐3.26] |
| 2 Aberrant Behavior Checklist (ABC) ‐ Hyperactivity subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐7.93 [‐10.98, ‐4.88] |
| 3 Aberrant Behavior Checklist (ABC) ‐ Stereotypy subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐2.66 [‐3.55, ‐1.77] |
| 4 Aberrant Behavior Checklist (ABC) ‐ Inappropriate Speech subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.60, ‐0.27] |
| 5 Aberrant Behavior Checklist (ABC) ‐ Lethargy/Withdrawal subscale: mean score changes Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.77, 0.40] |
| 6 Clinical Global Impression (CGI) ‐ Severity subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.96, ‐0.18] |
| 7 Clinical Global Impression (CGI) ‐ Improvement subscale: mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.75, ‐0.92] |
| 8 Any extrapyramidal symptom event (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.98, 3.66] |
| 9 Children's Yale‐Brown Obsessive Compulsive Scale (CY‐BOCS): mean scores Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.86, 0.00] |
| 10 Clinically relevant weight gain (side effect) Show forest plot | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [1.78, 8.02] |
| 11 Weight gain (side effect) Show forest plot | 2 | 308 | Mean Difference (IV, Random, 95% CI) | 1.13 [0.71, 1.54] |
| 12 Body mass index (BMI) change from baseline (side effect) Show forest plot | 2 | 313 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.27, 1.16] |
| 13 Elevated fasting triglycerides at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.62, 3.67] |
| 14 Elevated low‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 3.19 [0.13, 76.36] |
| 15 Decreased high‐density lipoprotein at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.11, 8.18] |
| 16 Elevated fasting blood glucose at endpoint (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.08, 32.11] |
| 17 Sedation (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.58, 11.60] |
| 18 Drooling (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 9.64 [1.29, 72.10] |
| 19 Tremor (side effect) Show forest plot | 2 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 10.26 [1.37, 76.63] |


