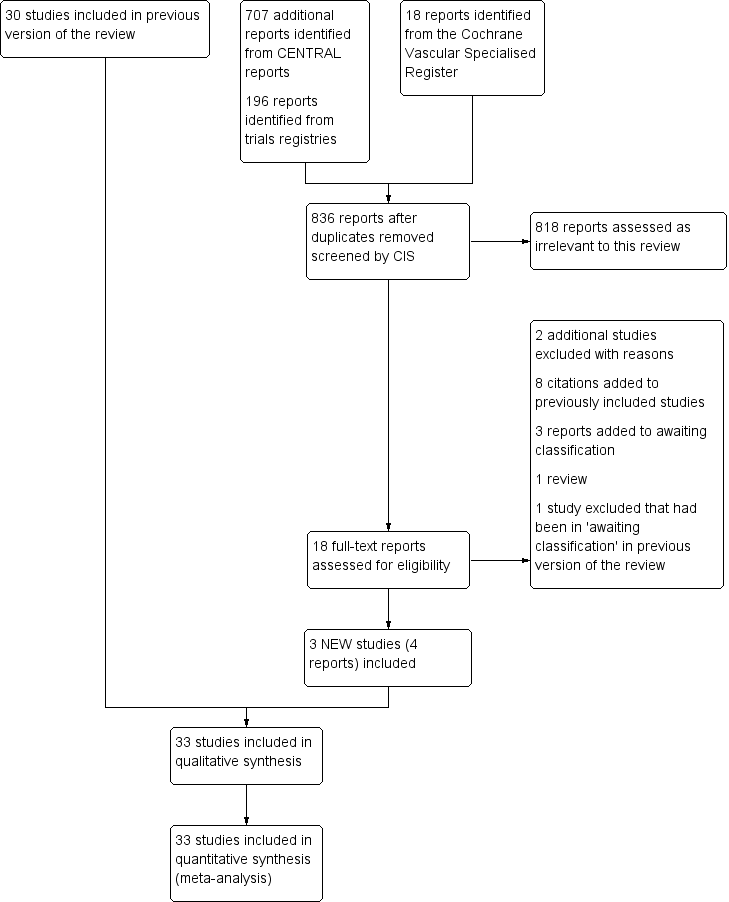
Treatment for superficial thrombophlebitis of the leg
Information
- DOI:
- https://doi.org/10.1002/14651858.CD004982.pub6Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 25 February 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Vascular Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
MDN: selected and assessed the quality of trials, extracted data, and wrote the review.
IW: selected and assessed the quality of trials, extracted data, and commented on the review.
SM: supervised the development of the review in all its phases.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
MDN: Dr Di Nisio reported participation to Advisory Boards for Daiichi‐Sankyo and Pfizer, and receiving consultancy fees from Daiichi‐Sankyo and Bayer Health Care.
IW: none known.
SM: Dr Middeldorp was a member of the Steering Committee of the CALISTO study, which was funded by GlaxoSmithKline (GSK) and which investigated the efficacy and safety of fondaparinux for superficial thrombophlebitis; funds were paid to Dr Middeldorp's institution. Dr Middeldorp's institution had also received funding from several pharmaceutical companies, including GSK, BMS, Bayer, Boehringer Ingelheim, Sanofi, and Pfizer to support some of her other educational and research activities. The first version of this review was written before the CALISTO study was designed.
Acknowledgements
We would like to thank the external peer referee Dr Benilde Cosmi for her comments and Mrs Carole Gibson for acting as the consumer on this review. We would also like to thank the Cochrane Consumer Network for their contribution to the 'Plain language summary.' We would like to thank the personnel from Cochrane Vascular, especially Marlene Stewart and Karen Welch for their invaluable assistance and advice.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Feb 25 | Treatment for superficial thrombophlebitis of the leg | Review | Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp | |
| 2013 Apr 30 | Treatment for superficial thrombophlebitis of the leg | Review | Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp | |
