Techniques for assisting difficult delivery at caesarean section
Abstract
Background
Caesarean section involves making an incision in the woman's abdomen and cutting through the uterine muscle. The baby is then delivered through that incision. Difficult caesarean birth may result in injury for the infant or complications for the mother. Methods to assist with delivery include vacuum or forceps extraction or manual delivery utilising fundal pressure. Medication that relaxes the uterus (tocolytic medication) may facilitate the birth of the baby at caesarean section. Delivery of the impacted head after prolonged obstructed labour can be associated with significant maternal and neonatal complication; to facilitate delivery of the head the surgeon may utilise either reverse breech extraction or head pushing.
Objectives
To compare the use of tocolysis (routine or selective use) with no use of tocolysis or placebo and to compare different extraction methods at the time of caesarean section for outcomes of infant birth trauma, maternal complications (particularly postpartum haemorrhage requiring blood transfusion), and long‐term measures of infant and childhood morbidity.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2015) and reference lists of retrieved studies.
Selection criteria
All published, unpublished, and ongoing randomised controlled trials comparing the use of tocolytic agents (routine or selective) at caesarean section versus no use of tocolytic or placebo at caesarean section to facilitate the birth of the baby. Use of instrument versus manual delivery to facilitate birth of the baby. Reverse breech extraction versus head pushing to facilitate delivery of the deeply impacted fetal head.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
Seven randomised controlled trials, involving 582 women undergoing caesarean section were included in this review. The risk of bias of included trials was variable, with some trials not adequately describing allocation or randomisation.
Three comparisons were included.
1. Tocolysis versus no tocolysis
A single randomised trial involving 97 women was identified and included in the review. Birth trauma was not reported. There were no cases of any maternal side‐effect reported in either the nitroglycerin or the placebo group. No other maternal and infant health outcomes were reported.
2. Reverse breech extraction versus head push for the deeply impacted head at full dilation at caesarean section
Four randomised trials involving 357 women were identified and included in the review. The primary outcome of birth trauma was reported by three trials and there was no difference between reverse breech extraction and head push for this rare outcome (three studies, 239 women, risk ratio (RR) 1.55, 95% confidence interval (CI) 0.42 to 5.73). Secondary outcomes including endometritis rate (three studies, 285 women, average RR 0.52, 95% CI 0.26 to 1.05, Tau I² = 0.22, I² = 56%), extension of uterine incision (four studies, 357 women, average RR 0.23, 95% CI 0.13 to 0.40), mean blood loss (three studies, 298 women, mean difference (MD) ‐294.92, 95% CI ‐493.25 to ‐96.59; I² = 98%) and neonatal intensive care unit (NICU)/special care nursery (SCN) admission (two studies, 226 babies, average RR 0.53, 95% CI 0.23 to 1.22, Tau I² = 0.27, I² = 74%) were decreased with reverse breech extraction. No differences were observed between groups for many of the other secondary outcomes reported (blood loss > 500 mL; blood transfusion; wound infection; mean hospital stay; average Apgar score).
There was significant heterogeneity between the trials for the outcomes mean blood loss, operative time and mean hospital stay, making comparison difficult. However the operation duration was significantly shorter for reverse breech extraction, which may correspond with ease of delivery and therefore, the amount of tissue trauma and therefore, significantly less blood loss. Given the heterogeneity, we cannot define the amount of difference in blood loss, operative time or hospital stay however.
3. Instrument (vacuum or forceps) versus manual extraction at elective caesarean section
Two randomised trials involving 128 women were identified and included in the review. Only one trial reported maternal and infant health outcomes as prespecified in this review. This trial reported birth trauma as an outcome but there were no instances of birth trauma in either comparison group. There were no differences found in mean fall in haemoglobin (Hb) between groups (one study, 44 women, MD 0.03, 95% CI ‐0.53 to 0.59), or in uterine incision extension (one study, 44 women, RR 0.70, 95% CI 0.13 to 3.73).
Authors' conclusions
There is currently insufficient information available from randomised trials to support or refute the routine or selective use of tocolytic agents or instrument to facilitate infant birth at the time of difficult caesarean section. There is limited evidence that reverse breech extraction may improve maternal and fetal outcomes, though there was no difference in primary outcome of infant birth trauma. Further randomised controlled trials are needed to answer these questions.
