银杏叶提取物治疗间歇性跛行
摘要
研究背景
间歇性跛行(intermittent claudication,IC)患者在运动时腿部肌肉疼痛,短时间休息后即可缓解。可以通过(有指导的)运动疗法和药物治疗来实现症状的缓解。银杏叶提取物是一种血管活性药物,并用于治疗IC。
研究目的
评估银杏叶提取物对间歇性跛行患者步行距离的影响。
检索策略
对于本次更新,Cochrane 周围血管疾病组检索统筹员检索了试验注册库(最后检索日期为2013年3月)和Cochrane对照中心注册库(2013年第2期)。
纳入排除标准
银杏叶提取物与安慰剂对比用于IC患者的随机对照试验,不限定剂量。
资料收集与分析
两位作者独立筛选文献、评估研究质量和提取资料。我们提取了患者数量、平均步行距离或时间及其标准差。为了标准化步行距离或步行时间,用热量消耗来表达不同跑步机方案之间的差异,这些差异是根据跑步机的速度和坡度计算出来的。
主要结果
共纳入了14项试验,涉及739名受试者。11项涉及477名受试者的试验比较了银杏叶提取物和安慰剂,并评估了绝对跛行距离(absolute claudication distance,ACD)。研究结束时,与安慰剂相比,使用银杏叶治疗后ACD增加,总体效应值为3.57千卡(95%置信区间(CI) [0.10,7.23],P=0.06)。换算出来,在平均速度为3.2公里/小时的水平跑步机上,只增加了64.5米(95%CI [1.8,130.7])。发表偏倚导致数据缺失或“阴性”试验可能夸大了效应量。
作者结论
总的来说,没有证据表明银杏叶提取物对外周动脉疾病患者有显著的临床获益。
PICOs
简语概要
银杏叶提取物治疗行走时腿疼(间歇性跛行)
外周动脉疾病(peripheral arterial disease,PAD)的主要症状是行走时一侧或两侧小腿疼痛。通常,这种疼痛发生在走路时,休息一段时间就可以缓解。这种临床现象称为间歇性跛行(intermittent claudication,IC)。外周动脉疾病是由一侧或双侧腿动脉的进行性狭窄引起的,是系统性动脉粥样硬化的一种表现,可能导致心血管事件。保守治疗包括运动疗法和药物治疗,与心血管危险因素和缓解症状的治疗一致。药物治疗的选择之一是银杏叶提取物,它是从银杏叶中提取的,几个世纪以来一直作为中药被使用。它是一种血管活性药物,人们认为它可以提升PAD患者的行走能力。本研究表明,使用银杏叶提取物的人可以多走64.5米,与安慰剂组相比没有显著差异。总的来说,没有证据表明银杏叶提取物对PAD患者有明显的临床获益。
Authors' conclusions
Background
Description of the condition
Patients with peripheral arterial disease (PAD) suffer from a progressive narrowing and hardening of the arteries in one or both legs (atherosclerosis). The most common symptom of PAD is intermittent claudication (IC) defined as pain in the muscles of the leg occurring during exercise which is relieved by a short period of rest. This clinical phenomenon affects 1.6% to 4.5% of all people in the general population (Fowkes 1991; Hooi 1998; Meijer 1998; Murabito 2002).
Description of the intervention
Basic conservative treatment for patients with IC includes risk‐factor modification and exercise therapy. Intermittent claudication is a manifestation of systemic atherosclerosis, thus all patients with this symptom should be treated with medical therapy for cardiovascular risk factors. Glucose and cholesterol metabolism and blood pressure should be optimised and, as smoking is considered the most important modifiable vascular risk factor, attention should be paid to smoking cessation. Antiplatelet therapy is indicated for all patients with IC to reduce the risk of a cardiovascular event (ATC 2002). Exercise therapy can improve the absolute claudication distance (ACD) by 150% (Leng 2004) and should be prescribed for symptom relief. A supervised exercise programme has more clinical benefits than an unsupervised exercise programme. A recent Cochrane review showed approximately a 150 metre difference in increased walking distance after three months in favour of supervised exercise therapy programmes compared with non‐supervised therapy (Bendermacher 2006). Pharmacological treatments reported to be associated with improvement of leg symptoms include cilostazol, pentoxifylline, naftidrofuryl, statins, angiotensin‐converting enzyme inhibitors and Ginkgo biloba extract (Bendermacher 2005; Hankey 2006).
