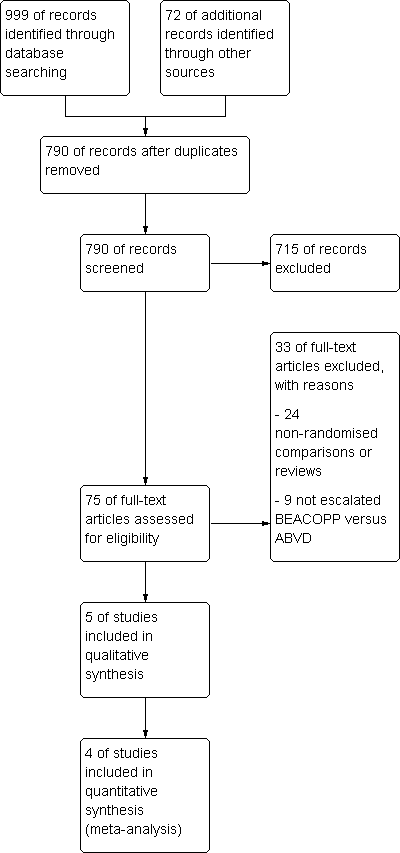
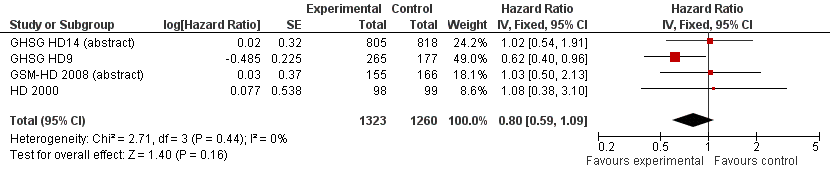
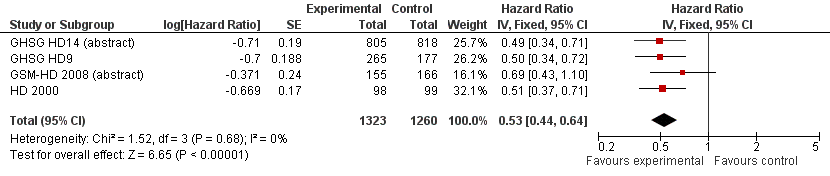
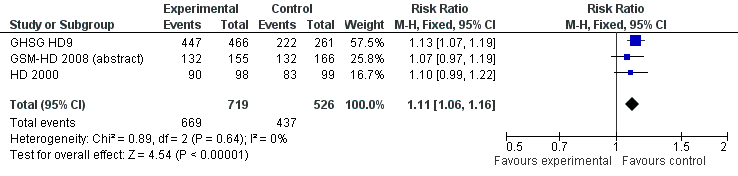
Related content
Related reviews and protocols
Nicole Skoetz, Andrea Will, Ina Monsef, Corinne Brillant, Andreas Engert, Bastian von Tresckow | 25 May 2017
Jeremy Franklin, Dennis A. Eichenauer, Ingrid Becker, Ina Monsef, Andreas Engert | 13 September 2017
Nicole Bergner, Ina Monsef, Gerald Illerhaus, Andreas Engert, Nicole Skoetz | 14 November 2012
Liat Vidal, Anat Gafter‐Gvili, Ronit Gurion, Pia Raanani, Martin Dreyling, Ofer Shpilberg | 12 September 2012
Arturo J Martí‐Carvajal, Andrés Felipe Cardona, Able Lawrence | 8 July 2009
Saurabh Parasramka, Goutham Talari, Myrna Rosenfeld, Jing Guo, John L Villano | 26 July 2017
Xia Zhang, Yunhai Chuai, Wei Nie, Aiming Wang, Guanghai Dai | 27 November 2017
Wahyu Wulaningsih, Ardyan Wardhana, Johnathan Watkins, Naomi Yoshuantari, Dimitra Repana, Mieke Van Hemelrijck | 12 February 2016
Frank Peinemann, Doreen A Kahangire, Elvira C van Dalen, Frank Berthold | 19 May 2015
Linyu Deng, Jing Zhang, Taixiang Wu, Theresa A Lawrie | 31 January 2013