PICOs
Plain language summary
Techniques for assisting difficult caesarean section
Caesarean section involves making an incision in the woman's abdomen and then cutting through the wall of the uterus. The baby is then born through these incisions. Numerous different ways have been suggested to facilitate the birth of the baby at difficult caesarean section and reduce the risk of injury to the baby, such as fractures and nerve damage. Some situations increase the likelihood of injury to mother and baby, especially if the woman has been in labour a long time or the baby's head is deep in the mother's pelvis.
This review includes a total of seven studies, involving 582 women and examines which techniques are safest for mother and baby. The risk of bias in trials was variable, with some trials not adequately describing the methods of randomisation.
At an emergency caesarean after a long labour, there is evidence from the developing world that delivery of the buttocks or feet of the baby first (reverse breech extraction) is safer than delivery of the head by pushing from the vagina back into the uterus. In four trials involving 357 women, delivery of the buttocks or feet first was associated with fewer adverse outcomes for the mother, including less bleeding, infection and a shorter operation duration. There was no significant difference in trauma to the baby but admission to special care or neonatal intensive care was decreased with delivery of the buttocks or feet first than when the head was pushed up from the vagina.
At a planned, non‐labouring caesarean section there is limited evidence to support techniques (forceps or vacuum extractor on the baby's head) other than the use of the surgeon's hands to deliver the head of the baby through the uterine incision. Two trials involving 128 women compared forceps/vacuum with manual delivery without any significance difference in outcomes.
There is also insufficient evidence to support the use of medication to relax the uterus (tocolysis) at the time of a caesarean to assist with safe delivery of the baby, with only one trial involving 97 women addressing this question.
Authors' conclusions
Background
Description of the condition
Caesarean section involves making an incision in the woman's abdomen and cutting through the uterine muscle. The baby is then delivered through that incision. There are many situations where caesarean section is indicated, and the clinical circumstances can cause potential difficulty when delivering the baby.
In most situations, the baby can be delivered without trauma through the uterine incision. In other circumstances, particularly after a woman been in labour for a long period of time, delivery may be more difficult, complicated by swelling of both maternal and fetal tissue, and the position of the fetal head deep within the maternal pelvis. In this situation, the baby may be difficult to deliver through the incision, with an increased risk of trauma for both the woman and her baby.
In some situations, the fetal head may not be well engaged within the maternal pelvis, and delivery of the baby through the uterine incision may be difficult and require manipulation of the baby.
Difficult caesarean birth may result in injury for the infant (such as fractures, peripheral nerve damage, spinal cord injury and subdural haematoma), resulting from direct trauma to the baby, or secondary to a delay in the timing of birth with subsequent reduction in oxygen delivery. The most common form of injury to the baby at the time of caesarean section is laceration to the skin. This is reported to occur in between 0.74% to 3.12% of all caesarean births (Dessole 2004; Haas 2002; Smith 1997). Difficult caesarean birth may also cause trauma to the mother, with injury to the broad ligament, cervix, vagina or disruption of the major uterine blood vessels or the urinary tract. The degree of damage and the amount of blood loss can impact on the duration of inpatient stay and maternal recovery.
Description of the intervention
Different techniques have been described to facilitate the delivery of the fetal head at the time of caesarean section at term (37 to 41 weeks of pregnancy). The technique depends on the location of the fetal head. At an elective caesarean, the head is often 'free floating' and methods to facilitate delivery include manual delivery utilising fundal pressure (De Costa 2006), vacuum extraction (Solomens 1962), and forceps (Acosta‐Sison 1938). Fundal pressure involves the assistant placing one or two hands on the uterine fundus and exerting downwards force while the obstetrician directs the head towards the uterine incision (De Costa 2006). Vacuum extraction was first described using a metal cup (Solomens 1962) and then with a soft cup (Boehm 1985; Pelosi 1984) applied directly to the fetal head through the uterine incision.
In contrast to the 'floating head' often found at elective caesarean procedures, at an emergency caesarean, the fetal head may be deeply impacted in the maternal pelvis, especially when the labour progress has been slow. Disengaging the impacted fetal head may result in extension of the uterine incision into the broad ligament, cervix or vagina, with possible damage to uterine blood vessels (De Costa 2006). Extraction and delivery of the fetal head in this situation can be achieved utilising either an abdominovaginal approach with head pushing from the vagina (Landesman 1984) or reverse breech extraction, where the baby is delivered by grasping the feet or buttocks and delivering them through the incision, with the head delivered last (Fong 1997). Other techniques described include use of a disimpaction system (Papanikolaou 2009) or Patwardhan technique, where the infants shoulders are delivered, then the trunk, breech, limbs then finally the head (Kumar Saha 2014; Mukhopadhyay 2005).