How the intervention might work
Ginkgo biloba extract is derived from the leaves of Ginkgo biloba, the only surviving species of the Ginkgoaceae family of trees. It has been used in traditional Chinese medicine for centuries. In the western world Ginkgo biloba extract is prescribed mainly for IC, cerebral insufficiency and tinnitus. The extract is made from dried leaves, and preparations for use are standardised to contain the same ingredients in similar doses of 24% ginkgo flavonoids and 6% terpenoids. Flavonoids are believed to contribute to Ginkgo's antioxidant properties. In vitro studies of Ginkgo biloba demonstrated free‐radical scavenging properties (Pincemail 1989) and prolongation of the half‐life of endothelial‐derived relaxing factor (Robak 1988) resulting in improved microcirculation by inducing relaxation of contracted blood vessels (Kleijnen 1992). In other in vitro experiments, the ginkgolides, components of the terpenoids, inhibit the activity of platelet‐activating factor (Korth 1988), resulting in diminished platelet aggregation, neutrophil degranulation and oxygen‐free radical production (Kleijnen 1992). Based on the use of Ginkgo biloba in traditional Chinese medicine and the vasoactive properties of Ginkgo biloba, Ginkgo biloba is being used in Western medicine for the relief of symptoms of PAD.
Why it is important to do this review
To establish the clinical effect in patients with PAD we performed a systematic review. Possible problems with such a review of Ginkgo biloba extract is the potential for small unpublished studies, especially from relatively inexperienced and small research groups. Moreover, strong beliefs and financial interest could hamper the publication of negative trials. Hence, it is important to explore the possibility of publication bias in detail.
Objectives
To determine the effect of Ginkgo biloba extract on the walking capacity of people with intermittent claudication.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of Ginkgo biloba extract versus placebo in people with IC (stage II according to Fontaine (Fontaine 1954); stages 1 to 3 according to Rutherford (Rutherford 1997).
Types of participants
Adults (18 years and older) with IC, according to Fontaine stage II or Rutherford stages 1 to 3.
Types of interventions
Ginkgo biloba extract was compared with placebo for IC. Incuded studies used standardised Ginkgo biloba extracts. All studies were included irrespective of dosage.
Types of outcome measures
Primary outcomes
The primary outcome measurement was the absolute claudication distance (ACD) at the end of the study.
Secondary outcomes
Secondary outcomes were initial claudication distance (ICD), ankle brachial index (ABI), quality of life (QoL) and adverse reactions.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (March 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 2, part of The Cochrane Library, (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the (Specialised Register) section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
For the previous version of the review the authors searched MEDLINE/PUBMED (January 1966 to May 2008) and EMBASE (January 1985 to May 2008) using the search strategy described in Appendix 2 which was adapted for each database.
Searching other resources
We checked the reference lists of all retrieved articles. In addition, we attempted to identify unpublished studies and contacted manufacturers of Ginkgo biloba products to ask for information about any published and unpublished studies. We did not apply any language restrictions.
Data collection and analysis
Selection of trials
For the 2013 update, two authors (RS and PB) independently assessed trials identified by the literature search. In the previous version, two authors (SN and LK) independently selected eligible trials identified by the literature search.
Quality of trials
Two authors (SN and LK) independently assessed the selected trials for methodological quality using The Cochrane Collaboration tool for assessing risk of bias (Higgins 2008). Trials were assessed with respect to sequence generation, allocation concealment and blinding in each group. We resolved discrepancies by discussion, or, if this failed, by consulting authors MP and JT. The results were checked for accuracy by BB.
Data extraction
Two authors (SN and LK) independently extracted data using standardised data extraction forms.
Data analysis
Data were analysed using Review Manager (RevMan 5) software. Treadmill testing is the main assessment used to quantify therapy effect and walking ability in patients with PAD. In studies concerning medical therapy for PAD, the change in walking distance is assessed by different treadmill protocols that vary in speed and incline of the treadmill. Owing to the diverse workloads of different protocols, it is difficult to compare increases in walking distance (metres) or time. To standardise walking distance caloric expenditure is used to express the difference. This was calculated from the speed and incline of the treadmill.
We explored publication bias by relating the observed treatment effect to the standard error of the mean difference. We used meta‐regression to assess statistically the effect of publication bias. For this purpose the square root of the size of the study was introduced as a covariate in the assessment of the size of the treatment effect. Three domains of quality assessment were applied according to The Cochrane Collaboration tool for assessing risk of bias (Figure 1; Figure 2) (Higgins 2008). As many studies were assessed as unclear risk of bias, all studies were included in the primary analysis (see Risk of Bias section). Additionally, we explored dose‐effect relationship using meta‐regression.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
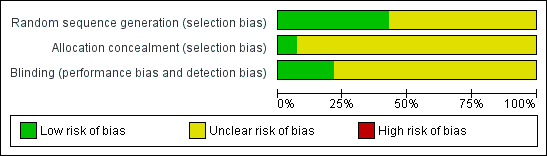
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Results
Description of studies
Results of the search
See Appendix 1.
No additional studies were identified for inclusion in this update. One additional study was excluded (Sandreau 1989).
Included studies
For details of included studies seeCharacteristics of included studies.
