补充维生素A用于降低1‐6月龄幼儿的发病率和死亡率。
Abstract
研究背景
维生素A缺乏是低收入和中等收入国家突出的公共卫生问题。给6月龄前的幼儿补充维生素A是一种改善维生素A缺乏高危幼儿营养状况的策略,翼此可同时降低他们的发病率和死亡率。
研究目的
为评价在低收入和中等收入国家中给1‐6月龄幼儿补充合成维生素A(不论其母是否在产前和产后补充维生素A)对发病率、死亡率的效果和副作用。
检索策略
我们使用了Cochrane新生儿(Cochrane Neonatal)的标准检索策略,检索了CENTRAL(Cochrane Central Register of Controlled Trials)数据库(2016第2期),经PubMed检索了MEDLINE(1966‐2016年3月5日),Embase数据库(1980‐2016年3月5日)和CINAHL库(1982‐2016年3月5日)。我们还检索了临床试验数据库、会议纪要和已获得文章的参考文献以寻找随机化对照试验和类随机化试验数据。。
标准/纳入排除标准
选择纳入随机化或类随机化、个体随机化或群体随机化的涉及给1‐6月龄幼儿补充合成性维生素A与安慰剂或无干预相比较的试验。
数据收集与分析
两名综述作者评价研究是否符合纳入标准、评价其偏倚的风险并收集结果数据。
主要结果
该综述包括12项研究(报告于22份公开出版物)。所含研究将24846名1‐6月龄的受试者分配入补充维生素A或对照组。基于包括7项研究的21339(85%)受试者的危险比(risk ratio, RR)为1.05(95% CI, 0.89‐1.25)的数值,补充维生素A对全死因死亡率这一主要终点没有效果;I2=0%;异质性检验:P=0.79;证据质量为中等。同时,因腹泻和呼吸道感染,补充维生素A对死亡率和发病率也无影响。与对照组相比,干预组补充维生素A后24‐72小时内前卤突出的风险增加,RR=3.10(95% CI, 1.89‐5.09);I2=9%,异质性检验:P=0.36;证据质量高等级。补充维生素A后前卤突出的幼儿中未见有死亡、抽搐或易激惹风险增加的后续报道,且绝大多数幼儿通常在72小时内缓解。与对照组相比,补充维生素A组未增加如呕吐、易激惹、腹泻、发热和抽搐等副作用的风险。补充维生素A对维生素A缺乏没有产生任何统计学上显著的效果,RR=0.86(95% CI, 0.70‐1.06);I2=27%,异质性检验:P=0.25;证据质量中等。
作者结论
目前尚缺乏令人信服的证据,支持给1‐6月龄幼儿补充维生素A会降低低收入或中等收入国家中幼儿的死亡率或发病率。在该年龄组中补充维生素A有增加前卤突出的风险;但没有因此产生后续并发症的报告。
PICOs
Plain language summary
给1‐6月龄幼儿补充维生素A以预防疾病和死亡。
背景
维生素A缺乏是低收入和中等收入国家中重大的公共卫生问题。在这些国家中给6月龄至5岁儿童补充维生素A减少其死亡。本综述关注1‐6月龄幼儿。
系统综述问题
给1‐6月龄幼儿补充维生素A有何益处或害处吗?
研究特征
综述作者检索了医学文献以确定比较了补充维生素A与对照相比在死亡、疾病和副作用方面对随机选择幼儿影响的相关研究。文献当前截止到2016年3月5日。检索获得了12项研究含24846名幼儿。绝大多数研究执行良好并且包括了从亚洲、非洲和拉丁美洲的儿童。
关键结果
这些研究的结果未能提供可信服的证据表明补充维生素A能减少1‐6月龄幼儿的死亡或疾病(证据质量:中等)。因腹泻或肺炎,补充维生素A没有减少死亡的益处。同样,从血液维生素A水平来看,补充维生素A无法减少维生素A缺乏儿童的比例(证据质量:中等)。接受维生素A补充的幼儿发生头顶柔部位突出(前卤突出)的风险增加且该副作用的证据质量为高等。但是这一不良反应不会增加其后的死亡或疾病发作风险。
总之,给1‐6月龄幼儿补充维生素A不能降低死亡率或发病率;而且增加前卤突出的风险。
Authors' conclusions
Summary of findings
| Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | ||||||
| Patient or population: infants 1 to 6 months of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with young infant vitamin A supplementation | |||||
| All‐cause mortality: longest follow‐up, i.e. until 1 year of age | Study population | RR 1.05 | 21,339 (9 RCTs) | ⊕⊕⊕⊝ | 2 studies contributed about 76% to the overall estimate (West 1995; WHO 1998). There was no substantial heterogeneity in the pooled data. Two studies were 2 x 2 factorial design trials and data were added as two data sets for each study. | |
| 25 per 1000 | 26 per 1000 | |||||
| Morbidity: diarrhoea: point prevalence | Study population | RR 0.99 | 9891 (2 RCTs) | ⊕⊕⊕⊝ | Even though the final quality assignment was moderate, the effect was from only 2 studies. In addition, prevalence was not as good an indicator as incidence to establish a causal association | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: bulging fontanelle within 48 to 72 hours | Study population | RR 3.10 | 13,493 | ⊕⊕⊕⊕ | Consistent effect across the studies | |
| 3 per 1000 | 8 per 1000 | |||||
| Adverse effects: vomiting 48 to 72 hours | Study population | RR 0.95 | 2187 | ⊕⊕⊝⊝ | ‐ | |
| 49 per 1000 | 47 per 1000 | |||||
| Adverse effects: diarrhoea 48 to 72 hours | Study population | RR 1.07 | 2176 | ⊕⊕⊝⊝ | ‐ | |
| 89 per 1000 | 95 per 1000 | |||||
| Adverse effects: fever 48 to 72 hours | Study population | RR 0.94 | 3187 | ⊕⊕⊝⊝ | ‐ | |
| 194 per 1000 | 183 per 1000 | |||||
| Vitamin A deficiency: retinol < 0.7 μmol/L | Study population | RR 0.86 (0.70 to 1.06) | 1204 | ⊕⊕⊕⊝ | ‐ | |
| 221 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious imprecision (confidence interval for summary estimate included unity). 2 Downgraded one level due to serious risk of bias. 3 Downgraded one level due to serious inconsistency (statistical heterogeneity was 94%). | ||||||
Background
Description of the condition
Vitamin A deficiency (VAD) is associated with increased risk of illness, death and blindness in young children (Wiseman 2016). The World Health Organization (WHO) estimates that about 190 million preschool children (about 33%) and 19.1 million pregnant women (about 15%) are vitamin A deficient (serum retinol less than 0.70 mmol/L) and among these about five million preschool children and nine million pregnant women had night blindness, as reported in the time period between 1995 and 2005 (WHO 2009a). More recent estimates suggest that VAD has decreased over time; however, it remains prevalent in south Asia and sub‐Saharan Africa (Stevens 2015).
Infants and young children in low‐ and middle‐income countries are at increased risk of VAD because of the following reasons: low liver stores at birth, decreased availability of vitamin A from breast milk due to maternal malnutrition or lack of adequate breastfeeding, increased demand due to rapid growth, decreased absorption and increased losses due to recurrent gastrointestinal infections (WHO 2011; Miller 2002). This leads to a sequence of reciprocal cause and effect where VAD lowers the immune system, increases the risk of gastrointestinal infection, which in turn decreases absorption of vitamin A and the cycle continues (Wiseman 2016).
Description of the intervention
Vitamin A is an essential element that cannot be produced by the human body internally by combination of raw materials, and it needs to be taken from external sources. There are two natural sources of vitamin A: provitamin A carotenoids and preformed vitamin A. Provitamin A carotenoids is found in plants, and mammals metabolise it into active vitamin A. Even though fruits and vegetables are rich sources of beta‐carotene, these might not be an adequate source of vitamin A, as the conversion ratio from beta‐carotene to active vitamin A is small (US IOM FNB 2000). The most active form of vitamin A, preformed vitamin A (i.e. retinol, retinal, retinoic acid and retinyl esters) is found in foods from animal sources and is most commonly used as supplementation.
Synthetic vitamin A supplementation has been associated with short‐term adverse effects including vomiting and bulging fontanelle (mainly in infants) (Baqui 1995; Imdad 2010). Bulging fontanelle, the outward curving of an infant's soft spot (fontanelle), can occur in normal healthy babies when they are crying or lying down, in which case, it is self limited with no long‐term consequences. Bulging fontanelle can also occur in certain pathological conditions when there is increased intracranial pressure, which can lead to potential long‐term complications. Therefore, bulging fontanelle itself is not always associated with adverse outcomes unless associated with underlying serious conditions such as meningitis, encephalitis and hydrocephalus etc. (Kiesler 2003).
How the intervention might work
VAD is believed to cause an increased susceptibility to infections by impeding normal regeneration of damaged mucosal barriers and by diminishing the function of neutrophils, macrophages and natural killer cells. Vitamin A is required for adaptive immunity and plays a role in the development of T‐helper (Th) cells and B‐cells (Stephensen 2001). VAD also diminishes antibody‐mediated responses directed by Th2 cells, although some aspects of Th1‐mediated immunity are also diminished (Stephensen 2001). These factors may account for the increased mortality seen in vitamin A‐deficient infants, young children and pregnant women. Deficiency of vitamin A causes xerophthalmia and significantly increases the risk of blindness (Christian 2001; Humphrey 1992). An estimated 250,000 to 500,000 vitamin A‐deficient children become blind every year, half of them dying within 12 months of losing their sight (West 2002).
Why it is important to do this review
Vitamin A supplementation in children aged 6 to 59 months has been shown to reduce all‐cause mortality and infection‐related morbidity (Imdad 2010). This review is an update of the previous review where effectiveness of vitamin A supplementation was assessed in infants aged under six months including neonatal and maternal supplementation; however, neonatal and maternal vitamin A supplementation is assessed in separate Cochrane reviews (Haider 2011; McCauley 2015). Therefore, this review focuses on infants one to six months of age only. The period of one to six months of age is important as there is a potential platform for delivery of vitamin A along with routine vaccination, usually given at the ages of 6, 10 and 14 weeks according to WHO recommended extended programme of immunisation. Therefore, it is important to determine any beneficial or harmful effects of vitamin A when given during this age period.
Objectives
To evaluate the effect of synthetic vitamin A supplementation in infants one to six months of age in low‐ and middle‐income countries, irrespective of maternal antenatal or postnatal vitamin A supplementation status, on mortality, morbidity and adverse reactions.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised controlled trials (RCT) or quasi‐randomised trials for inclusion. We included trials if randomisation was done at individual or cluster (such as village) level that involved synthetic vitamin A supplementation to infants one to six months of age.
Types of participants
We included trials that studied apparently healthy infants from low‐ and middle‐income countries (as defined by World Bank), breastfed or non‐breastfed, receiving vitamin A supplementation initiated between the ages of one and six months. The exact age limit was one month (more than first 28 days of life) to five months and 29 days.
We excluded trials that recruited neonates, children over six months of age or trials focusing only on maternal supplementation before pregnancy, during pregnancy or the postpartum period. We also excluded trials that included only selected subgroups of infants, such as infants who were very low birth weight (less than 1500 g), who were born to known HIV‐positive mothers, or who were sick or hospitalised. Although such studies may be of clinical interest, they do not address the research question of this review and have been the subject of previously published Cochrane reviews (Darlow 2011; Haider 2011; Imdad 2010; McCauley 2015; Oliveira 2016; Wiysonge 2011).
Types of interventions
Synthetic vitamin A supplementation compared to administration of placebo or no supplementation in breastfed or non‐breastfed infants one to six months of age irrespective of maternal postpartum vitamin A supplementation status.
We considered trials providing additional interventions if the only difference between the treatment arms was vitamin A supplementation. In studies assessing different doses of vitamin A and placebo, we combined the intervention groups to create a single pair‐wise comparison in order to avoid double‐counting data. We excluded studies that evaluated food fortification, consumption of vitamin A‐rich foods or beta‐carotene supplementation.
Types of outcome measures
Primary outcomes
All‐cause mortality during infancy, in the period between initiation of intervention and the last follow‐up, until the age of one year.
Secondary outcomes
Cause‐specific mortality (as defined by the study authors, irrespective of ascribing a single or multiple causes of death) due to:
-
diarrhoea;
-
acute respiratory infections;
-
meningitis;
-
measles.
Morbidity during infancy (as defined by the study authors, irrespective of ascribing a single or multiple causes) in the period between initiation of intervention and the last follow‐up, until the age of one year:
-
diarrhoea;
-
acute respiratory infection or respiratory difficulty;
-
fever.
Adverse effects within one week following the intervention:
-
bulging fontanelle;
-
vomiting;
-
irritability;
-
diarrhoea;
-
fever.
Search methods for identification of studies
Electronic searches
For the 2016 update, we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 2); MEDLINE via PubMed (1966 to 5 March 2016); Embase (1980 to 5 March 2016) and CINAHL (1982 to 5 March 2016) using the following search terms: (vitamin A OR retinol OR retinoid OR retinoic OR vitamin A[MeSH]), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov); the WHO International Trials Registry and Platform (www.whoint/ictrp/search/en/), and the ISRCTN Registry (www.isrctn.com/).
The previous publications of this review used the standard search strategy of the Cochrane Neonatal Review Group using CENTRAL (2010, Issue 3), MEDLINE and Embase (1966 to 15 October 2010) via PubMed and clinical trials websites (e.g. clinicaltrials.gov) using the following search terms: (Newborn OR infan* OR neonat*) AND ("vitamin A" OR retino*).
We limited the search to "humans" and "clinical trial" without language restriction. We did a lateral search using the related articles link in PubMed for the articles initially included from the search strategy.
Searching other resources
We reviewed the reference lists of identified articles and handsearched reviews, bibliographies of books and abstracts.
Data collection and analysis
Selection of studies
Two review authors (AI and ZA) independently assessed the eligibility of the trials. We first screened the titles and abstracts to select potential studies for inclusion and reviewed these studies further by reading full texts to decide about final inclusion. We resolved any potential conflicts about inclusion of studies by contacting a third review author (ZAB).
Data extraction and management
We used a data collection sheet. This included information on study characteristics such as: year of publication, location (country, urban/rural), method of recruitment, inclusion criteria, unit of analysis, risk of bias, details of intervention and comparison group, time points for collection and report of outcomes. Two review authors (AI and ZA) extracted the data independently and resolved any discrepancies by discussion. We consulted a third review author (ZAB) if we could not reach mutual agreement. For dichotomous outcomes, we extracted the total number of participants for each group and the number of participants experiencing an event. We extracted the risk ratios (RR) and 95% confidence intervals (CI) (or standard errors) of treatment effects for the outcomes.
The main analyses included the longest reported follow‐up in each study. We pre‐determined the order of preferences of extraction of outcomes when data were available in different formats. We used raw numbers when possible to avoid any manipulation from study authors. This means that we extracted raw values (e.g. means and standard deviations) rather than calculated effect sizes (e.g. Cohen's d). For mortality data, we gave preference to denominators in the following order: number with definite outcome known, number randomised and child‐years. For other dichotomous outcomes to which both survivors and non‐survivors may have contributed data (e.g. incidence of measles), we gave preference to child‐years, number with definite outcome known and number randomised.
In the case of cluster RCTs, we used adjusted estimates reported by the study authors or adjusted the sample size based on methods described in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
We assessed the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the included studies for their risk of bias against the following key criteria:
-
sequence generation;
-
allocation concealment;
-
blinding;
-
incomplete outcome data;
-
selective outcome reporting;
-
other biases.
Two review authors independently evaluated and agreed the risk of bias for the individual studies. We resolved any disagreements by discussion.
Measures of treatment effect
For mortality outcomes, we used author‐reported cause‐specific mortality. In the case of morbidity, we combined all available data whenever possible if outcomes were measured in different ways. For example, we included all types of diarrhoea (mild, moderate and severe). In the case of pneumonia, we included lower respiratory tract infections.
Unit of analysis issues
Cluster randomised trials
When participants were randomised based on clusters rather than individuals, we followed guidelines given in the Cochrane Handbook for Systematic Reviews of Interventions to adjust for cluster design (Higgins 2011). We used design effect or the intra‐cluster correlation (ICC) estimates as given by study authors to adjust for clustering. If design effect or ICC were not available, we used design effect as calculated previously by Beaton 1993.
Dealing with missing data
In cases of differential drop‐out from studies, biased estimates of effect size may be generated. We assessed risk of bias related to attrition as described in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When analyses were reported for completers as well as controlling for drop‐out (e.g. imputed using regression methods), we extracted the drop‐outs.
Assessment of heterogeneity
We assessed statistical heterogeneity by visual inspection of forest plots, by performing the Chi2 test (assessing the P value) and by calculating the I2 statistic. Statistical heterogeneity was considered substantial if the P value was less than 0.10 or I2 value exceeded 50%.
Assessment of reporting biases
In order to assess reporting bias from small studies, we drew funnel plots when data were available from at least 10 studies.
Data synthesis
We used Review Manager 5 software to perform the meta‐analysis (RevMan 2014). We used the generic inverse‐variance method of meta‐analysis to pool the studies when data were available in different formats (e.g. when data were available in the form of raw numbers from one study and only summary estimate from another study). This method requires data input in the form of natural log of effect estimate (e.g. RR) and standard error, which were calculated by built in calculator in Review Manager 5 (RevMan 2014). All the analyses used fixed‐effect models.
We reported the pooled results with 95% CI. We calculated RR values for dichotomous outcomes and mean differences (MD) for continuous variables.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: all‐cause mortality, diarrhoea, bulging fontanelle, vomiting, fever and VAD.
Two review authors independently assessed the quality of the evidence for each of the outcomes. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2014 Guideline Development Tool to create summary of findings Table for the main comparison to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
The proposed subgroup analyses for the infant mortality component in the infant supplementation analysis were:
-
cumulative vitamin A dose received by the infant until the age of six months: low dose (less than 50,000 international units (IU)) versus high dose (50,000 IU or greater);
-
maternal vitamin A supplementation: received versus not received;
-
birth weight: less than 2500 g (low birth weight) versus 2500 g or greater (normal birth weight);
-
vitamin A given with vaccination versus given independent of vaccination.
Investigation of heterogeneity
We considered heterogeneity to be substantial if the I2 statistic exceeded 50% and visual inspection of the forest plot was indicative. We sought to explain heterogeneity in terms of sensitivity analyses, which considered the possible sources of variation as:
-
cumulative dose of vitamin A supplementation;
-
vitamin A status of mother;
-
birth weight of neonate;
-
high baseline infant mortality.
Results
Description of studies
Results of the search
The search strategy identified 1110 titles. Initial screening identified 52 potentially eligible trials; of these we excluded 40 studies after reviewing full texts (Figure 1).
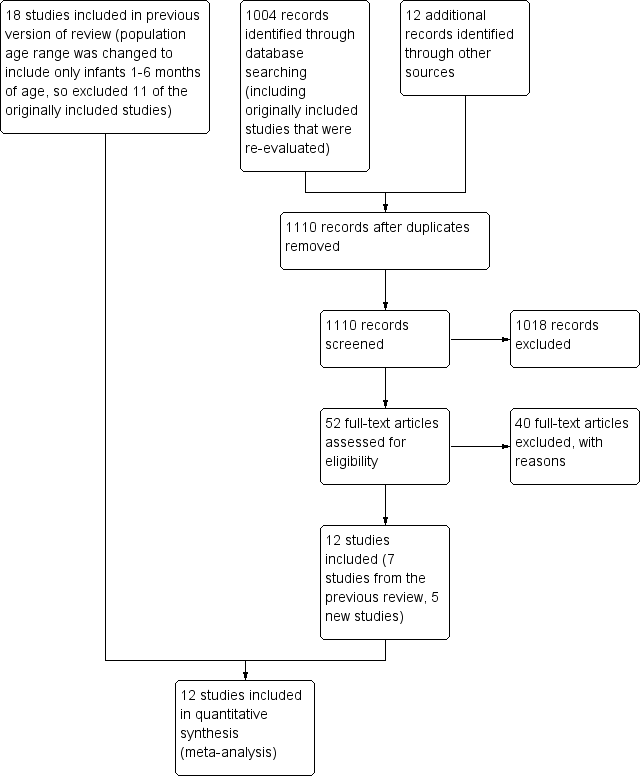
Study flow diagram: review update.
Included studies
The review included 12 studies reported in 22 publications. We treated two factorial design studies as two studies (however, counted as one overall) [(Ayah 2007; Ayah 2007 (2) one study); (Newton 2005; Newton 2005 (2) one study)]. Five (41%) of the included studies had more than one report (Mahalanabis 1997; Newton 2010; Semba 2001; West 1995; WHO 1998). When data were available from more than one publication, we extracted relevant data from all the reports; however, we counted the study as one overall. See Characteristics of included studies table for more details of the included studies.
All the studies contributed data to a meta‐analysis for at least one of the outcomes studied in this review.
Sample size
Trials assigned 24,846 participants aged one to six months, with sample sizes ranging between 89 (Kutukculer 2000) and 10,297 (West 1995). The three largest studies contributed about 83% of the total sample size (Daulaire 1992; West 1995; WHO 1998). We adjusted the original sample size for two studies to account for the cluster design (Daulaire 1992; West 1995).
Comparisons
Eight (66%) studies compared vitamin A supplementation to placebo and the remainder compared to "no intervention".
Multiple trial arms
Four trials (33%) had multiple arms (Ayah 2007; Kutukculer 2000; Newton 2005; Semba 2001), and three of these trials used a factorial design (Ayah 2007; Kutukculer 2000; Newton 2005). Two factorial design trials combined vitamin A with or without placebo between mother and infant and we used these studies as two individual studies in our review (Ayah 2007; Ayah 2007 (2); Newton 2005; Newton 2005 (2)). In these studies, there were individual groups where mother and infant were treated in combinations as follows: mother treated/infant untreated, mother untreated/infant treated, mother treated/infant treated and mother untreated/infant untreated. We included these analyses as follows: mother treated/infant treated versus mother treated/infant untreated and mother untreated/infant treated versus mother untreated/infant untreated. Therefore, in these two trials, the comparisons were considered in a way that mother received the same intervention in both groups and the only difference between groups was infant supplementation with vitamin A. One factorial design trial combined groups in terms of vitamin A and vitamin E (Kutukculer 2000). This study contributed data for only one outcome and reported values for only vitamin A and control group (irrespective of vitamin E), so we included the study that way. The fourth trial studied two doses of vitamin A and compared them with placebo (Semba 2001). We combined two vitamin A groups for outcomes of adverse effects as data were available separately for each vitamin A group and placebo. For morbidity outcomes, there were no data (nominators/denominators) available for each group separately but the summary estimates (relative risks) and so we included only one group (dose 25, 000 IU) to avoid double counting of control group data.
Unit of randomisation
Two studies randomised participants in clusters (Daulaire 1992; West 1995). We combined these trials with individually randomised studies after adjustment for cluster design. We reduced the effective sample size as methods given in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the ICC as calculated previously by Beaton 1993.
Allocation ratio
Participants were evenly allocated to the intervention and control groups in almost all the studies. One study allocated 53% of participants to the vitamin A group and the remainder to the control group (Newton 2010).
Location/setting
Trials were conducted in eight countries: eight (66%) in Asia, four of these in Bangladesh (Baqui 1995; de Francisco 1993; Mahalanabis 1997; Rahman 1995), two in Nepal (Daulaire 1992; West 1995), and one each in Indonesia (Semba 2001) and Turkey (Kutukculer 2000); three (25%) in Africa, two of them in Ghana (Newton 2005; Newton 2010) and one in Kenya (Ayah 2007). There was one trial that was conducted in three countries, India, Ghana and Peru (WHO 1998), representing Asia, Africa and Latin America. Seven of the studies were conducted in rural settings (Ayah 2007; Daulaire 1992; de Francisco 1993; Newton 2005; Newton 2010; Semba 2001; West 1995), three in urban/peri‐urban settings (Baqui 1995; Mahalanabis 1997; Rahman 1995), and the setting was unclear in two studies (Kutukculer 2000; WHO 1998).
Age
The age range was one to six months in most of the trials. One trial included neonates and children over six months of age; however, separate data were available for ages one to five months and we included these data (Daulaire 1992). Another trial included infants less than six months of age including neonates and reported data separately for each month of age (West 1995). We included data only for infants one to five months of age from this study. In WHO 1998 study, vitamin A was given at ages of 6, 10 and 14 weeks and an additional dose was given at nine months of age. Follow‐up data were available for participants before nine months of age and we included them to avoid confounding by later dose of vitamin A.
Sex
Sex was reported in nine trials (75%). The majority assigned approximately equal numbers of boys and girls. One study included 58% boys (Rahman 1995). The median for boys was 51%.
Time
Most of the studies reported outcomes until six months of age. Three studies had follow‐up (and/or participants) beyond six months of age; however, data for clinical outcomes were available below six months of age and we included these (Daulaire 1992; Semba 2001; WHO 1998).
Intervention
The dose of vitamin A ranged from 25,000 IU to 100,000 IU. The most commonly used dose regimen was 25,000 IU/dose given three times at the time of vaccination at ages 6, 10 and 14 weeks (Baqui 1995; Newton 2005; Rahman 1995; Semba 2001; WHO 1998). The second most common dose regimen was 50,000 IU/dose given three times at the time of vaccination at ages 6, 10 and 14 weeks (de Francisco 1993; Mahalanabis 1997; Newton 2010). One study supplemented a single dose of 100,000 IU (Ayah 2007), and two studies supplemented single doses of 50,000 IU (Daulaire 1992; West 1995). The route of supplementation was oral in all studies, and vitamin A was given in liquid form.
Co‐intervention
Ten of the included studies supplemented vitamin A at the time of vaccination (Ayah 2007; Baqui 1995; de Francisco 1993; Kutukculer 2000; Mahalanabis 1997; Newton 2005; Newton 2010; Rahman 1995; Semba 2001; WHO 1998), and two studies supplemented vitamin A independent of vaccination (Daulaire 1992; West 1995). Three studies supplemented both mothers and infants (vitamin A or placebo) (Ayah 2007; Newton 2005; WHO 1998).
Excluded studies
See Characteristics of excluded studies table for the reasons for excluding 40 studies. The most common reason was that vitamin A was supplemented to neonates or to mothers only.
Risk of bias in included studies
See the 'risk of bias' table for individual included studies in the Characteristics of included studies table and Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
From a total of 12 trials, the risk of bias for sequence generation was low in nine trials (Ayah 2007; Baqui 1995; Daulaire 1992; de Francisco 1993; Mahalanabis 1997; Rahman 1995; Semba 2001; West 1995; WHO 1998), while it was unclear in two trials (Kutukculer 2000; Newton 2005). One study had high risk of sequence generation as randomisation was done based on date of birth (Newton 2010). Seven studies successfully concealed allocation, while it was at high risk of bias in two studies (de Francisco 1993; Newton 2010). Three studies provided insufficient information to make an assessment about risk of bias related to allocation concealment (Kutukculer 2000; Newton 2005; Rahman 1995).
Blinding
Eight of the included studies were at low risk of bias for blinding (Ayah 2007; Baqui 1995; de Francisco 1993; Mahalanabis 1997; Newton 2005; Semba 2001; West 1995; WHO 1998), while two were at high risk (Daulaire 1992; Newton 2010). There was insufficient information from two studies to make an assessment about risk of bias for blinding (Kutukculer 2000; Rahman 1995).