| 2012 Mar 14 | Treatment for superficial thrombophlebitis of the leg | Review | Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp | |
| 2007 Apr 18 | Treatment for superficial thrombophlebitis of the leg | Review | Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp | |
| 2007 Jan 24 | Treatment for superficial thrombophlebitis of the leg | Review | Marcello Di Nisio, Saskia Middeldorp, Iris M Wichers | |
| 2004 Oct 18 | Treatment for superficial thrombophlebitis of the leg | Protocol | M Di Nisio, Saskia Middeldorp, Iris M Wichers, Marcello di Nisio | |
Differences between protocol and review
We planned to evaluate publication bias using funnel plots and heterogeneity of treatment effects between trials using the Chi2 test and the I2 statistic. However, despite the relatively broad number of comparisons found, no funnel plots or tests of heterogeneity were performed since most studies did not evaluate the same treatment comparisons on the same study outcomes. For the same reason, subgroup analysis and sensitivity analysis to take into account possible sources of bias (e.g. open‐label design, incomplete follow‐up, high levels of exclusions unbalanced between the groups, or inadequate allocation concealment) were not possible. For most of the treatment comparisons, standardised mean differences (SMD) could not be calculated for continuous variables since the standard deviations of the means were not reported.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti‐Inflammatory Agents, Non‐Steroidal [*therapeutic use];
- Anticoagulants [*therapeutic use];
- Factor Xa Inhibitors [therapeutic use];
- Fondaparinux;
- Hemorrhage [chemically induced];
- Heparin, Low‐Molecular‐Weight [*therapeutic use];
- Polysaccharides [therapeutic use];
- Randomized Controlled Trials as Topic;
- Rivaroxaban [therapeutic use];
- Stockings, Compression;
- Thrombectomy;
- Thromboembolism [prevention & control];
- Thrombophlebitis [drug therapy, surgery, *therapy];
- Venous Thromboembolism [*prevention & control];
Medical Subject Headings Check Words
Humans;
PICOs
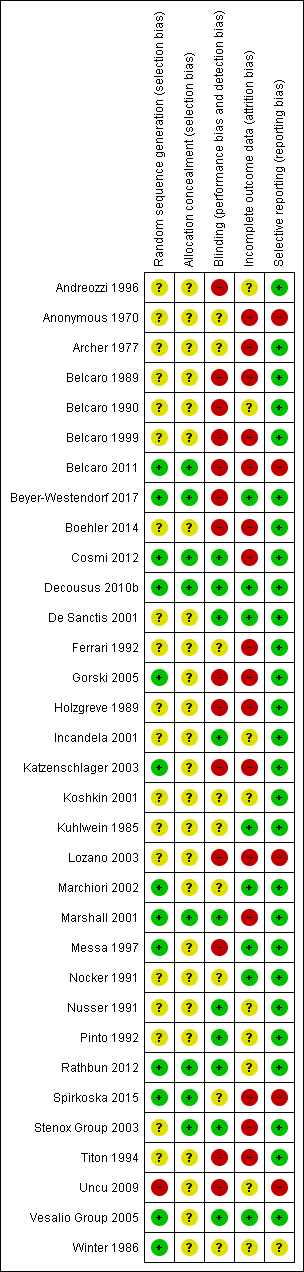
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fondaparinux versus placebo, Outcome 1 Pulmonary embolism.