We included 14 randomised controlled trials; 11 published trials and three unpublished trials. Five of the published articles were published in German (Bauer 1984; Blume 1996; Blume 1998; Bulling 1991; Salz 1980); five in English (Gardner 2008; Mouren 1994; Peters 1998; Thomson 1990; Wang 2007); and one in Danish (Drabaek 1996). One clinical trial was published as an abstract (Schoop 1997). Two internal reports with unpublished data (Diehm 1990; Natali 1985) which were mentioned in other published meta‐analyses (Horsch 2004; Letzel 1992; Pittler 2000; Schneider 1992) were requested from the manufacturers. We contacted all authors of trials who did not report means and standard deviation for walking distances. However, we only received additional data for one trial (Gardner 2008). The two most recent trials were published in 2007 and 2008 (Gardner 2008; Wang 2007). The remaining 12 trials were published between 1984 and 1998.
The fourteen included trials involved a total of 739 participants. All these studies compared Ginkgo biloba with placebo in people with IC. The trials were conducted in Germany (8), France (2), Denmark (1), United States of America (1), United Kingdom (1) and Australia (1).The number of participants included in each trial varied between 18 and 209. The majority of trials included less than 50 participants and only two trials included more than 100 patients (Peters 1998; Schoop 1997). The mean age of the participants in the included trials varied between 60.9 and 71.6 years. Eleven trials included male and female participants. In three trials the gender of the participants was not described (Drabaek 1996; Mouren 1994; Thomson 1990).
In one trial maximal trained participants who did not further improve after a walking program of three to four times per week were included (Blume 1996). In another trial all participants received exercise therapy in combination with participation in the study (Bulling 1991), and one trial offered exercise therapy to all patients (Peters 1998). In this trial no information was given regarding how many patients actually participated in the exercise training program.
Inclusion criteria
In all 14 trials the patients had PAD according to Fontaine stage II (Fontaine 1954). Six trials mentioned that the situation should have been stable for a given period (three months to one year) (Bauer 1984; Blume 1998; Drabaek 1996; Mouren 1994; Natali 1985; Wang 2007), and in three trials the diagnosis was confirmed by angiography (Blume 1996; Blume 1998; Peters 1998). Four trials applied an age restriction; 40 to 75 years (Diehm 1990); 35 to 75 years (Mouren 1994); 30 to 75 years (Natali 1985); and 50 to 80 years (Wang 2007). In nine studies an upper limit of the ACD or ICD was used as an inclusion criterion. Inclusion criterion for the ACD were an ACD < 300 metres (Bauer 1984), ACD between 50 and 500 metres (Drabaek 1996; Gardner 2008) or an ACD between 100 and 500 metres (Mouren 1994) as assessed by treadmill testing. Inclusion criteria for the ICD were an ICD < 150 metres (Blume 1996; Peters 1998), ICD < 300 metres (Thomson 1990) or an ICD between 50 and 200 metres (Bulling 1991; Diehm 1990).
Exclusion criteria
The exclusion criteria were slightly variable between trials (seeCharacteristics of included studies). In all studies patients with PAD according to Fontaine stage III or IV were excluded. Patients with other reasons for a limited walking distance, e.g. cardiopulmonary or orthopaedic co‐morbidities were also excluded.
Dosage and duration of treatment
There were no dose‐related restrictions to the included trials in this review. Six trials included a comparison of 120 mg Ginkgo biloba daily versus placebo (Bauer 1984; Blume 1996; Diehm 1990; Drabaek 1996; Peters 1998; Schoop 1997) and three trials included the comparison of 160 mg Ginkgo biloba daily versus placebo (Blume 1998; Natali 1985; Salz 1980). Three trials, including the two most recent ones, compared daily dosages of 240 mg (Wang 2007), 300 mg (Gardner 2008) and 320 mg (Mouren 1994) Ginkgo biloba extract with placebo. The maximal follow‐up period of eight trials was 24 weeks, of one trial 16 weeks, of three trials 12 weeks, of one trial six weeks, and of one trial four weeks.
Outcome measurements
The primary outcome measurement of the study was ACD. In 13 trials walking distances were mentioned. Data on the walking distances could not be retrieved from one trial (Mouren 1994). In two other studies, only data from ICD were presented (Schoop 1997; Thomson 1990). In 12 trials treadmill testing with different protocols was used to assess the ACD and/or ICD. In one trial (Salz 1980) walking distance was measured by walking on a flat floor with two steps per second.
In six trials data from ankle brachial index (ABI) or ankle pressures were presented (Bauer 1984; Bulling 1991; Drabaek 1996; Gardner 2008; Thomson 1990; Wang 2007).Only one study reported quality of life as administered by the MOS SF‐36 questionnaire (Gardner 2008). Four trials presented data of a Visual Analogue Scale (VAS‐scale) for the subjective estimate of pain (Bauer 1984; Blume 1996; Drabaek 1996; Thomson 1990) and one trial presents a VAS‐scale for functional impairment (Mouren 1994). Nine trials reported on tolerance and adverse reactions of the medication and the placebo (Bauer 1984; Blume 1996; Blume 1998; Bulling 1991; Diehm 1990; Natali 1985; Peters 1998; Schoop 1997; Thomson 1990).