If the fetus cannot be delivered through a standard transverse incision through the lower segment, the uterine incision may need to be extended, utilising either a J shape or inverted T shape incision that commonly involves the upper segment or main part of the uterus (De Costa 2006). Such an incision is associated with more complications for the woman, especially during a subsequent pregnancy and delivery (Landon 2004).
The breech presentation may also pose difficulties for delivery of the baby at caesarean section in both the emergency and elective settings. Techniques utilised for delivery of a breech presentation at caesarean section include grasping a foot or both feet to facilitate delivery of the buttocks (De Costa 2006). The shoulders and head can then be delivered utilising the same manoeuvres employed for vaginal breech birth, including the Lovsett and Mauriceau‐Smellie‐Veit manoeuvres, or forceps can be applied to the aftercoming head.
During difficult delivery of the baby at the time of caesarean section, the uterus may contract and contribute to mechanical impediments with the delivery. A number of medications have been used in order to relax the uterus (tocolysis) and facilitate the birth of the infant. Tocolytic agents act through a variety of mechanisms to relax the uterus and prevent uterine contractions and have been used mainly in treating preterm labour and include betamimetics (such as salbutamol or terbutaline) (Gyetvai 1999); calcium channel blockers (such as nifedipine) (Flenady 2014a); non‐steroidal anti‐inflammatory drugs (such as indomethacin) (Reinebrant 2015); magnesium sulphate (Crowther 2014); nitric oxide donors (Duckitt 2014); and oxytocin receptor antagonists (Flenady 2014b). These drugs have side‐effects for the woman and, as they are able to cross the placenta, may affect the infant (Gyetvai 1999; Neilson 2014). Side‐effects for the woman include tachycardia (increased heart rate), hypotension (low blood pressure), nausea and vomiting, hyperglycaemia (high blood sugar levels), and pulmonary oedema (fluid accumulation in the lungs) (Crowther 2014; Duckitt 2014; Flenady 2014b; Gyetvai 1999; Neilson 2014).
While uterine relaxation at caesarean section may be a beneficial effect as it relaxes the uterus to facilitate birth of the infant, it can be followed by uterine atony (where the uterus does not contract after birth) causing subsequent postpartum haemorrhage.
How the intervention might work
This review compares three interventions for delivery of the fetus at caesarean section. Firstly, the use of tocolysis (routine or selective) with no use of tocolysis or placebo at the time of caesarean birth, secondly, the use of either forceps, vacuum or manual delivery with fundal pressure at the time of elective caesarean birth and finally, the use of head pushing versus reverse breech extraction for the deeply impacted fetal head at the time of emergency caesarean section.
These interventions may expedite the delivery of the fetus and may reduce the risk of birth trauma. Any benefit of increased ease of infant birth and reduction in birth trauma has to be balanced against the potential complications for the woman, including postpartum haemorrhage and damage to the urinary tract.
Outcome measures for infant and childhood, and short‐term and long‐term maternal morbidity, for women undergoing caesarean birth are all important to consider and potentially impacted by such interventions.
Why it is important to do this review
Rates of caesarean section are increasing, and techniques used at this common operation should be supported by evidence to ensure rare complications, including birth trauma, are decreased.
Caesarean section at full dilatation with obstructed labour can be difficult, with potential complications for mother and baby. Evidence is needed to guide clinicians as to which technique, reverse breech extraction or head push best facilitates delivery with the least complications for mother and baby.
There are risks associated with the use of medication to relax the uterus, including excessive bleeding and possible interference with the blood the baby receives from the placenta at birth. There is currently limited evidence available to help with informed decisions around the use of these drugs at difficult caesareans. Until there is evidence that these drugs do more good than harm, they should not be used.
Objectives
-
To compare the use of tocolysis (routine or selective use) with no use of tocolysis, or placebo at the time of caesarean section when a difficult delivery is encountered or anticipated.
-
To compare head pushing with reverse breech extraction for the deeply impacted fetal head after prolonged obstructed labour.
-
To compare the use of either forceps, vacuum or manual delivery with fundal pressure at the time of elective caesarean birth when the fetal head is difficult to deliver.
All comparisons utilise outcomes of infant birth trauma, maternal complications (particularly postpartum haemorrhage requiring blood transfusion), and long‐term measures of infant and childhood morbidity.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished, and ongoing randomised controlled trials comparing:
-
use of any tocolytic (routine or selective) at caesarean section with no use of tocolytic or placebo;
-
reverse breech extraction versus head pushing at caesarean section for the deeply impacted fetal head in obstructed labour;
-
use of an instrument (forceps or vacuum) to assist with delivering the fetal head at elective caesarean section with no instrument.