Comparison 1 Fondaparinux versus placebo, Outcome 2 Deep vein thrombosis.

Comparison 1 Fondaparinux versus placebo, Outcome 3 Deep vein thrombosis or pulmonary embolism.

Comparison 1 Fondaparinux versus placebo, Outcome 4 Extension of superficial thrombophlebitis.

Comparison 1 Fondaparinux versus placebo, Outcome 5 Recurrence of superficial thrombophlebitis.

Comparison 1 Fondaparinux versus placebo, Outcome 6 Mortality.

Comparison 1 Fondaparinux versus placebo, Outcome 7 Major bleeding.

Comparison 1 Fondaparinux versus placebo, Outcome 8 Clinically relevant non‐major bleeding.

Comparison 1 Fondaparinux versus placebo, Outcome 9 Minor bleeding.

Comparison 1 Fondaparinux versus placebo, Outcome 10 Arterial thromboembolic complication.
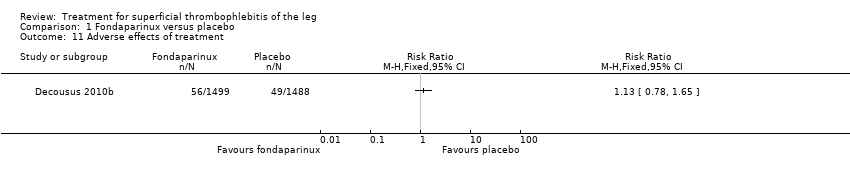
Comparison 1 Fondaparinux versus placebo, Outcome 11 Adverse effects of treatment.

Comparison 1 Fondaparinux versus placebo, Outcome 12 Non‐fatal serious adverse event.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 1 Pulmonary embolism.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 2 Deep vein thrombosis.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 3 Deep vein thrombosis or pulmonary embolism.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 4 Extension of superficial thrombophlebitis.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 5 Recurrence of superficial thrombophlebitis.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 6 Mortality.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 7 Major bleeding.
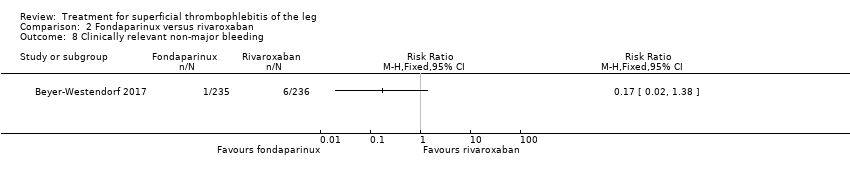
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 8 Clinically relevant non‐major bleeding.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 9 Minor bleeding.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 10 Serious adverse events.

Comparison 2 Fondaparinux versus rivaroxaban, Outcome 11 Adverse effects of treatment.

Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 4 Major bleeding.

Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 5 Heparin‐induced thrombocytopenia.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 4 Major bleeding.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 5 Heparin‐induced thrombocytopenia.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.

Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.

Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.

Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 1 Symptomatic venous thromboembolism.

Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 2 Symptomatic pulmonary embolism.

Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 3 Superficial thrombophlebitis progression into deep vein thrombosis.

Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 4 Major bleeding.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 1 Superficial thrombophlebitis or venous thromboembolism.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 2 Venous thromboembolism.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 3 Superficial thrombophlebitis.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 4 Swelling disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 5 Tenderness disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 6 Pain disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 7 Pitting oedema disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 8 Collateral veins disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 9 Redness disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 10 Palpable cord disappearance.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 11 Major bleeding.

Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 12 Heparin‐induced thrombocytopenia.

Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 1 Venous thromboembolism.

Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 3 Major bleeding.

Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 4 Complications.

Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism.

Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Major bleeding.

Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Heparin‐induced thrombocytopenia.

Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism.

Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Major bleeding.

Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Heparin‐induced thrombocytopenia.

Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism end‐of‐treatment.

Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Venous thromboembolism 3‐month follow‐up.

Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Major bleeding.

Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 5 Heparin‐induced thrombocytopenia.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 1 Pulmonary embolism.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 2 Deep vein thrombosis.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 3 Extension of superficial thrombophlebitis.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 4 Pain reduction.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 5 Hyperaemia reduction.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 6 Tenderness reduction.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 7 Palpable cord reduction.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 8 Mortality.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 9 Major bleeding.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 10 Minor bleeding.

Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 11 Adverse events.

Comparison 15 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.