Excluded studies
For this update one additional study (Sandreau 1989) was excluded because it only included patients diagnosed with PAD stage III according to Fontaine. Twelve randomised controlled trials were excluded from analysis in 2009. Five of these trials compared Ginkgo biloba with another pharmacological treatment; dextran (Baitsch 1986), buflomedil (Berndt 1987), pentoxifylline (Bohmer 1988), naftidrofuryl (Horsch 1998), and the combination of Ginkgo biloba extract with magnesium and Levo‐arginine compared with acetylsalicylic acid (Michelini 1998). In four trials, conducted in 1975, 1977 and 1981, the inclusion criterion to include patients only with PAD stage II according to Fontaine was not met and separate data for patients diagnosed with PAD stage II according to Fontaine were not available (Ambrosi 1975; Courbier 1977; Frileux 1975; Garzya 1981). In one trial the primary aim was to compare diabetic and non‐diabetic PAD patients (Li 1998) and one double blind randomised cross‐over study evaluated the short term effect (within 60 minutes) of Ginkgo biloba extract versus placebo in patients with PAD stage II according to Fontaine (Rudofsky 1987). One study compared two dosages of Ginkgo biloba extract without a placebo group (Schweizer 1999).
Risk of bias in included studies
Allocation
All 14 trials were randomised trials, however explicit generation of the allocation sequence was only described in six trials (Blume 1996; Diehm 1990; Gardner 2008; Mouren 1994; Salz 1980; Wang 2007) where a random number generator was used.
Blinding
Only one trial explicitly described an adequate allocation concealment. In this trial the allocation sequence was administered by a third party who was not involved in the study (Wang 2007). All trials were reported to be double blinded. However, only three trials describe explicitly how this blinding was performed and stated that participants and trial personnel were blinded to treatment assignment until the end of the study (Blume 1996; Gardner 2008; Wang 2007). Because most of the studies were assessed as unclear risk of bias, all studies were included in the primary analysis (Figure 1; Figure 2).
Effects of interventions
Absolute claudication distance
Data for the ACD at the end of the study were available in 11 trials with a total sample size of 477 participants. The effect was calculated after standardisation of the presented walking distances. The total caloric expenditure was calculated from the speed and incline of the treadmill and if possible the weight of the participants. At the end of the study the ACD increased with an overall effect size of 3.57 kcal (confidence interval (CI) ‐0.10 to 7.23, P = 0.06) (Figure 3), in favour of the Ginkgo biloba group, using a random‐effects model. For a 70 kg weighted person this can be translated to an increase of 64.5 metres (CI ‐1.8 to 130.7) on a flat treadmill with an average speed of 3.2 km/h, which is most comparable with walking in daily life.
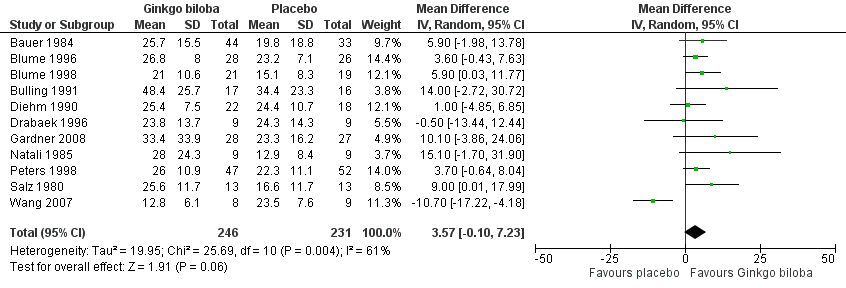
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study.