We excluded quasi‐randomised trials (e.g. those randomised by date of birth or hospital number) from the analysis. Studies presented in abstract form only will not be included until the full report becomes available to assess methodological quality and relevance to the scope of the review. If identified, cluster‐randomised trials will be considered for inclusion in future updates.
Types of participants
Women undergoing a caesarean birth with anticipated/possible difficulty in delivering the fetus.
Types of interventions
-
Use of tocolytic agents (routine or selective) at caesarean section versus no use of tocolytic or placebo at caesarean section.
-
Use of reverse breech extraction versus head push at caesarean section for obstructed labour.
-
Use of forceps or vacuum at caesarean section versus no use of forceps or vacuum at caesarean section.
Types of outcome measures
Primary outcomes
Infant birth trauma (any of: subdural or intracerebral haemorrhage, spinal cord injury, basal skull fracture, other fracture, peripheral nerve injury).
Secondary outcomes
Outcome measures for the woman
-
Extension of uterine incision
-
Blood loss > 500 mL at time of caesarean birth
-
Amount of blood loss at caesarean (mL)
-
Mean fall in haemoglobin (Hb) (not prespecified)
-
Blood transfusion
-
Endometritis/endometrial infection
-
Wound infection (as defined by trial authors)
-
Ureteral/bladder/cervical injury
-
Operative time (duration of surgery)
-
Mean hospital stay (not prespecified)
-
Pain scores post operatively (as defined/measured by trial authors)
-
Maternal side‐effects (not prespecified)
Outcome measures for the infant
-
Apgar less than four at five minutes
-
Average Apgar score (not prespecified)
-
Cord pH less than 7.10 or low pH/low base excess as defined by trialists
-
Admission to neonatal special care or intensive care unit
-
Neonatal death ‐ (defined as death within the first 28 days of life)
-
Length of stay in a neonatal special care or intensive care unit
-
Intraventricular haemorrhage
-
Periventricular leukomalacia
Longer‐term outcomes for the infant
-
Cerebral palsy
-
Developmental delay in childhood (as defined by trial authors)
Measures of satisfaction include
-
Women's satisfaction
-
Women's preferences for care
Health service use
-
Length of postoperative stay for the woman and infant
-
Readmission to hospital of the woman or infant, or both
Only outcomes with available data appear in the analysis tables.
Only outcome data that were prestated by the review authors have been used.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2015).
For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full texts of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
[For additional searching carried out in a previous version of the review (Dodd 2006), see Appendix 1.]
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeDodd 2006.
For this update we used the following methods when assessing the reports identified by the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference for outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. In future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not considered eligible for inclusion in this review.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we planned to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we planned to attempt to include all participants randomised to each group in the analyses, and all participants would be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial would be the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots. We planned to assess funnel plot asymmetry visually, If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was not carried out due to insufficient data. In future updates we plan to carry out the following prespecified subgroup analyses:
-
gestational age at caesarean section (less than 34 weeks' gestation or greater than 34 weeks' gestation);
-
type of uterine incision at caesarean (lower segment transverse incision (with or without extension of the incision), midline uterine incision);
-
type of tocolytic used;
-
effect of labour (caesarean section prior to labour versus in labour);
-
or multiple versus singleton gestation.
We will carry out subgroup analyses on the following outcome:
-
infant birth trauma.
We will assess subgroup differences by interaction tests available within RevMan 2014.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (greater than 20%), or both, with poor‐quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result. Sensitivity analysis was not carried out due to insufficient number of trials being included in meta‐analyses.
Results
Description of studies
Results of the search
The search strategy identified 32 trial reports for consideration in this review. Seven trials (nine reports) were included and 19 trials (23 reports) were excluded.
Included studies
Seven randomised controlled trials, involving 582 women undergoing caesarean section were included in this review.
Tocolysis versus no tocolysis
The search identified a single randomised controlled trial of 97 women, that was able to be included in the review. This study involving a three‐way comparison between nitroglycerin (comparing two different doses 0.25 mg and 0.5 mg), and placebo administered at the time of caesarean section to facilitate fetal extraction (David 1998). Data were analysed as a single‐pair comparison; nitroglycerin (either 0.25 mg or 0.5 mg) compared with placebo.
