Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery
Information
- DOI:
- https://doi.org/10.1002/14651858.CD006364.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 13 February 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Eyes and Vision Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Conceiving the review: EG, AB, RC, IL, PM, SV.
Designing the review: EG, AB, RC, IL, PM, SV.
Coordinating the review: EG.
Data collection for the review:
-
Undertaking manual searches: EG, KL, IL, AN;
-
Screening search results: EG, KL, IL, RC, AN;
-
Organizing retrieval of papers: KL, AN;
-
Screening retrieved papers against inclusion criteria: EG, KL, IL, AN;
-
Appraising quality of papers: EG, KL, AB;
-
Extracting data from papers: EG, KL, IL, AN;
-
Writing to authors of papers for additional information: not applicable;
-
Providing additional data about papers: not applicable;
-
Obtaining and screening data on unpublished studies: not applicable.
Data management for the review:
-
Entering data into Review Manager 5: KL, EG.
Analysis of data: EG, KL.
Interpretation of data: EG, KL, AN, IL, PM.
Writing the review: EG, KL.
Securing funding for the review: PM.
Performing previous work that was the foundation of current study: AB, PM, EG.
Updating the review: EG, KL, ST, AN, IL, PM.
Sources of support
Internal sources
-
Discretionary funds, Wilmer Eye Institute, Johns Hopkins University, USA.
External sources
-
Cochrane Eyes and Vision US Project supported by grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, National Health Service, or the Department of Health.
-
Declarations of interest
EG: none known.
KL: none known.
ST: none known.
AN: none known.
IL: none known.
PM: none known.
Acknowledgements
We acknowledge Iris Gordon, Information Specialist for Cochrane Eyes and Vision (CEV), who designed strategies for and conducted the electronic searches. We acknowledge Niall Crosby, Barbara Hawkins, and Tianjing Li for their comments on the protocol and Lisa Herrinton, Neal Shorstein, and Barbara Hawkins for peer reviewing the original review.
We are grateful to Roy Chuck (RC), Ashley Behrens (AB), and Satyanarayana Vedula (SV) for their contributions to developing and publishing the protocol for this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Feb 13 | Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery | Review | Emily W Gower, Kristina Lindsley, Samantha E Tulenko, Afshan A Nanji, Ilya Leyngold, Peter J McDonnell | |
| 2013 Jul 15 | Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery | Review | Emily W Gower, Kristina Lindsley, Afshan A Nanji, Ilya Leyngold, Peter J McDonnell | |
| 2007 Jan 24 | Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. | Protocol | Ilya Leyngold, Afshan A Nanji, Roy S Chuck, Ashley Behrens, Satyanarayana S Vedula, Peter J McDonnell, Emily W Gower | |
Differences between protocol and review
We added methods for the assessment of the certainty of evidence and presentation of outcomes in a 'Summary of findings' table according to revised Cochrane standards.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Disease;
- Anti‐Bacterial Agents [*administration & dosage];
- Cataract Extraction [*adverse effects];
- Endophthalmitis [*prevention & control];
- Injections, Intraocular [methods];
- Ophthalmic Solutions [administration & dosage];
- Postoperative Complications [*prevention & control];
- Randomized Controlled Trials as Topic;
- Therapeutic Irrigation [methods];
- Visual Acuity;
Medical Subject Headings Check Words
Adult; Humans;
PICOs
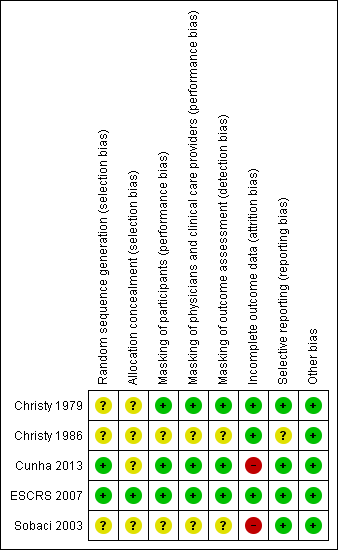
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
| Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Comparison (intervention vs comparator) | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ | |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ | |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ | |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) | Certainty of the evidence | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ | |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ | |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence | ||||||||
| BSS: balanced salt solution; CI: confidence interval; RR: risk ratio. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR) 1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups. 2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported). 3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide. 4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially. 5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population). | ||||||||
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) | |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
| CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity. *Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis. **Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR). | |||||||||