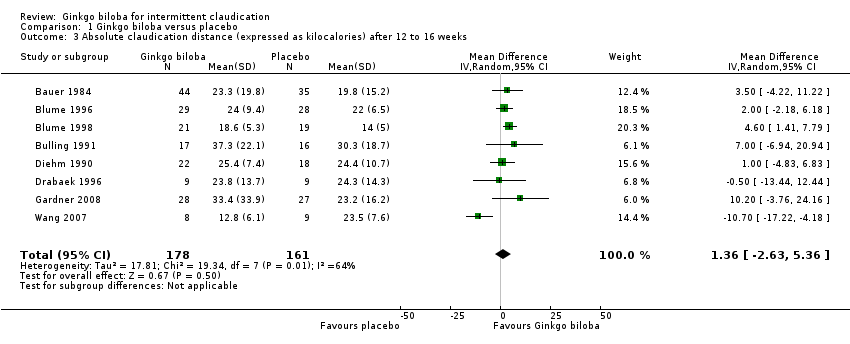
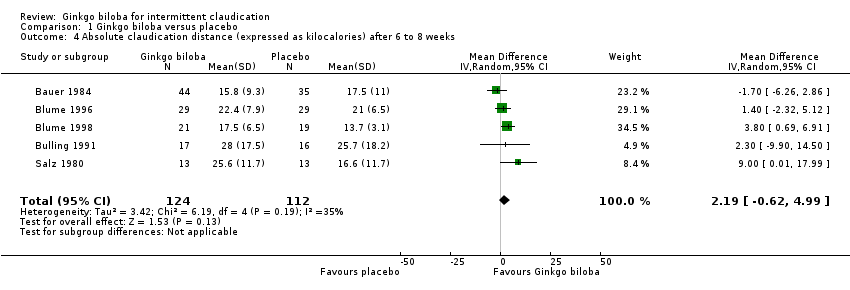
Furthermore, the effect size was calculated for 24 weeks of treatment (Figure 4), 12 to 16 weeks of treatment (Figure 5) and 6 to 8 weeks of treatment (Figure 6) with Ginkgo biloba extract versus placebo. After a follow‐up period of 24 weeks, data for six trials for ACD were available with a sample size of 321 participants. The ACD was increased with an overall effect size of 4.72 kcal (CI 2.27 to 7.16, P = 0.0002), in favour of the Ginkgo biloba group, using a random‐effects model. This can be translated to 85.3 metres (CI 41.0 to 129.4) on a flat treadmill with an average speed of 3.2 km/h. After 12 to 16 weeks of follow‐up data of eight trials (n = 339) present an increase in ACD with an overall effect size of 1.36 kcal (CI ‐2.63 to 5.36, P = 0.50). This was comparable with the overall effect size of 2.19 kcal ( CI ‐0.62 to 4.99, P = 0.13) after 6 to 8 weeks of follow up (n = 236).
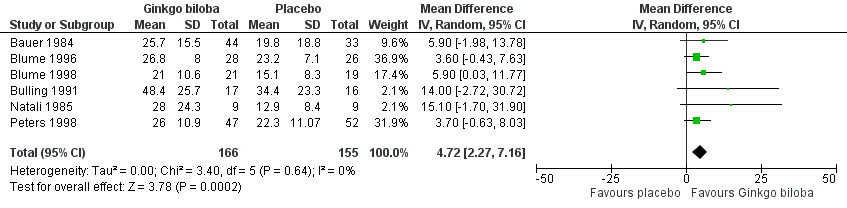
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks.
Initial claudication distance
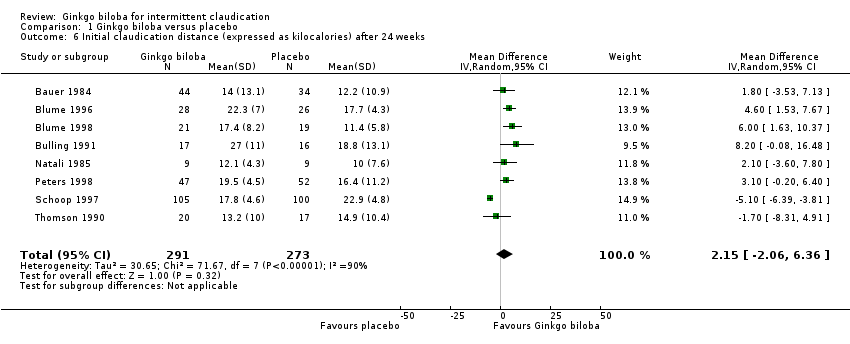
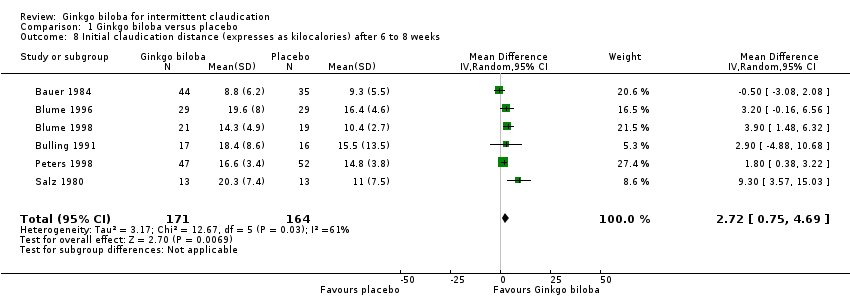
Data for the ICD were present in 13 trials at the end of the study within a total of 723 participants (Figure 7). The ICD increased with a overall effect size of 1.84 kcal (CI ‐0.92 to 4.61, P = 0.19) with a random‐effects model in favour of the Ginkgo biloba group. This corresponds with an increase in 33.3 metres (CI ‐16.7 to 83.3) for a 70 kg weighted adult with 3.2 km/h on a flat treadmill. After 24 weeks of treatment the overall effect size was 2.15 kcal (CI ‐2.06 to 6.36, P = 0.32) in favour of the Ginkgo biloba group (Figure 8). After 12 to 16 weeks (Figure 9) and 6 to 8 weeks (Figure 10) the overall effect sizes were 1.54 kcal (CI ‐0.04 to 3.12, P = 0.06) and 2.72 kcal (CI 0.75 to 4.69, P = 0.007), respectively.
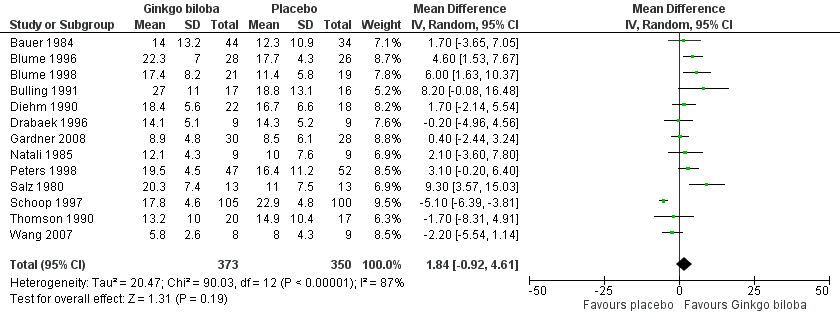
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.5 Initial claudication distance (expressed as kilocalories) at the end of the study.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.6 Initial claudication distance (expressed as kilocalories) after 24 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks.
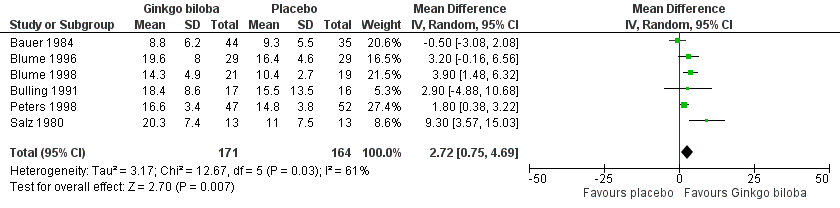
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks.
Ankle brachial index
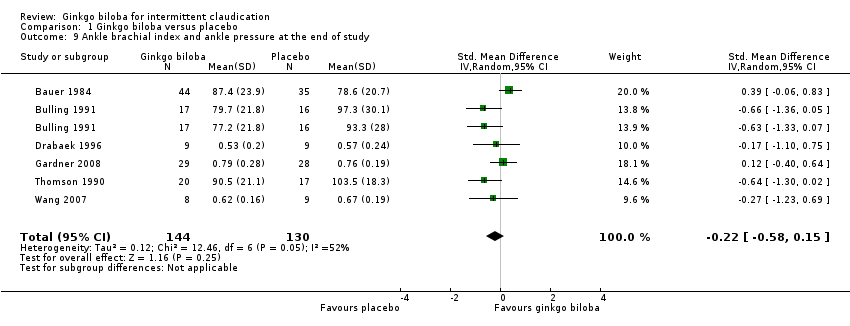
In three trials the ABI (Drabaek 1996; Gardner 2008; Wang 2007) and in three trials ankle pressures (Bauer 1984; Bulling 1991; Thomson 1990) were presented. Therefore, the standardised mean differences were used to show an overall effect size of ‐0.22 (CI –0.58 to 0.15, P = 0.25) on the ABI or ankle pressures in favour of the placebo group (Figure 11).
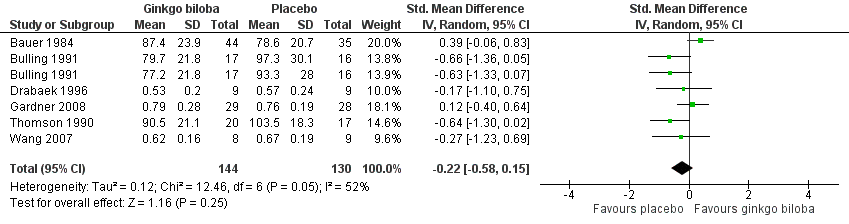
Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.9 Ankle brachial index and ankle pressure at the end of study.
Quality of life
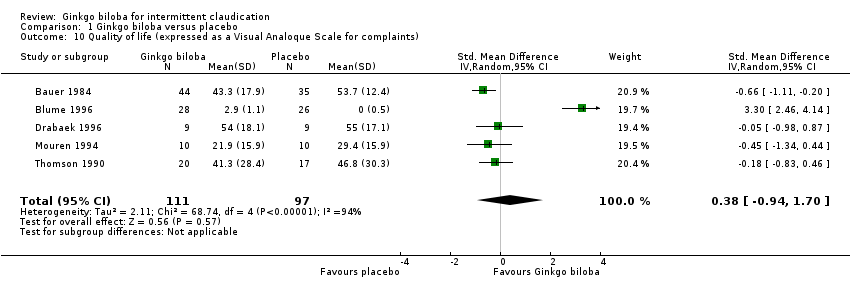
One study collected QoL data using the standardised MOS SF‐36 questionnaire (Gardner 2008). This study showed a significant improvement for the “role physical” domain in favour of the Ginkgo biloba study group (P = 0.03). The subjective assessment of pain and functional impairment administered by a VAS scale increased with an overall effect of 0.38 (CI –0.94 to 1.70, P = 0.57) in the Ginkgo biloba group (Bauer 1984; Blume 1996; Drabaek 1996; Mouren 1994; Thomson 1990) (Figure 12).