Comparison 15 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
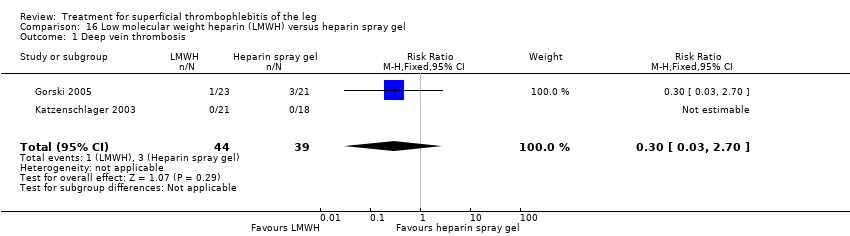
Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 1 Deep vein thrombosis.

Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 2 Participants with thrombus at 21 days.

Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 3 Allergic reaction or elevated sedimentation rate.

Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 1 Incidence of venous thromboembolism.

Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 3 Major bleeding.

Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 4 Heparin‐induced thrombocytopenia.

Comparison 18 Heparin calcium plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.

Comparison 19 Heparin subcutaneous (sc) versus defibrotide, Outcome 1 Decrease in the analogue score.

Comparison 19 Heparin subcutaneous (sc) versus defibrotide, Outcome 2 Adverse effects of treatment.

Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 1 Venous thromboembolism.

Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 3 Major bleeding.

Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 4 Heparin‐induced thrombocytopenia.

Comparison 21 Indomethacin versus placebo, Outcome 1 Adverse effects.

Comparison 22 Nimesulide versus diclofenac sodium, Outcome 1 Gastric pain.

Comparison 23 Essaven gel versus placebo, Outcome 1 Intolerance.

Comparison 24 Thrombectomy plus venoruton plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.

Comparison 25 Thrombectomy plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.

Comparison 26 Ligation plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.

Comparison 26 Ligation plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 27 Prophylactic unfractionated heparin (UFH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.

Comparison 27 Prophylactic unfractionated heparin (UFH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 28 Oral vasotonin versus placebo, Outcome 1 Cured or substantially better.

Comparison 28 Oral vasotonin versus placebo, Outcome 2 Poor tolerability.

Comparison 29 Elastic compression bandage (ECB) plus venoruton versus ECB alone, Outcome 1 Deep vein thrombosis.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 1 Redness disappearance.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 2 Pain disappearance.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 3 Disappearance of itching.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 4 Oedema improvement.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 5 Trophism improvement.

Comparison 31 Oxyphenbutazone versus placebo, Outcome 1 Tenderness improvement.

Comparison 32 Vitamin K antagonist (VKA) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.

Comparison 32 Vitamin K antagonist (VKA) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 33 Stripping plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.

Comparison 33 Stripping plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.

Comparison 34 Enzyme therapy versus placebo, Outcome 1 Pain reduction.

Comparison 34 Enzyme therapy versus placebo, Outcome 2 Responders.

Comparison 35 Desmin intramuscular (im) 200 versus desmin 100, Outcome 1 Adverse events.

Comparison 35 Desmin intramuscular (im) 200 versus desmin 100, Outcome 2 Adverse drug reactions.

Comparison 36 Desmin subcutaneous (sc) 2 × 100 versus desmin 100, Outcome 1 Adverse events.

Comparison 36 Desmin subcutaneous (sc) 2 × 100 versus desmin 100, Outcome 2 Adverse drug reactions.

Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 1 Pain (VAS, cm) at 3 weeks.

Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 2 Skin erythema (cm2) at 3 weeks.

Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 3 SF‐36 physical score at 3 weeks.

Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 4 SF‐36 mental score at 3 weeks.
| Fondaparinux compared to placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Fondaparinux | |||||
| Symptomatic VTE | 13 per 1000 | 2 per 1000 | RR 0.15 | 3002 | ⊕⊕⊕⊝ | ‐ |
| Major bleeding | 1 per 1000 | 1 per 1000 | RR 0.99 | 2987 | ⊕⊕⊕⊝ | ‐ |
| Extension of ST | 34 per 1000 | 3 per 1000 | RR 0.08 | 3002 | ⊕⊕⊕⊝ | ‐ |
| Recurrence of ST | 16 per 1000 | 3 per 1000 | RR 0.21 | 3002 | ⊕⊕⊕⊝ | ‐ |
| Mortality | 1 per 1000 | 1 per 1000 | RR 2 | 3002 | ⊕⊕⊕⊝ | ‐ |
| Minor bleeding | 4 per 1000 | 6 per 1000 | RR 1.49 | 2987 | ⊕⊕⊕⊝ | ‐ |
| Adverse effects of treatment | 33 per 1000 | 37 per 1000 | RR 1.13 | 2987 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Evidence downgraded one level for imprecision due to a low number of events. | ||||||
| Prophylactic LMWH versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Prophylactic LMWH | |||||
| Symptomatic VTE | 45 per 1000 | 54 per 1000 | RR 1.22 | 222 | ⊕⊕⊝⊝ | ‐ |
| Major bleeding | See comment | See comment | Not estimable | 222 | ⊕⊕⊝⊝ | 0 episodes of major bleeding |
| Extension or recurrence (or both) of ST | 330 per 1000 | 145 per 1000 | RR 0.44 | 222 | ⊕⊕⊝⊝ | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Evidence downgraded one level due to unclear random sequence generation and incomplete outcome data. | ||||||
| Therapeutic LMWH versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic LMWH | |||||
| Symptomatic VTE | 45 per 1000 | 38 per 1000 (10 to 137) | RR 0.85 | 218 | ⊕⊕⊝⊝ | ‐ |
| Major bleeding | See comment | See comment | Not estimable | 218 | ⊕⊕⊝⊝ | No episodes of major bleeding |
| Extension or recurrence (or both) of ST | 330 per 1000 | 152 per 1000 (89 to 254) | RR 0.46 | 218 | ⊕⊕⊝⊝ | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Evidence downgraded one level due to an unclear random sequence generation and incomplete outcome data. | ||||||
| NSAIDs versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with superficial thrombophlebitis of the leg | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NSAIDs | |||||
| Symptomatic VTE | 45 per 1000 | 41 per 1000 | RR 0.91 | 211 | ⊕⊕⊝⊝ | ‐ |
| Major bleeding | See comment | See comment | Not estimable | 211 | ⊕⊕⊝⊝ | 0 episodes of major bleeding |
| Extension or recurrence (or both) of ST | 330 per 1000 | 152 per 1000 | RR 0.46 | 211 | ⊕⊕⊝⊝ | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Evidence downgraded one level due to an unclear random sequence generation and incomplete outcome data. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis or pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Extension of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Clinically relevant non‐major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Arterial thromboembolic complication Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse effects of treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Non‐fatal serious adverse event Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis or pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Extension of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Clinically relevant non‐major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse effects of treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Superficial thrombophlebitis progression into deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Superficial thrombophlebitis or venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism Show forest plot | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.72] |
| 3 Superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Swelling disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Tenderness disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pain disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Pitting oedema disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Collateral veins disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Redness disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Palpable cord disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Major bleeding Show forest plot | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Heparin‐induced thrombocytopenia Show forest plot | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 16.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Complications Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.24, 3.63] |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.58, 1.78] |
| 3 Major bleeding Show forest plot | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Heparin‐induced thrombocytopenia Show forest plot | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism end‐of‐treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pain reduction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Hyperaemia reduction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Tenderness reduction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Palpable cord reduction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Minor bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis Show forest plot | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.70] |
| 2 Participants with thrombus at 21 days Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Allergic reaction or elevated sedimentation rate Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Decrease in the analogue score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects of treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gastric pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intolerance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cured or substantially better Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Poor tolerability Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Deep vein thrombosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Redness disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pain disappearance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Disappearance of itching Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Oedema improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Trophism improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tenderness improvement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Venous thromboembolism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain reduction Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Responders Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse drug reactions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse drug reactions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain (VAS, cm) at 3 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Skin erythema (cm2) at 3 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 SF‐36 physical score at 3 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 SF‐36 mental score at 3 weeks Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |