Vitamin D supplementation for prevention of cancer in adults
Information
- DOI:
- https://doi.org/10.1002/14651858.CD007469.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 23 June 2014see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Metabolic and Endocrine Disorders Group
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Goran Bjelakovic (GB): initiated the review, drafted the protocol, performed the literature search, data extraction, and statistical analyses, and drafted the review.
Lise Lotte Gluud (LLG): revised the protocol, performed data extraction, and revised the review.
Dimitrinka Nikolova (DN): revised the protocol, performed data extraction, and revised the review.
Kate Whitfield (KW): developed the search strategy, revised the protocol, performed data extraction, and revised the review.
Goran Krstic (GK): joined the team of authors during the preparation of the review, performed data extraction, and revised the review.
Jørn Wetterslev (JW): revised the protocol, performed data extraction, and revised the review.
Christian Gluud (CG): revised the protocol, acted as arbiter for disagreements, and revised the review.
Sources of support
Internal sources
-
The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
-
Ministry of Education, Science and Technological Development of the Republic of Serbia, Serbia.
Declarations of interest
Goran Bjelakovic (GB): None known.
Lise Lotte Gluud (LLG): None known.
Dimitrinka Nikolova (DN): None known.
Kate Whitfield (KW): None known.
Goran Krstic (GK): None known.
Jørn Wetterslev (JW): None known.
Christian Gluud (CG): None known.
Acknowledgements
We extend our gratitude to all participants and investigators who took part in the randomised clinical trials. We are grateful to the many authors who kindly responded to our requests for further information on the trials they were involved in. We thank the individual trial authors in our Characteristics of included studies tables. We thank Sarah Louise Klingenberg, the Cochrane Hepato‐Biliary Group, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark, for her help with paper copies of publications.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Jun 23 | Vitamin D supplementation for prevention of cancer in adults | Review | Goran Bjelakovic, Lise Lotte Gluud, Dimitrinka Nikolova, Kate Whitfield, Goran Krstic, Jørn Wetterslev, Christian Gluud | |
| 2008 Oct 08 | Vitamin D supplementation for prevention of cancer in adults | Protocol | Goran Bjelakovic, Lise Lotte Gluud, Dimitrinka Nikolova, Kate Whitfield, Jørn Wetterslev, Christian Gluud | |
Differences between protocol and review
1. Methods section. Criteria for considering studies for this review. Types of participants. We have now added the following categories of participants, which were excluded in order to be more precise: people with secondary induced osteoporosis (for example, glucocorticoid‐induced osteoporosis, thyroidectomy, primary hyperparathyroidism, chronic kidney disease, liver cirrhosis, Crohn's disease, and gastrointestinal bypass surgery). Firstly, all of these conditions are accompanied by deranged vitamin D metabolism, and by an increase in bone resorption and by a decrease in bone formation. Secondly, we decided to follow exclusion criteria applied in our previous Cochrane review on vitamin D supplementation for prevention of mortality (Bjelakovic 2014).
2. Methods section. Criteria for considering studies for this review. Types of interventions. We have now deleted the following types of interventions: in combination with other vitamins or trace elements; in combination with calcium and other vitamins and trace elements. Our intention was to eliminate the influence of other co‐interventions on our results. We wanted to obtain results that would reflect the pure influence of vitamin D on the outcome measures.
3. We changed QUORUM (Moher 1999) into PRISMA (Moher 2009) as the guideline was updated.
4. Data collection and analysis. Assessment of risk of bias in included studies. We have now added the following risk of bias domains: incomplete outcome data; selective outcome reporting; for‐profit bias; and risk of other bias as the guidelines for risk of bias were updated.
5. Data collection and analysis. Data synthesis. We also planned to conduct trial sequential analyses with diversity‐adjusted required information size instead of heterogeneity‐adjusted required information size. The reason is that the diversity‐adjusted required information size seems to give less biased estimates of the required information size than the inconsistency‐adjusted required information size (Wetterslev 2009).
6. Data collection and analysis. Dealing with missing data. Regarding the primary outcomes, we included participants with incomplete or missing data in sensitivity analyses by imputing them according to the extreme case analysis favouring the experimental intervention ('best‐worst' case scenario): none of the dropouts/participants lost from the experimental arm, but all of the dropouts/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator, and extreme case analysis favouring the control ('worst‐best' case scenario): all dropouts/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator.
7. Data collection and analysis. Subgroup analysis. We have decided against performing the following subgroup analysis: "trials without risk of for‐profit bias compared to trials with risk of for‐profit bias", due to the introduction of this source of bias in the review.
8. Data collection and analysis. Sensitivity analysis. We have now decided against performing the following sensitivity analyses: repeating the analysis excluding unpublished trials; repeating the analysis excluding trials using the following filters: diagnostic criteria, language of publication, country. The reason is that all included trials used the same diagnostic criteria. We did not find unpublished trials. All included trials came from high‐income countries and were published in the English language.
9. Goran Krstic joined the team of authors during the preparation of the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Dietary Supplements;
- Calcitriol [administration & dosage];
- Cholecalciferol [administration & dosage];
- Hydroxycholecalciferols [administration & dosage];
- Neoplasms [epidemiology, *prevention & control];
- Randomized Controlled Trials as Topic;
- Vitamin D [*administration & dosage, analogs & derivatives];
- Vitamins [*administration & dosage];
Medical Subject Headings Check Words
Aged; Female; Humans; Male; Middle Aged;
PICOs

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
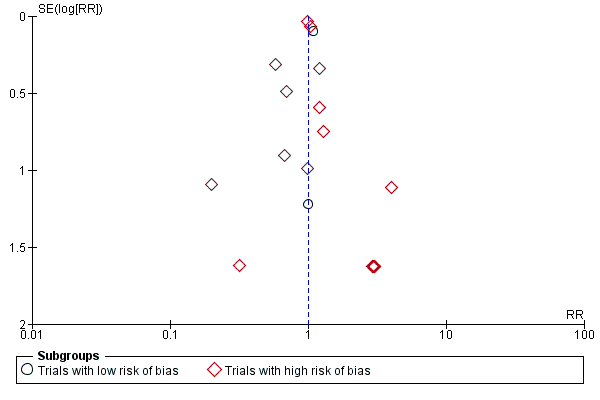
Funnel plot of comparison: 1 Vitamin D versus placebo or no intervention, outcome: 1.1 Cancer occurrence in trials with a low or high risk of bias.

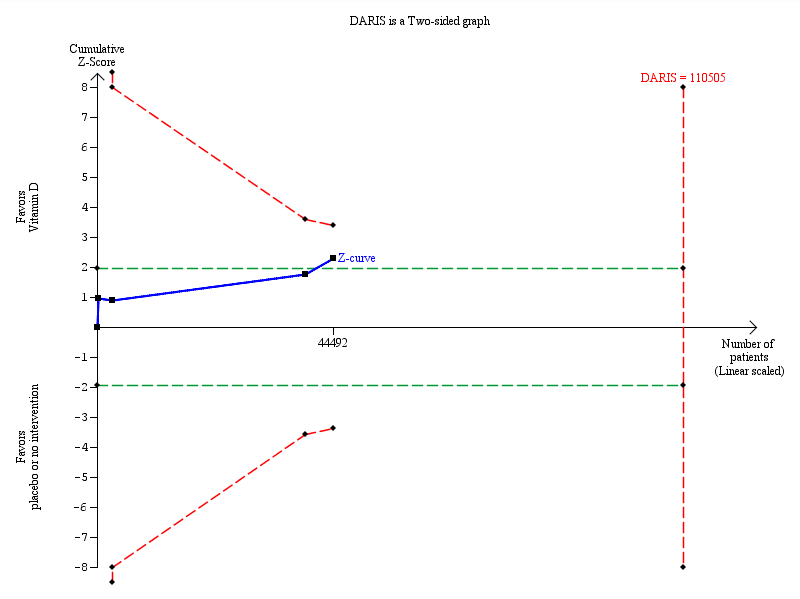
Trial sequential analysis on cancer occurrence in the 18 vitamin D trials was performed based on cancer occurrence of 10% in the control group, a relative risk reduction of 5% with vitamin D supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. This resulted in a required information size of 110,505 participants. Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.
Trial sequential analysis on cancer mortality in the four vitamin D trials was performed based on cancer mortality of 3% in the control group, a relative risk reduction of 10% with vitamin D₃ supplementation, a type I error of 5%, and a type II error of 20% (80% power). There was no diversity. The required information size was 110,505 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundary (red line) after the fourth trial. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries. The red inward sloping lines represent the trial sequential monitoring boundaries.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 1 Cancer occurrence in trials with a low or high risk of bias.
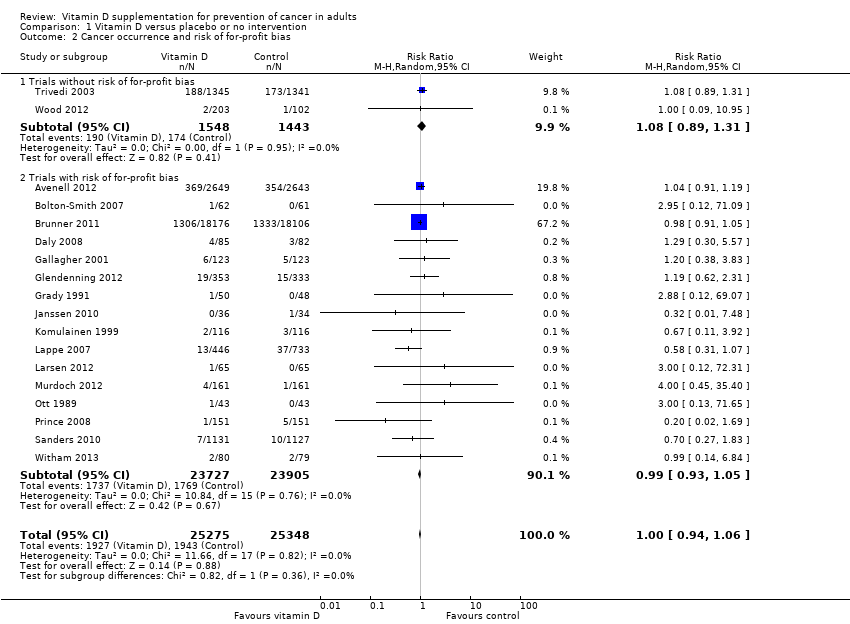
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 2 Cancer occurrence and risk of for‐profit bias.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 3 Cancer occurrence in primary and secondary prevention trials.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 4 Cancer occurrence and vitamin D status.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario).
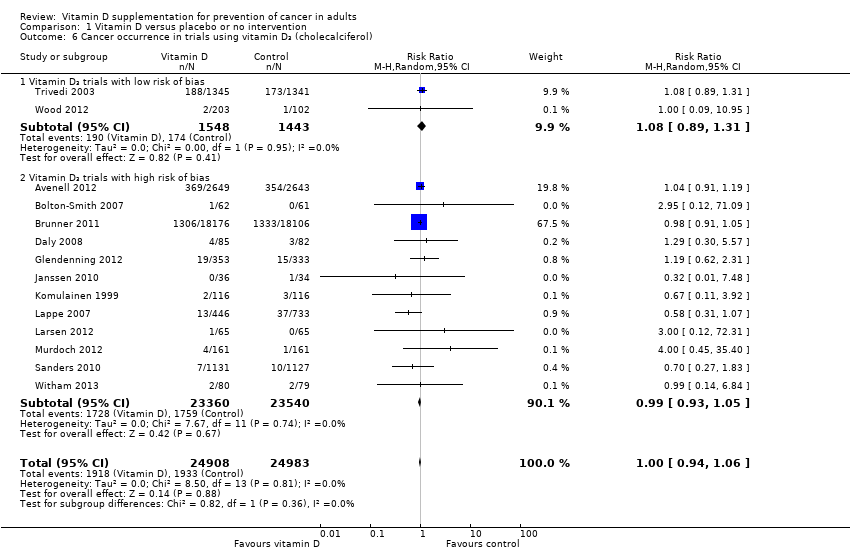
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium.
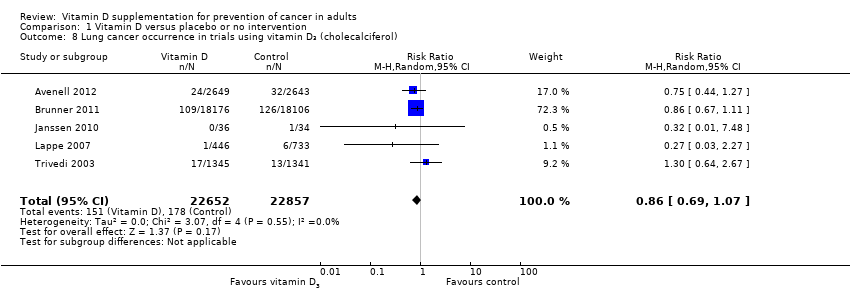
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol).
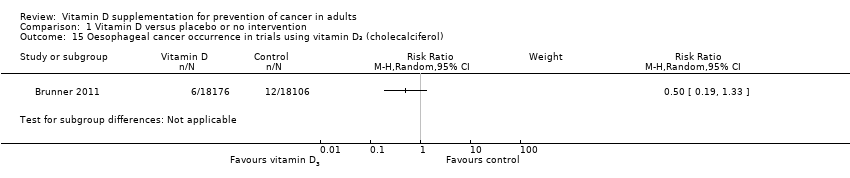
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 19 Cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 20 Breast cancer occurrence in trials using calcitriol.
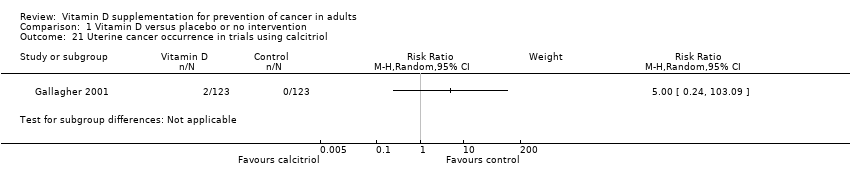
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 21 Uterine cancer occurrence in trials using calcitriol.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 22 Stomach cancer occurrence in trials using calcitriol.
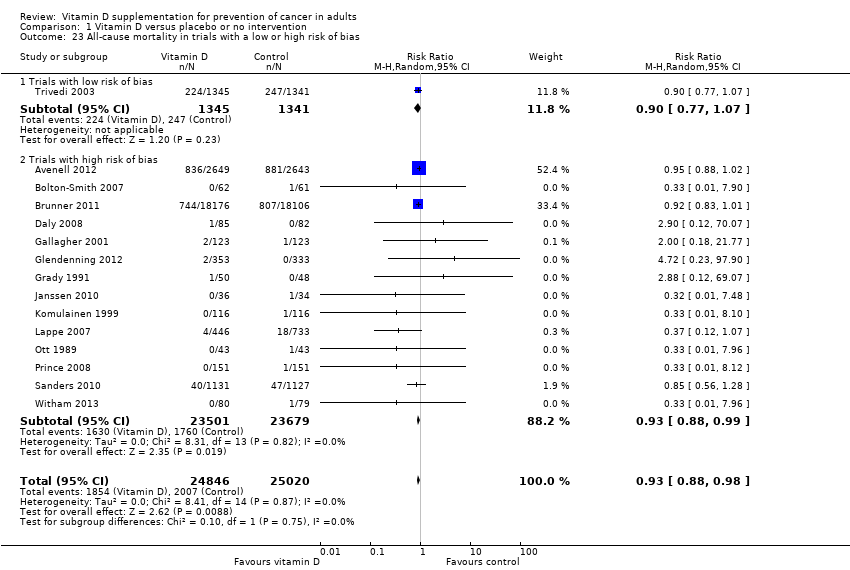
Comparison 1 Vitamin D versus placebo or no intervention, Outcome 23 All‐cause mortality in trials with a low or high risk of bias.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 25 Cancer mortality.

