Antibiotic therapy for preventing infections in people with acute stroke
Information
- DOI:
- https://doi.org/10.1002/14651858.CD008530.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 22 January 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Stroke Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
J‐D Vermeij (JDV): performed the search, extracted data, and analysed and interpreted data.
WF Westendorp (WW): performed the search, extracted data, analysed and interpreted data, and drafted the review.
DWJ Dippel (DWJD): conceived and designed the review, assessed methodological quality, and provided final approval of the review for publication.
PJ Nederkoorn (PJN): performed analysis and interpretation of the data and commented on the review for its intellectual content.
D van de Beek (DVDB): commented on the review for intellectual content and provided final approval of the review for publication.
Sources of support
Internal sources
-
Academic Medical Center, Netherlands.
Department of Neurology
-
Erasmus Medical Center, Netherlands.
Department of Neurology
External sources
-
ZonMW, Netherlands.
Grant: Netherlands Organisation for Health Research and Development (ZonMW): 171002302
-
Netherlands Heart Foundation, Netherlands.
Grant: Netherlands Heart Foundation (Hartstichting): 2009B095
-
Netherlands Organization for Health Research and Development, Netherlands.
Netherlands Organization for Health Research and Development (ZonMw; NWOVeni grant 2006 [916.76.023], NWO‐Vidi grant 2010 [016.116.358])
Declarations of interest
J‐D Vermeij (JDV): member of the study group of the Preventive Antibiotics in Stroke Study (ISRCTN66140176).
WF Westendorp (WW): member of the study group of the Preventive Antibiotics in Stroke Study (ISRCTN66140176).
DWJ Dippel (DWJD): member of the study group of the Preventive Antibiotics in Stroke Study (ISRCTN66140176).
PJ Nederkoorn (PJN): principal investigator of the Preventive Antibiotics in Stroke Study (ISRCTN66140176).
D van de Beek (DVDB): principal investigator of the Preventive Antibiotics in Stroke Study (ISRCTN66140176).
Acknowledgements
This work is supported by grants from the Netherlands Organization for Health Research and Development (ZonMW; 171002302) and the Netherlands Heart Foundation (Hartstichting; 2009B095). We especially thank Prof A Meisel for permitting us to use unpublished data from the STRAWINSKI trial in favour of this meta‐analysis. We also thank Dr A Chamorro, Dr M Boaz, and Dr Y Lampl, for providing additional data from their studies. Finally, we thank B van de Veen (BvdV) for assessing risk of bias of the manuscript by Westendorp et al.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jan 22 | Antibiotic therapy for preventing infections in people with acute stroke | Review | Jan‐Dirk Vermeij, Willeke F Westendorp, Diederik WJ Dippel, Diederik van de Beek, Paul J Nederkoorn | |
| 2012 Jan 18 | Antibiotic therapy for preventing infections in patients with acute stroke | Review | Willeke F Westendorp, Jan‐Dirk Vermeij, Frederique Vermeij, Heleen M Den Hertog, Diederik WJ Dippel, Diederik van de Beek, Paul J Nederkoorn | |
| 2010 Jun 16 | Antibiotic therapy for preventing infections in patients with acute stroke | Protocol | Frederique Vermeij, Paul J Nederkoorn, Heleen M Den Hertog, Diederik van de Beek, Diederik WJ Dippel | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti-Bacterial Agents [*therapeutic use];
- Antibiotic Prophylaxis [methods];
- Bacterial Infections [mortality, *prevention & control];
- Brain Ischemia [complications];
- Pneumonia [epidemiology];
- Randomized Controlled Trials as Topic;
- Stroke [*complications, mortality];
- Urinary Tract Infections [epidemiology];
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study, using the Cochrane 'Risk of bias' tool. '+' is defined as low risk of bias, '‐' as high risk of bias, '?' as unclear risk of bias.
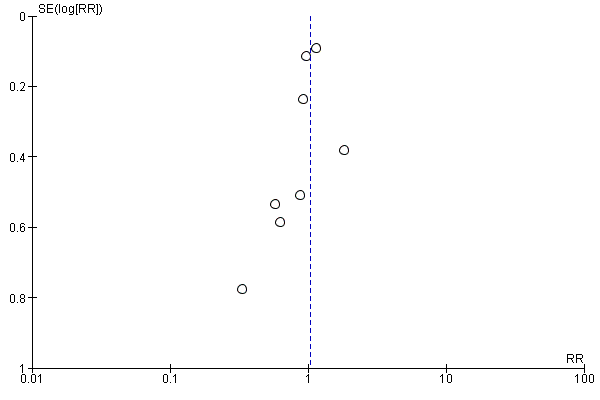
Funnel plot of comparison: 1 Forest plot of comparison: primary outcomes, outcome: 1.1 Case fatality at the end of follow‐up.

Funnel plot of comparison: 1 Forest plot of comparison: primary outcomes, outcome: 1.2 Death or dependency at the end of follow‐up.