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.10 Quality of life (expressed as a Visual Analogue Scale for complaints).
Adverse reactions
Most common side effects of Ginkgo biloba included gastro‐intestinal complaints, headache, allergic skin reactions. Furthermore, Ginkgo biloba may increase the risk of bleeding. Nine out of 14 studies reported tolerance on Ginkgo biloba, as assessed by adverse reactions. In five trials no side effects were observed (Blume 1998; Bulling 1991; Diehm 1990; Peters 1998; Schoop 1997). In the other four trials the following side effects were reported; nausea (2), gastro‐intestinal complaints (2), blood in the urine (1), headache (1) (Bauer 1984; Blume 1996; Natali 1985; Thomson 1990). Overall, a low percentage of adverse reactions, which were all minor side effects, were found.
Assessment of publication bias
To explore publication bias two funnel plots in which the standard error of the mean difference was plotted against the mean difference were performed. The funnel plots for the ACD at the end of the study and the ACD after 24 weeks of follow up suggested publication bias as many smaller studies had more positive results. (Figure 13; Figure 14).
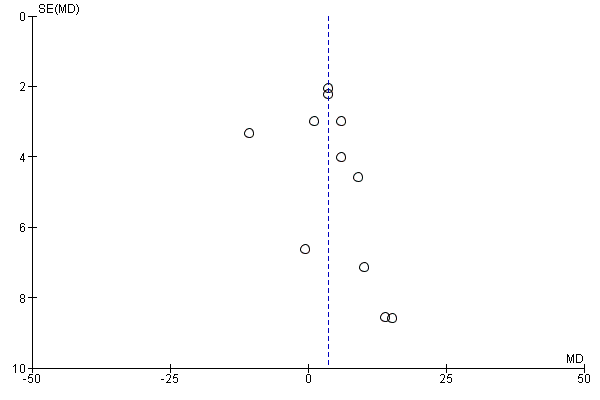
Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance at the end of the study is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.

Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance after 24 weeks is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.
Dosage of Ginkgo biloba
Meta‐regression analysis did not show a significant effect on the effect of Ginkgo biloba on walking distance; neither did the dosage of the Ginkgo biloba extract.
Discussion
Summary of main results
Eleven included trials with a total sample size of 477 patients did reveal non significant increases in ACD at the end of the study with a total effect size of 3.57 kcal. This corresponds with an increase of 64.5 metres on a flat treadmill (average speed 3.2 km/h). However, in clinical studies, most frequently a treadmill protocol of 3.2 km/h and 10% incline is used to assess therapy effect. Applying this protocol, 3.57 kcal represents an increase of 31 metres.
After 24 weeks of treatment we found a significant improvement in ACD with an overall effect size of 4.72 kcal, corresponding with 85.3 metres. However, in this analysis the number of patients who contributed was smaller, n = 321, and this analysis is likely to be more prone to publication bias (Figure 14).
For comparison, exercise therapy which is the main conservative treatment for symptomatic relief, is associated with 150% overall improvement of the ACD (Leng 2004). This translates into a benefit of 19.1 to 39.9 kcal, depending on the applied treadmill protocol to assess the improvement in walking distance. (19.1 kcal for 3.2 km/h and 0% increase, 39.9 kcal for 3.2 km/h and 10%, energy expenditures of graded protocols lie in between both). Hence, the effect of exercise therapy can be estimated to be approximately 5 to10 times greater than that of Ginkgo biloba extract. Moreover, supervised exercise therapy programs have an additional and significantly greater benefit than non‐supervised regimens (Bendermacher 2006).
Other meta‐analyses comparing pharmacological therapy (cilostazol, buflomedil, pentoxifylline and naftidrofuryl) with placebo for IC show similar results (Robless 2008; de Backer 2008; de Backer 2008a; Girolami 1999; Moher 2000). Possibly, the results on walking distance for buflomedil are most conclusive. Although all these comparisons are indirect, they support the generally accepted view that (supervised) exercise therapy is the main conservative therapy for patients with IC.
Clinical relevance
The clinical relevance of a non significant improvement of 64.5 metres in ACD is questionable. Quality of life, which is probably more important than the regained walking distance (for the patients' well being), was evaluated in only one trial. In this single study there was a borderline significant improvement of QoL (Gardner 2008). However, five trials assessed subjective assessment of pain and functional impairment by a VAS scale, which failed to show a statistically significant effect (Bauer 1984; Blume 1996; Drabaek 1996; Mouren 1994; Thomson 1990).