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario).

Comparison 1 Vitamin D versus placebo or no intervention, Outcome 27 Adverse events.
| Vitamin D versus placebo or no intervention for prevention of cancer in adults | ||||||
| Patient or population: healthy participants or recruited among the general population; individuals diagnosed with a specific disease in a stable phase or with vitamin D deficiency Settings: outpatients | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vitamin D versus placebo or no intervention | |||||
| Cancer occurrence Follow‐up: 0.5 to 7 years | Study population | RR 1.00 | 50623 | ⊕⊕⊕⊝ moderatea | Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 | |||||
| Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Follow‐up: 0.5 to 7 years | Study population | RR 1.00 | 49891 | ⊕⊕⊕⊝ moderatea | Trial sequential analysis of all vitamin D trials suggests that the futility area is reached after the 10th trial allowing us to conclude that any possible intervention effect, if any, is lower than a 5% relative risk reduction. | |
| 77 per 1000 | 77 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 28 per 1000 | |||||
| All‐cause mortality Follow‐up: 0.5 to 7 years | Study population | RR 0.93 | 49866 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 80 per 1000 | 75 per 1000 | |||||
| Moderate | ||||||
| 16 per 1000 | 15 per 1000 | |||||
| Cancer mortality in trials using vitamin D₃(cholecalciferol) Follow‐up: 5 to 7 years | Study population | RR 0.88 | 44492 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 29 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 37 per 1000 | 33 per 1000 | |||||
| Adverse events: nephrolithiasis in trials using vitamin D₃(cholecalciferol) combined with calcium Follow‐up: 0.5 to 7 years | Study population | RR 1.17 | 42753 | ⊕⊕⊝⊝ lowb | Trial sequential analysis of all trials irrespective of bias risks showed that the required information size had not yet been reached and that the cumulative Z‐curve did not cross the trial sequential monitoring boundary for benefit. | |
| 18 per 1000 | 21 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 | |||||
| Health‐related quality of life | See comment | Not investigated. | ||||
| Health economics | See comment | Not investigated. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by one level because of risk of attrition bias bDowngraded by two levels because of risk of attrition bias and imprecision | ||||||
| Characteristic | Intervention(s) and comparator(s) | Screened/eligible | Randomised | ITT | Finishing study | Randomised finishing study |
| (1) Avenell 2012
| I1: vitamin D₃ | 15,024 | 1343 | 1343 | 1813 | 68 |
| I2: vitamin D₃ plus calcium | 1306 | 1306 | ||||
| C1: calcium | 1311 | 1311 | 1762 | 67 | ||
| C2: matched placebo tablets | 1332 | 1332 | ||||
| total: | 5292 | 5292 | 3575 | 68 | ||
| (2) Bolton‐Smith 2007
| I1: vitamin D₃ plus calcium | ‐ | 62 | 62 | 50 | 81 |
| C1: matched placebo | 61 | 61 | 56 | 92 | ||
| total: | 123 | 123 | 106 | 86 | ||
| (3) Brunner 2011
| I1: vitamin D₃ plus calcium | 68,132 | 18,176 | 18,176 | 16,936 | 93 |
| C1: matched placebo | 18,106 | 18,106 | 16,815 | 93 | ||
| total: | 36,282 | 36,282 | 33,751 | 93 | ||
| (4) Daly 2008
| I1: calcium‐vitamin D₃‐fortified milk plus calcium | 422 | 85 | 85 | 76 | 89 |
| C1: usual diet | 82 | 82 | 73 | 89 | ||
| total: | 167 | 167 | 149 | 89 | ||
| (5) Gallagher 2001
| I1: calcitriol | 1905 | 123 | 123 | 101 | 82 |
| C1: matched placebo | 123 | 123 | 112 | 91 | ||
| total: | 246 | 246 | 213 | 87 | ||
| (6) Glendenning 2012
| I1: cholecalciferol | 2110 | 353 | 353 | 331 | 94 |
| C1: placebo vitamin D | 333 | 333 | 307 | 92 | ||
| total: | 686 | 686 | 638 | 93 | ||
| (7) Grady 1991
| I1: calcitriol | 98 | 50 | 50 | 49 | 98 |
| C1: placebo vitamin D | 48 | 48 | 48 | 100 | ||
| total: | 98 | 50 | 97 | 99 | ||
| (8) Janssen 2010
| I1: vitamin D₃ plus calcium | 91 | 36 | 36 | 18 | 50 |
| C1:placebo vitamin D₃ plus calcium | 34 | 34 | 31 | 91 | ||
| total: | 70 | 70 | 49 | 70 | ||
| (9) Komulainen 1999
| I1: vitamin D₃ plus calcium | 13,100 | 116 | 116 | 112 | 97 |
| C1: placebo | 116 | 116 | 115 | 99 | ||
| total: | 232 | 232 | 227 | 98 | ||
| (10) Lappe 2007
| I1: vitamin D₃ plus calcium | 1180 | 446 | 446 | 403 | 90 |
| C1: vitamin D₃ placebo plus calcium | 445 | 445 | 416 | 93 | ||
| C2: vitamin D₃ placebo plus calcium placebo | 288 | 288 | 266 | 92 | ||
| total: | 1179 | 1179 | 1085 | 92 | ||
| (11) Larsen 2012 | I1: vitamin D₃ | 136 | 65 | 65 | 55 | 85 |
| C1: vitamin D placebo | 65 | 65 | 57 | 88 | ||
| total: | 130 | 130 | 112 | 86 | ||
| (12) Murdoch 2012 | I1: vitamin D₃ | 351 | 161 | 161 | 148 | 92 |
| C1: vitamin D placebo | 161 | 161 | 146 | 91 | ||
| total: | 322 | 322 | 294 | 91 | ||
| (13) Ott 1989
| I1: calcitriol plus calcium | ‐ | 43 | 43 | 39 | 91 |
| C1: placebo vitamin D plus calcium | 43 | 43 | 37 | 86 | ||
| total: | 86 | 86 | 76 | 88 | ||
| (14) Prince 2008
| I1: vitamin D₂ plus calcium | 827 | 151 | 151 | 144 | 95 |
| C1: placebo vitamin D plus calcium | 151 | 151 | 145 | 96 | ||
| total: | 302 | 302 | 289 | 95 | ||
| (15) Sanders 2010
| I1: vitamin D₃ | 7204 | 1131 | 1131 | 1015 | 90 |
| C1: vitamin D placebo | 1127 | 1127 | 1017 | 90 | ||
| total: | 2258 | 2258 | 2032 | 90 | ||
| (16) Trivedi 2003
| I1: vitamin D₃ | ‐ | 1345 | 1345 | 1262 | 94 |
| C1:placebo vitamin D | 1341 | 1341 | 1264 | 94 | ||
| total: | 2686 | 2686 | 2526 | 94 | ||
| (17) Witham 2013 | I1: vitamin D₃ | 341 | 80 | 80 | 73 | 91 |
| C1: placebo vitamin D | 79 | 79 | 69 | 87 | ||
| total: | 159 | 159 | 142 | 89 | ||
| (18) Wood 2012 | I1: vitamin D₃ | 424 | 102 | 102 | 84 | 82 |
| I2: vitamin D₃ | 101 | 101 | 90 | 89 | ||
| C1: placebo vitamin D | 102 | 102 | 91 | 89 | ||
| total: | 305 | 305 | 265 | 87 | ||
| Grand total | All interventions | 25,275 | 22,799 | 90 | ||
| All controls | 25,348 | 22,827 | 90 | |||
| All interventions and controls | 50,623 | 45,626 | 90 | |||
| "‐" denotes not reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cancer occurrence in trials with a low or high risk of bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 1.1 Trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 1.2 Trials with high risk of bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 2 Cancer occurrence and risk of for‐profit bias Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 2.1 Trials without risk of for‐profit bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 2.2 Trials with risk of for‐profit bias | 16 | 47632 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 3 Cancer occurrence in primary and secondary prevention trials Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.1 Primary prevention trials | 16 | 50334 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 3.2 Secondary prevention trials | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.26, 6.96] |
| 4 Cancer occurrence and vitamin D status Show forest plot | 18 | 50623 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Vitamin D insufficiency | 7 | 44668 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 4.2 Vitamin D adequacy | 9 | 4544 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 4.3 Unknown vitamin status | 2 | 1411 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 5 Cancer occurrence ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 'Best‐worst' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.54] |
| 5.2 'Worst‐best' case scenario | 17 | 49444 | Risk Ratio (M‐H, Random, 95% CI) | 2.76 [1.97, 3.86] |
| 6 Cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 14 | 49891 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 6.1 Vitamin D₃ trials with low risk of bias | 2 | 2991 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.89, 1.31] |
| 6.2 Vitamin D₃ trials with high risk of bias | 12 | 46900 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.93, 1.05] |