Funnel plot of comparison: 2 Forest plot of comparison: secondary outcomes, outcome: 2.1 Number of infections at the end of follow‐up.
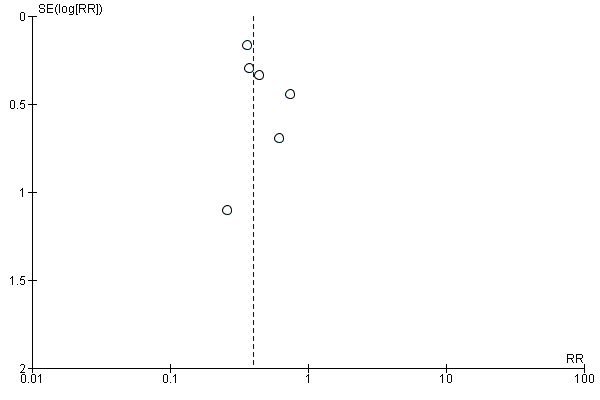
Funnel plot of comparison: 2 Forest plot of comparison: secondary outcomes, outcome: 2.2 Number of UTIs at the end of follow‐up.

Funnel plot of comparison: 2 Forest plot of comparison: secondary outcomes, outcome: 2.3 Number of pneumonias at the end of follow‐up.

Comparison 1 Forest plot of comparison: primary outcomes, Outcome 1 Case fatality at the end of follow‐up.

Comparison 1 Forest plot of comparison: primary outcomes, Outcome 2 Death or dependency at the end of follow‐up.

Comparison 2 Forest plot of comparison: secondary outcomes, Outcome 1 Number of infections at the end of follow‐up.
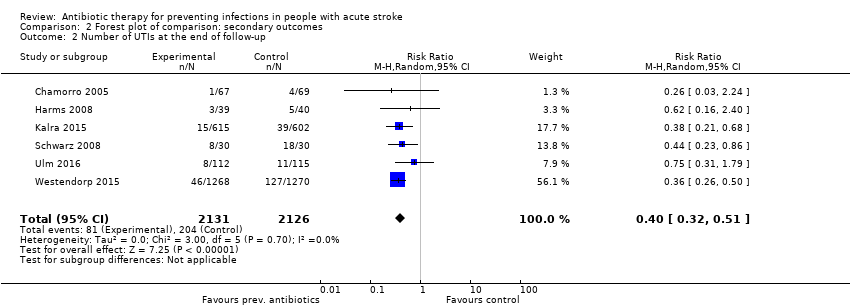
Comparison 2 Forest plot of comparison: secondary outcomes, Outcome 2 Number of UTIs at the end of follow‐up.

Comparison 2 Forest plot of comparison: secondary outcomes, Outcome 3 Number of pneumonias at the end of follow‐up.
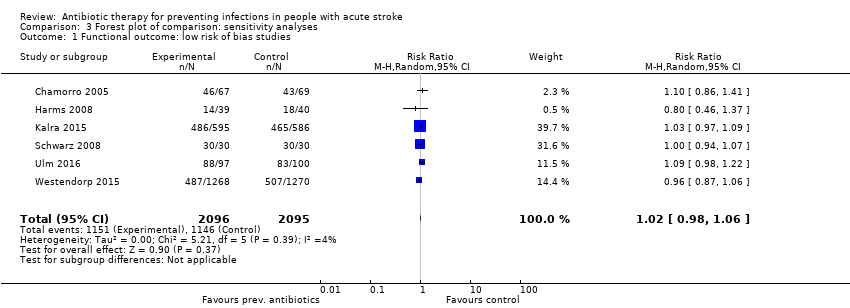
Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 1 Functional outcome: low risk of bias studies.
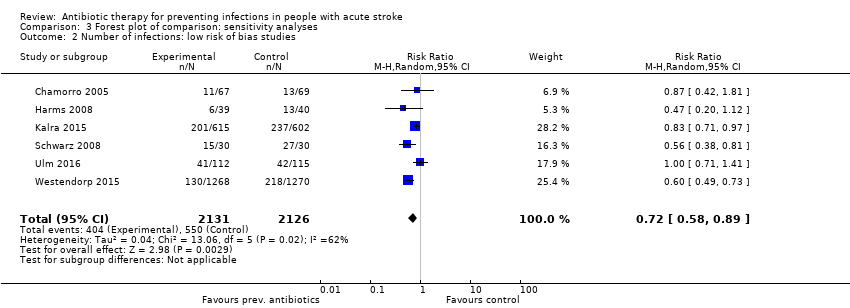
Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 2 Number of infections: low risk of bias studies.
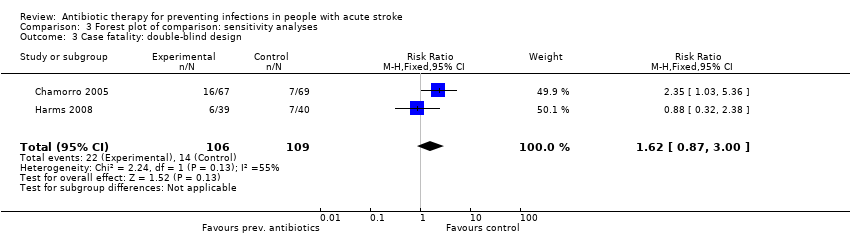
Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 3 Case fatality: double‐blind design.
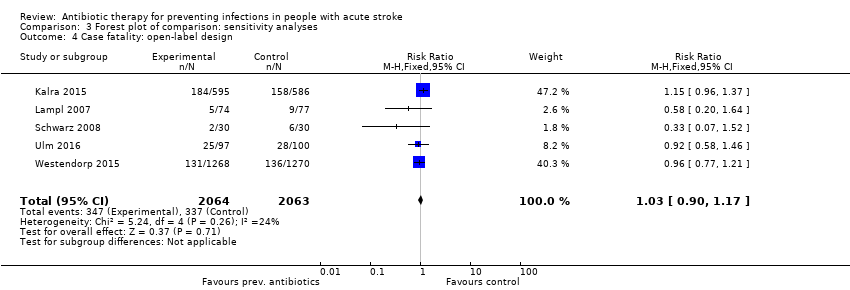
Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 4 Case fatality: open‐label design.
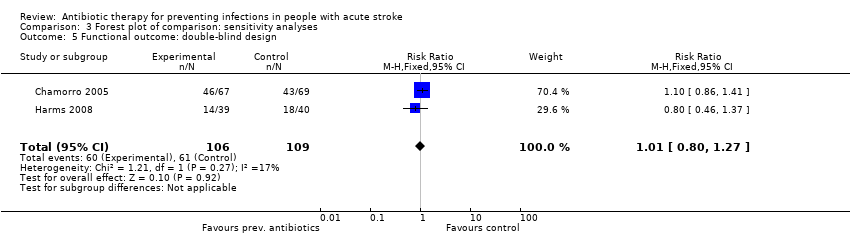
Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 5 Functional outcome: double‐blind design.

Comparison 3 Forest plot of comparison: sensitivity analyses, Outcome 6 Functional outcome: open‐label design.
| Preventive antibiotic therapy compared with placebo and/or conventional management in acute stroke | ||||||
| Patient or population: patients with acute ischaemic or haemorrhagic stroke Setting: acute stroke management Intervention: preventive antibiotic therapy for systemic use, at any dose or length of treatment Comparison: placebo and/or conventional acute stroke management | ||||||
| Outcomes | Absolute risk | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo and/or conventional management | Risk with preventive antibiotic treatment | |||||
| Case fatality at the end of follow‐up | Study population | RR 1.03 (0.87 to 1.21) | 4422 (8) | ⊕⊕⊕⊕ | ||
| 163 per 1000 | 169 per 1000 | |||||
| Poor functional outcome at the end of follow‐up | Study population | RR 0.99 (0.89 to 1.10) | 4332 (7) | ⊕⊕⊕⊕ | ||
| 547 per 1000 | 535 per 1000 | |||||
| Number of infections at the end of follow‐up | Study population | RR 0.71 (0.58 to 0.88) | 4317 (7) | ⊕⊕⊕⊕ | ||
| 259 per 1000 | 189 per 1000 | |||||
| Number of UTIs at the end of follow‐up | Study population | RR 0.40 (0.32 to 0.51) | 4257 (6) | ⊕⊕⊕⊕ | ||
| 96 per 1000 | 39 per 1000 | |||||
| Number of pneumonias at the end of follow‐up | Study population | RR 0.95 (0.80 to 1.13) | 4257 (6) | ⊕⊕⊕⊕ | ||
| 111 per 1000 | 105 per 1000 | |||||
| Occurrence of elevated body temperature | Insufficient data. Assessed qualitatively in only 2 studies | |||||
| Rate of serious adverse events | No major side effects of preventive antibiotic therapy were reported. | |||||
| *The absolute risk is calculated using the absolute numbers of events in both study arms. | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aLarge number of included studies, large number of participants, and small confidence interval (ultimately low risk of bias). Good applicability in clinical practice. bLimited publication bias cannot be excluded, as funnel plots for primary outcomes were skewed at the base, towards good outcomes. eDowngraded owing to multiple remarks on GRADE considerations, despite the fact that all remarks can be explained and rectified. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Case fatality at the end of follow‐up Show forest plot | 8 | 4422 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.87, 1.21] |
| 2 Death or dependency at the end of follow‐up Show forest plot | 7 | 4332 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.89, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of infections at the end of follow‐up Show forest plot | 7 | 4317 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.88] |
| 2 Number of UTIs at the end of follow‐up Show forest plot | 6 | 4257 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.32, 0.51] |
| 3 Number of pneumonias at the end of follow‐up Show forest plot | 6 | 4257 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional outcome: low risk of bias studies Show forest plot | 6 | 4191 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.06] |
| 2 Number of infections: low risk of bias studies Show forest plot | 6 | 4257 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.58, 0.89] |
| 3 Case fatality: double‐blind design Show forest plot | 2 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.87, 3.00] |
| 4 Case fatality: open‐label design Show forest plot | 5 | 4127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 5 Functional outcome: double‐blind design Show forest plot | 2 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 6 Functional outcome: open‐label design Show forest plot | 5 | 4117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.03] |