Limitations of this review
Eleven out of fourteen studies reported data on ACD. Authors of the three other studies were contacted for the walking distances. However, they did not respond (Mouren 1994; Schoop 1997; Thomson 1990). In two articles only the median was presented with the corresponding 95% confidence intervals (CI) (Blume 1996; Drabaek 1996). The authors were contacted but did not reply and the median was used instead of the mean and the standard deviation was calculated from the 95% CI. In one study the standard deviations were not directly available and had to be derived from the reported P values (Blume 1998). Unfortunately, in two studies only the differences to the ACD at the start of the study were available with the corresponding P values. The ACD at baseline was calculated with the known proportions of the ICD and ACD in the other included studies (Peters 1998; Salz 1980).
Figure 13 and Figure 14 suggested publication bias as many smaller studies had more positive results. This publication bias leading to missing data or "negative" trials is likely to have inflated the effect size. Furthermore, most included studies were performed with a small sample size; the majority included less than 50 participants. Special attention should be paid for a more recent study by Wang et al. (Wang 2007) that presented data of 17 participants and is the only study that reported results contrary to the other studies (Figure 3).

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.5 Initial claudication distance (expressed as kilocalories) at the end of the study.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.6 Initial claudication distance (expressed as kilocalories) after 24 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.9 Ankle brachial index and ankle pressure at the end of study.

Forest plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.10 Quality of life (expressed as a Visual Analogue Scale for complaints).
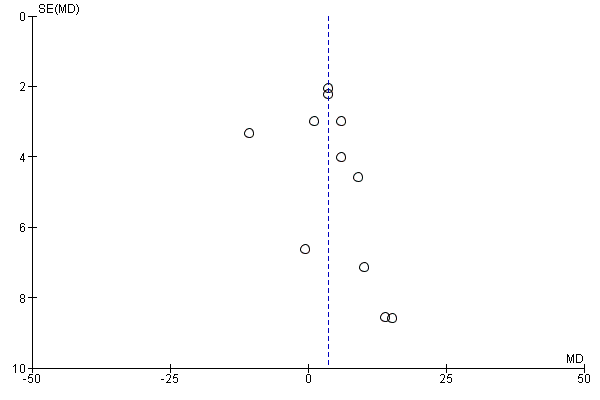
Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.1 Absolute claudication distance (expressed as kilocalories) at the end of the study. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance at the end of the study is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.

Funnel plot of comparison: 1 Ginkgo biloba versus placebo, outcome: 1.2 Absolute claudication distance (expressed as kilocalories) after 24 weeks. On the horizontal axis the effect estimate of Ginkgo biloba versus placebo on the absolute claudication distance after 24 weeks is presented as the mean difference. The vertical axis presents the standard error of the intervention effect on a reversed scale.

Comparison 1 Ginkgo biloba versus placebo, Outcome 1 Absolute claudication distance (expressed as kilocalories) at the end of the study.

Comparison 1 Ginkgo biloba versus placebo, Outcome 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks.
Comparison 1 Ginkgo biloba versus placebo, Outcome 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks.
Comparison 1 Ginkgo biloba versus placebo, Outcome 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 5 Initial claudication distance (expressed as kilocalories) at the end of the study.
Comparison 1 Ginkgo biloba versus placebo, Outcome 6 Initial claudication distance (expressed as kilocalories) after 24 weeks.

Comparison 1 Ginkgo biloba versus placebo, Outcome 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks.
Comparison 1 Ginkgo biloba versus placebo, Outcome 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks.
Comparison 1 Ginkgo biloba versus placebo, Outcome 9 Ankle brachial index and ankle pressure at the end of study.
Comparison 1 Ginkgo biloba versus placebo, Outcome 10 Quality of life (expressed as a Visual Analoque Scale for complaints).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Absolute claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 11 | 477 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.10, 7.23] |
| 2 Absolute claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 6 | 321 | Mean Difference (IV, Random, 95% CI) | 4.72 [2.27, 7.16] |
| 3 Absolute claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 8 | 339 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐2.63, 5.36] |
| 4 Absolute claudication distance (expressed as kilocalories) after 6 to 8 weeks Show forest plot | 5 | 236 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐0.62, 4.99] |
| 5 Initial claudication distance (expressed as kilocalories) at the end of the study Show forest plot | 13 | 723 | Mean Difference (IV, Random, 95% CI) | 1.84 [‐0.92, 4.61] |
| 6 Initial claudication distance (expressed as kilocalories) after 24 weeks Show forest plot | 8 | 564 | Mean Difference (IV, Random, 95% CI) | 2.15 [‐2.06, 6.36] |
| 7 Initial claudication distance (expressed as kilocalories) after 12 to 16 weeks Show forest plot | 9 | 441 | Mean Difference (IV, Random, 95% CI) | 1.54 [‐0.04, 3.12] |
| 8 Initial claudication distance (expresses as kilocalories) after 6 to 8 weeks Show forest plot | 6 | 335 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.75, 4.69] |
| 9 Ankle brachial index and ankle pressure at the end of study Show forest plot | 6 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.15] |
| 10 Quality of life (expressed as a Visual Analoque Scale for complaints) Show forest plot | 5 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.94, 1.70] |