| 7 Cancer occurrence in trials using vitamin D₃ singly or combined with calcium Show forest plot | 14 | 49870 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.04] |
| 7.1 Vitamin D₃ singly | 8 | 9200 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.90, 1.17] |
| 7.2 Vitamin D₃ combined with calcium | 7 | 40670 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 8 Lung cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45509 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.69, 1.07] |
| 9 Breast cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 7 | 43669 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 10 Colorectal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 5 | 45598 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.92, 1.34] |
| 11 Pancreatic cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 2 | 36405 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 12 Prostate cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Uterine cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Ovarian cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Oesophageal cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16 Stomach cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17 Liver cancer occurrence in trials using vitamin D₃ (cholecalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18 Cancer occurrence in trials using vitamin D₂ (ergocalciferol) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19 Cancer occurrence in trials using calcitriol Show forest plot | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.52, 4.06] |
| 20 Breast cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21 Uterine cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22 Stomach cancer occurrence in trials using calcitriol Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23 All‐cause mortality in trials with a low or high risk of bias Show forest plot | 15 | 49866 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.98] |
| 23.1 Trials with low risk of bias | 1 | 2686 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.77, 1.07] |
| 23.2 Trials with high risk of bias | 14 | 47180 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 24 All‐cause mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 'Best‐worst' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.31, 0.60] |
| 24.2 'Worst‐best' case scenario | 14 | 48687 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.47, 2.80] |
| 25 Cancer mortality Show forest plot | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.78, 0.98] |
| 26 Cancer mortality ('best‐worst case' and 'worst‐best case' scenario) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 'Best‐worst' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.33, 0.70] |
| 26.2 'Worst‐best' case scenario | 4 | 44492 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.04, 2.75] |
| 27 Adverse events Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Hypercalcaemia in trials using supplemental forms of vitamin D | 4 | 5879 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.64, 3.09] |
| 27.2 Hypercalcaemia in trials using active forms of vitamin D | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.56, 29.22] |
| 27.3 Nephrolithiasis in trials using vitamin D₃ combined with calcium | 3 | 42753 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.03, 1.34] |
| 27.4 Nephrolithiasis in trials using calcitriol | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.10] |
| 27.5 Hypercalciuria | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 12.49 [0.72, 215.84] |
| 27.6 Renal insufficiency | 3 | 5549 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.82] |
| 27.7 Cardiovascular disorders | 8 | 4938 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 27.8 Gastrointestinal disorders | 7 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.88, 1.59] |
| 27.9 Psychiatric disorders | 2 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.46, 4.38] |