Incomplete outcome data
Nine trials reported attrition data adequately and were at low risk of bias (Ayah 2007; Daulaire 1992; de Francisco 1993; Mahalanabis 1997; Newton 2010; Rahman 1995; Semba 2001; West 1995; WHO 1998). Two studies were at high risk attrition bias (Kutukculer 2000; Newton 2005), and there was insufficient information to make an assessment in one study (Baqui 1995).
Selective reporting
Most of the studies did not have study protocols available to make an assessment about risk of bias for selective reporting.
Other potential sources of bias
Most of the included studies did not have any noticeable 'other' risk of bias except two studies that had high risk of bias. These studies recruited participants from a centre for diarrhoea treatment (Mahalanabis 1997; Rahman 1995). The primary aim of these studies was to assess adverse effects of vitamin A supplementation when it was given along with vaccination. These studies utilised a practice of giving vaccination at time of discharge at this diarrhoea treatment centre and offered vitamin A supplements to both participants and their siblings if they fell in the age range. We assumed that all the babies included in this study did not have diarrhoea because siblings were given vitamin A as well and also all the participants received two additional doses of vitamin A after the first dose when they might not have had diarrhoea.
Effects of interventions
Primary outcomes
All‐cause mortality (Outcome 1.1)
Seven studies reported all‐cause mortality (two of which had 2 x 2 factorial design, and data were added as 'two sets' from each study) for an overall 21,339 (85%) participants with 279 deaths in vitamin A group and 259 deaths in comparison group (pooled effect size RR 1.05, 95% CI 0.89 to 1.25; I2 = 0%, test for heterogeneity: P = 0.79) (Analysis 1.1) (Ayah 2007; Ayah 2007 (2); Daulaire 1992; Mahalanabis 1997; Newton 2005; Newton 2005 (2); Newton 2010; West 1995; WHO 1998). The funnel plot drawn for this outcome was symmetrical.
Out of four 'a priori' subgroup analyses, we performed two: maternal vitamin A supplementation (Analysis 1.15) and supplementation given at the time of vaccination (Analysis 1.16). There was no differential effect of vitamin A supplementation in these subgroup analyses and overall effect and statistical significance remained almost the same. The subgroup analysis for 'dose' was not performed because all the studies of infants one to six months of age had a cumulative dose of 50, 000 IU or greater. Subgroup analysis for 'low birth weight' was not performed as segregated data were not available for low birth weight infants from any of the individual studies.
Sensitivity analyses were not performed as there was no substantial statistical heterogeneity.
Secondary outcomes
Cause‐specific mortality due to diarrhoea (Outcome 1.2)
One trial reported mortality due to diarrhoea with an RR of 0.59 (95% CI 0.20 to 1.70) (Analysis 1.2) (Mahalanabis 1997).
Cause‐specific mortality due to acute respiratory infections (Outcome 1.3)
One trial reported cause‐specific mortality due to acute respiratory infections with an RR of 2.12 (95% CI 0.40 to 11.33) (Analysis 1.3) (Mahalanabis 1997).
Cause‐specific mortality due to meningitis (Outcome 1.4)
One trial contributed data for cause‐specific mortality due to meningitis with an RR of 0.35 (95% CI 0.01 to 8.58) (Analysis 1.4) (Mahalanabis 1997).
Morbidity due to diarrhoea (Outcome 1.5)
Two trials provided data for morbidity due to diarrhoea (Semba 2001; WHO 1998). The pooled RR was 0.99 (95% CI 0.93 to 1.05; I2 = 0%, test for heterogeneity: P = 0.49) (Analysis 1.5).
Morbidity due to acute respiratory tract infection (Outcome 1.6)
One trial reported morbidity due to acute respiratory tract infection with an effect size of 0.98 (95% CI 0.81 to 1.19) (Analysis 1.6) (WHO 1998).
Morbidity due to fever (Outcome 1.7)
One study contributed data for morbidity due to fever with an RR of 0.69 (95% CI 0.56 to 0.85) (Analysis 1.7) (Semba 2001).
Adverse effects: bulging fontanelle (Outcome 1.8)
Nine trials reported bulging fontanelle (Baqui 1995; de Francisco 1993; Kutukculer 2000; Mahalanabis 1997; Newton 2010; Rahman 1995Semba 2001; West 1995; WHO 1998). The pooled RR was 3.10 (95% CI 1.89 to 5.09; I2 = 9%, test for heterogeneity: P = 0.36) (Analysis 1.8). Subgroup analysis for maternal supplementation showed no statistically significant different when vitamin A was given to both mothers and infants compared to infants only (Analysis 1.17). All the included studies in this analysis supplemented vitamin A at the time of vaccination, so no subgroup analyses were performed for this aspect of the intervention.
Adverse effects: vomiting (Outcome 1.9)
Two trials provided data on vomiting with a pooled RR of 0.95 (95% CI 0.67 to 1.35; I2 = 94%, test for heterogeneity: P < 0.0001) (Analysis 1.9) (Semba 2001; West 1995).
Adverse effects: irritability (Outcome 1.10)
Four trials provided data on irritability with a pooled RR of 0.88 (95% CI 0.65 to 1.20; I2 = 0%, test for heterogeneity: P = 0.63) (Analysis 1.10) (Mahalanabis 1997; Newton 2010; Rahman 1995; West 1995).
Adverse effects: diarrhoea (Outcome 1.11)
Three trials provided data on diarrhoea with a pooled RR of 1.07 (95% CI 0.82 to 1.40; I2 = 0%, test for heterogeneity: P = 0.39) (Analysis 1.11) (Mahalanabis 1997; Rahman 1995; West 1995).
Adverse effects: fever (Outcome 1.12)
Three trials provided data on fever with a pooled RR of 0.94 (95% CI 0.83 to 1.07; I2 = 83%, test for heterogeneity: P = 0.003) (Analysis 1.12) (Newton 2010; Semba 2001; West 1995).
Convulsions (Outcome 1.13)
One study contributed data for convulsions with an RR of 0.19 (95% CI 0.01 to 3.85) (Analysis 1.13) (Newton 2010).
Vitamin A deficiency (Outcome 1.14)
Three trials reported VAD with a pooled RR of 0.86 (95% CI 0.70 to 1.06; I2 = 27%, test for heterogeneity: P = 0.25) (Analysis 1.14) (Ayah 2007; Mahalanabis 1997; WHO 1998).
Discussion
Summary of main results
Vitamin A supplementation for infants one to six months of age showed no effect on all‐cause mortality during infancy. The pooled effect size suggested an increased risk of 5%; however, results were not statistically significant (RR 1.05, 95% CI 0.89 to 1.25). There were no significant effects on mortality or morbidity due to diarrhoea or respiratory tract infection. There was a three times increased risk of bulging fontanelle in the vitamin A supplemented group compared to control (RR 3.10, 95% CI 1.89 to 5.09). There was no increased risk of other adverse effects such as vomiting, irritability, diarrhoea, fever and convulsions. Vitamin A supplementation did not decrease VAD in the intervention group compared to control.
Overall completeness and applicability of evidence
This review used comprehensive methods to evaluate risks and benefits associated with vitamin A supplementation in infants one to six months of age. The 12 included studies contributed 24,846 participants and were conducted in low‐ and middle‐incomes countries. Most studies contributed data for at least two outcomes (i.e. all‐cause mortality and adverse effect of bulging fontanelle); results were consistent among all the studies with no substantial statistical heterogeneity.
Does vitamin A supplementation have 'any' benefit in terms of protection against mortality or morbidity when given between ages one and six months? Based on the findings of this review, the most likely answer is 'no'. There were no beneficial effects on all‐cause or cause‐specific mortality and morbidity. The primary analysis for all‐cause mortality included seven trials and 21,877 (88%) participants and had no statistical heterogeneity in the pooled data. The number of studies in cause‐specific mortality and morbidity were small, and no solid conclusion can be made from them except that current evidence does not support any beneficial effect of vitamin A supplementation in this age group.
Is there any risk or harmful effects associated with vitamin A supplementation in infants one to six months of age? The most important finding of this review was that the incidence of bulging fontanelle was three times higher in vitamin A group compared to control in the first 24 to 72 hours post supplementation. Almost all the studies that reported this outcome also reported that it was self resolved in all the cases, and there was no increased risk of mortality, convulsions or irritability. It is not clear if there was a dose‐response relationship for this outcome as two small studies reported higher incidence with subsequent doses (Baqui 1995; de Francisco 1993); however, a subsequent larger study did not reproduce these results (WHO 1998).
Does vitamin A supplementation at the time of vaccination have any beneficial or harmful effects for response to vaccines? Ten of the included studies supplemented vitamin A at the time of vaccination mostly at ages of 6, 10 and 14 weeks. Five of these studies reported serum levels of antibodies to different vaccines and there was no differential effect of vitamin A in favour or against the antibody response to tetanus toxoid, polio, measles, hepatitis B and influenza vaccines (Table 1).
| Study ID | Intervention | Type of vaccination | Response to vaccine |
| Vitamin A dose and frequency: vitamin A 30,000 IU for 3 days just after each 3 doses of DPT Control: no vitamin A supplementation | DPT | Vitamin A administered orally for 3 consecutive days after each 3 doses of DPT for primary immunisation did not affect the specific antibody response against tetanus toxoid | |
| Vitamin A dose and frequency: vitamin A 25,000 IU RE at 6, 10 and 14 weeks Control: placebo | DPT/OPV | Vitamin A supplementation does not affect infants' antibody responses to tetanus toxoid or OPV delivered at EPI contacts | |
| Vitamin A dose and frequency: vitamin A 50,000 IU at 6, 10 and 14 weeks Control: no vitamin A supplementation | Hib + Hep | No significant difference (P = 0.93) in the geometric mean concentration of Haemophilus influenzae type b antibodies in the intervention (2.45) and in the control group (2.51); ratio of geometric mean concentration 0.98 (95% CI 0.59 to 1.62). Similarly, no significant difference (P = 0.29) in the geometric mean concentration of hepatitis B antibodies in the intervention (1.28) and in the control group (1.71); ratio of geometric mean concentration 0.74 (95% CI 0.43 to 1.28) | |
| Vitamin A dose and frequency: vitamin A 25,000 RE; vitamin A 50,000 IU at 6, 10 and 14 week of age; vitamin A 100 000 IU at 9 months of age Placebo | DPT/OPV and measles | There was no differential effect of vitamin A supplementation in favour or against measles vaccination | |
| Vitamin A dose and frequency: vitamin A 25,000 IU with the first, second and third doses of DPT/OPV at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru; vitamin A 25,000 IU at 9 months Placebo: soybean oil. Received vitamin A 100,000 IU at 9 months | DPT/OPV and measles | "Vitamin A given to the mothers in the postpartum period and their infants with OPV did not interfere with the antibody response to any of the three polioviruses and enhanced the response to poliovirus type 1" (Data from Indian site only) |
DPT: diphtheria, pertussis (whooping cough) and tetanus; EPI: extended programme of immunisation; Hep: hepatitis; Hib: Haemophilus influenzae type b; IU: international unit; OPV: oral polio vaccine; RE: retinol equivalent.
Quality of the evidence
summary of findings Table for the main comparison shows overall quality assessment of pooled estimates. We graded most of the outcomes as moderate or low quality. These assessments considered type of studies, risk of bias, consistency, indirectness, imprecision and strength of association and publication bias, etc. (Balshem 2011). Most of the assessments for outcomes of this review were influenced by imprecision of summary estimate and risk of bias in included studies. In terms of risk of bias, the study by Newton 2010 was at high risk of bias for sequence generation because randomisation was done based on date of birth. Also blinding was not done in this study. Two studies were at high risk of 'other' bias because the participants were recruited at time of discharge from a centre that treated children with diarrhoea (Mahalanabis 1997; Rahman 1995). We assumed that most of these infants had recovered from diarrhoea before receiving the first dose of vitamin A supplementation, and these infants were given two subsequent doses when they did not have diarrhoea. Therefore, it is less likely that all of these infants were having diarrhoea at the time of vitamin A supplementation, and they received at least two doses when they were diarrhoea‐free.
Potential biases in the review process
This review applied standard Cochrane guidelines for clearly defined inclusion/exclusion criteria, and a comprehensive search strategy for identification of relevant studies. We combined cluster randomised trials with individual randomised trials for meta‐analyses and applied an appropriate adjustment to reduce their effective sample size.
The comprehensive search strategy aimed to identify all published and unpublished studies, though none of the included studies were unpublished. It is more likely that a study with positive results will be published compared to a study that had negative results; however, the funnel plot for the primary outcome was symmetrical (data not shown).
Most of the studies that reported the primary outcome did not report secondary outcomes that might lead to selective outcome reporting bias.
Agreements and disagreements with other studies or reviews
The findings of lack of effect of vitamin A supplementation for infants one to six months of age for all‐cause mortality are in agreement with a previous review done by our team (Imdad 2011). The previous review by our team mainly focused on mortality outcome as part of an exercise to develop summary estimates for the Lives Saved Tool (LiST 2014; Imdad 2011). The current review not only assessed mortality outcomes but also those of morbidity and adverse effects. A previous version of this review included infants under six months of age, including neonates. A subgroup analysis for all‐cause mortality for infants one to six months of age in that review showed similar results for all‐cause mortality (RR 1.05, 95% CI 0.84 to 1.32) (Gogia 2011).
It is surprising to note that there is strong evidence that vitamin A supplementation reduces morbidity and mortality in children 6 to 59 months of age (Imdad 2010) and not in infants one to six months of age. Vitamin A is thought to work by reducing mortality and morbidity related to infectious diseases, mainly diarrhoea and measles. There were not enough studies that reported cause‐specific mortality or morbidity in this review; therefore, it is difficult to make a statement if vitamin A had any differential effect on these infectious causes.
Recently published literature has described the development of human gastrointestinal microbiota over time and how it can determine risk for certain diseases (Blanton 2016; Cho 2012). It was shown that human gastrointestinal microbiota changes significantly in the first two years of life and younger children had certain bacteria that are essential for growth (Blanton 2016). Is it that fecal microbiota of younger infants is not mature enough to have detrimental effect from vitamin A supplementation? Future research might provide further insight into this question.

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 1 All‐cause mortality: longest follow‐up.
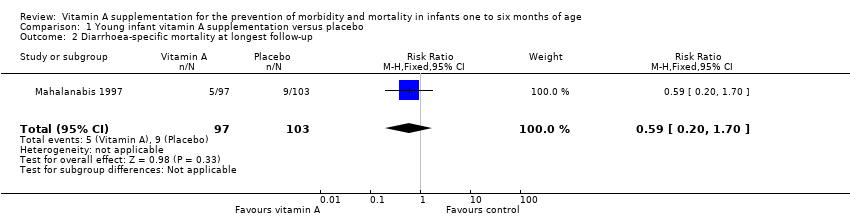
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 2 Diarrhoea‐specific mortality at longest follow‐up.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 3 Acute respiratory infection‐specific mortality at longest follow‐up.
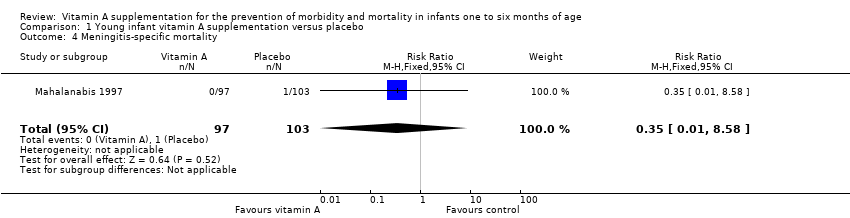
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 4 Meningitis‐specific mortality.
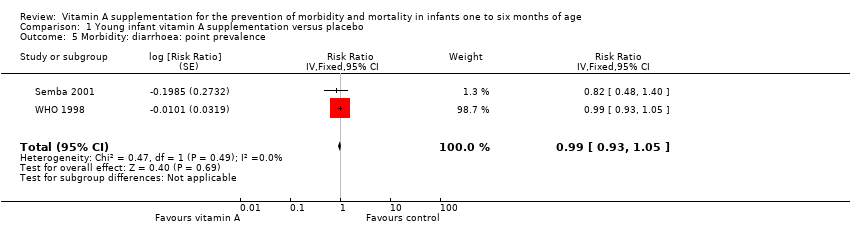
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 5 Morbidity: diarrhoea: point prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 6 Morbidity: lower respiratory tract infection: period prevalence.
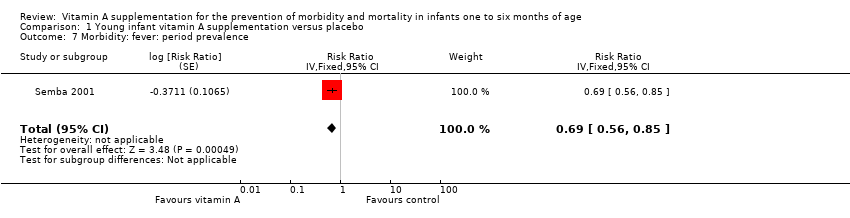
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 7 Morbidity: fever: period prevalence.
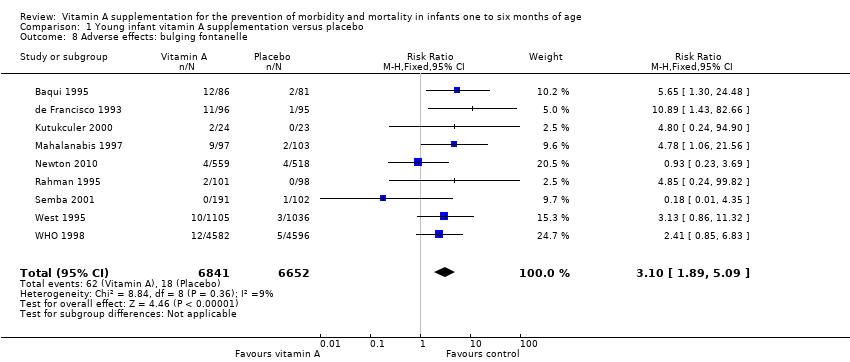
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 8 Adverse effects: bulging fontanelle.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 9 Adverse effects: vomiting.
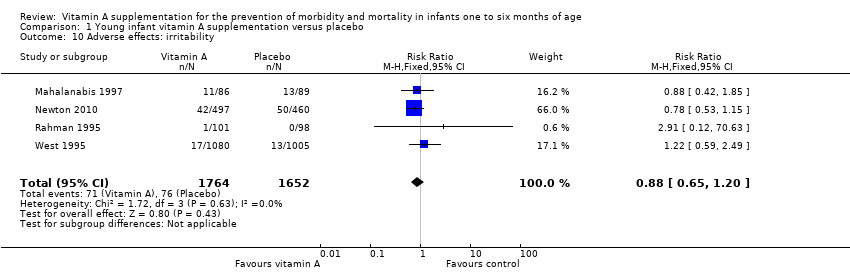
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 10 Adverse effects: irritability.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 11 Adverse effects: diarrhoea.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 12 Adverse effects: fever.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 13 Adverse effects: convulsions.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 14 Vitamin A deficiency: retinol < 0.7 μmol/L.
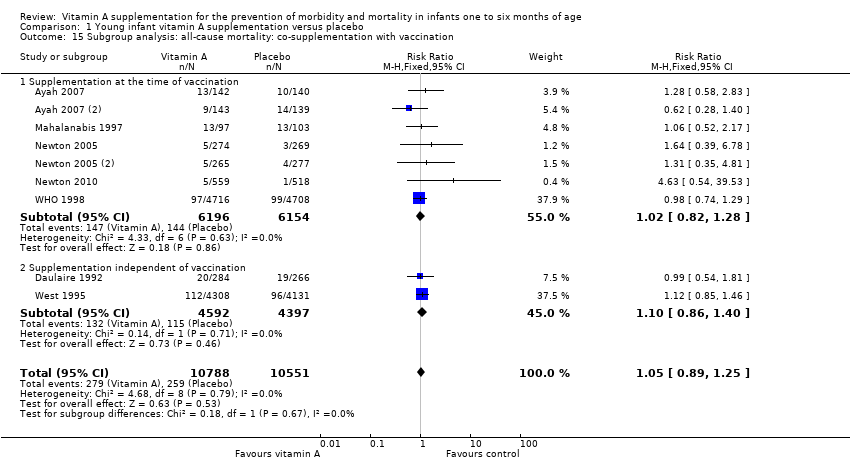
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination.
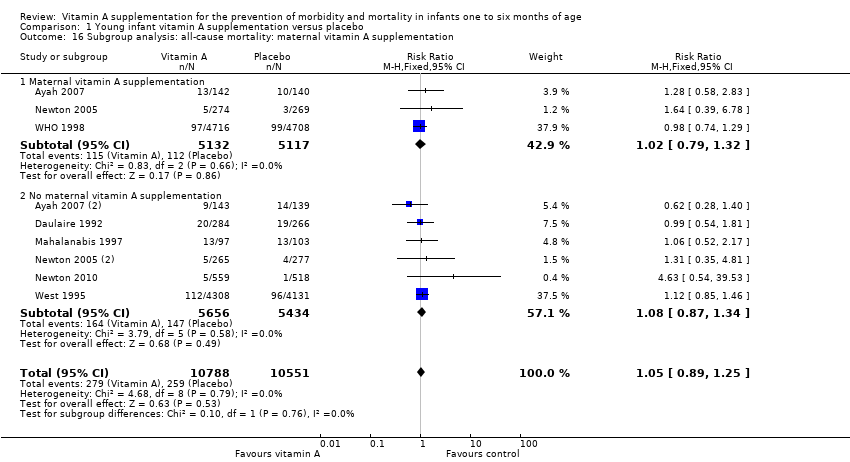
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination.
| Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | ||||||
| Patient or population: infants 1 to 6 months of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with young infant vitamin A supplementation | |||||
| All‐cause mortality: longest follow‐up, i.e. until 1 year of age | Study population | RR 1.05 | 21,339 (9 RCTs) | ⊕⊕⊕⊝ | 2 studies contributed about 76% to the overall estimate (West 1995; WHO 1998). There was no substantial heterogeneity in the pooled data. Two studies were 2 x 2 factorial design trials and data were added as two data sets for each study. | |
| 25 per 1000 | 26 per 1000 | |||||
| Morbidity: diarrhoea: point prevalence | Study population | RR 0.99 | 9891 (2 RCTs) | ⊕⊕⊕⊝ | Even though the final quality assignment was moderate, the effect was from only 2 studies. In addition, prevalence was not as good an indicator as incidence to establish a causal association | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: bulging fontanelle within 48 to 72 hours | Study population | RR 3.10 | 13,493 | ⊕⊕⊕⊕ | Consistent effect across the studies | |
| 3 per 1000 | 8 per 1000 | |||||
| Adverse effects: vomiting 48 to 72 hours | Study population | RR 0.95 | 2187 | ⊕⊕⊝⊝ | ‐ | |
| 49 per 1000 | 47 per 1000 | |||||
| Adverse effects: diarrhoea 48 to 72 hours | Study population | RR 1.07 | 2176 | ⊕⊕⊝⊝ | ‐ | |
| 89 per 1000 | 95 per 1000 | |||||
| Adverse effects: fever 48 to 72 hours | Study population | RR 0.94 | 3187 | ⊕⊕⊝⊝ | ‐ | |
| 194 per 1000 | 183 per 1000 | |||||
| Vitamin A deficiency: retinol < 0.7 μmol/L | Study population | RR 0.86 (0.70 to 1.06) | 1204 | ⊕⊕⊕⊝ | ‐ | |
| 221 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious imprecision (confidence interval for summary estimate included unity). 2 Downgraded one level due to serious risk of bias. 3 Downgraded one level due to serious inconsistency (statistical heterogeneity was 94%). | ||||||
| Study ID | Intervention | Type of vaccination | Response to vaccine |
| Vitamin A dose and frequency: vitamin A 30,000 IU for 3 days just after each 3 doses of DPT Control: no vitamin A supplementation | DPT | Vitamin A administered orally for 3 consecutive days after each 3 doses of DPT for primary immunisation did not affect the specific antibody response against tetanus toxoid | |
| Vitamin A dose and frequency: vitamin A 25,000 IU RE at 6, 10 and 14 weeks Control: placebo | DPT/OPV | Vitamin A supplementation does not affect infants' antibody responses to tetanus toxoid or OPV delivered at EPI contacts | |
| Vitamin A dose and frequency: vitamin A 50,000 IU at 6, 10 and 14 weeks Control: no vitamin A supplementation | Hib + Hep | No significant difference (P = 0.93) in the geometric mean concentration of Haemophilus influenzae type b antibodies in the intervention (2.45) and in the control group (2.51); ratio of geometric mean concentration 0.98 (95% CI 0.59 to 1.62). Similarly, no significant difference (P = 0.29) in the geometric mean concentration of hepatitis B antibodies in the intervention (1.28) and in the control group (1.71); ratio of geometric mean concentration 0.74 (95% CI 0.43 to 1.28) | |
| Vitamin A dose and frequency: vitamin A 25,000 RE; vitamin A 50,000 IU at 6, 10 and 14 week of age; vitamin A 100 000 IU at 9 months of age Placebo | DPT/OPV and measles | There was no differential effect of vitamin A supplementation in favour or against measles vaccination | |
| Vitamin A dose and frequency: vitamin A 25,000 IU with the first, second and third doses of DPT/OPV at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru; vitamin A 25,000 IU at 9 months Placebo: soybean oil. Received vitamin A 100,000 IU at 9 months | DPT/OPV and measles | "Vitamin A given to the mothers in the postpartum period and their infants with OPV did not interfere with the antibody response to any of the three polioviruses and enhanced the response to poliovirus type 1" (Data from Indian site only) | |
| DPT: diphtheria, pertussis (whooping cough) and tetanus; EPI: extended programme of immunisation; Hep: hepatitis; Hib: Haemophilus influenzae type b; IU: international unit; OPV: oral polio vaccine; RE: retinol equivalent. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality: longest follow‐up Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 2 Diarrhoea‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.70] |
| 3 Acute respiratory infection‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.40, 11.33] |
| 4 Meningitis‐specific mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.58] |
| 5 Morbidity: diarrhoea: point prevalence Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.93, 1.05] | |
| 6 Morbidity: lower respiratory tract infection: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.81, 1.19] | |
| 7 Morbidity: fever: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] | |
| 8 Adverse effects: bulging fontanelle Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 9 Adverse effects: vomiting Show forest plot | 2 | 2187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.35] |
| 10 Adverse effects: irritability Show forest plot | 4 | 3416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 11 Adverse effects: diarrhoea Show forest plot | 3 | 2176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 12 Adverse effects: fever Show forest plot | 3 | 3187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 13 Adverse effects: convulsions Show forest plot | 1 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| 14 Vitamin A deficiency: retinol < 0.7 μmol/L Show forest plot | 4 | 1204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 15.1 Supplementation at the time of vaccination | 7 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 15.2 Supplementation independent of vaccination | 2 | 8989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 16.1 Maternal vitamin A supplementation | 3 | 10249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 16.2 No maternal vitamin A supplementation | 6 | 11090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 17.1 Supplementation at the time of vaccination | 8 | 11352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.81, 5.30] |
| 17.2 Supplementation independent of vaccination | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.32] |