Reverse breech versus head push for obstructed labour
The search identified four randomised controlled trials including 357 women. All four trials compared the morbidity and mortality associated with the push method and the pull (reverse breech) method to deliver the impacted head at caesarean section for obstructed labour (Bastani 2012; Fasubaa 2002; Frass 2011; Veisi 2012).
Elective use of instrument versus fundal pressure
The search identified a single randomised controlled trial of 44 women that utilised a three‐way comparison between vacuum, forceps and manual delivery to deliver the cephalic fetus at caesarean section (Bofill 2000), comparing safety and ease of delivery. Vacuum and forceps were combined as a single experimental group (instrumental delivery) for comparison with manual delivery.
A further randomised controlled trial was identified that compared the transplacental microtransfusion from mother to baby with manual versus instrumental delivery at caesarean section in 84 women (Owens 2003).
For details of the included studies, see the table of Characteristics of included studies.
Excluded studies
We excluded 19 trials from the review, with four studies excluded as abstracts only were available and no further information could be obtained to allow inclusion (Naghibi 2008; Papanikolaou 2009; Seal 2013; Wright 1995). Bellad 2013 is excluded at present because it is not yet published and although contact has been made with the author, data are not yet finalised. Three studies were excluded as they were retrospective cohort studies (Chopra 2009Kumar Saha 2014; Levy 2005), and two were excluded as they were prospective cohort studies and not randomised (Kadhum 2009; Mukhopadhyay 2005). The trials Burke 1989, Magann 1993, Kaukinen 1978, Kulier 1997 and Visser 1979 were excluded as they involved administration of tocolytic agents for intrauterine fetal resuscitation prior to a caesarean section for fetal distress. The trial by Hong 1993 was excluded as it involved the use of tocolytic agents in women with hypertension for the prevention of hypertension in response to laryngeal stimulation at the time of intubation prior to caesarean section. The trial by Eisler 1999 was excluded as it involved continuous tocolytic infusion for two hours prior to birth, for the improvement of infant respiratory effort and glucose metabolism. The Buhimschi 2002 trial was excluded as it did not involve women who required caesarean section. The trial by Fothergill 1971 was excluded because it assessed anaesthetic duration, not tocolysis effect.
For details of the excluded studies, see the table of Characteristics of excluded studies.
Risk of bias in included studies
Overall, the methodological quality of the trials was variable (see Description of studies and Characteristics of included studies).There was variable reporting of the prespecified outcomes of the review, with all seven trials presenting information that could be incorporated into the meta‐analysis (Bastani 2012; Bofill 2000; David 1998; Fasubaa 2002; Frass 2011; Owens 2003; Veisi 2012).
SeeFigure 1 and Figure 2 for a summary of 'Risk of bias' assessments.
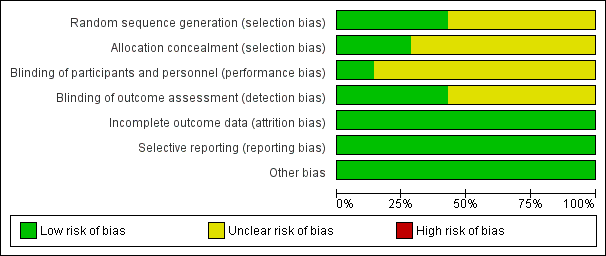
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
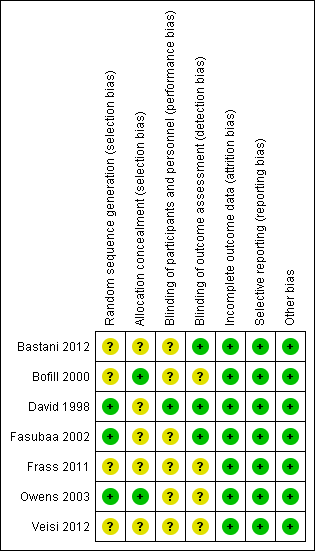
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
While all of the studies were stated to be randomised, the method of randomisation was adequately described in three trials as involving either computer‐generated randomisation sequences or tables of random numbers (David 1998; Fasubaa 2002; Owens 2003).
The method of allocation concealment was assessed as adequate in two trials, both utilising sequentially numbered, sealed, opaque envelopes (Bofill 2000; Owens 2003), no trials utilised telephone randomisation.
Four trials (Bastani 2012; Bofill 2000; Frass 2011; Veisi 2012) stated they were randomised but did not describe the randomisation process.
Blinding
Blinding of outcome assessor was indicated in only three of the trials (Bastani 2012; David 1998; Fasubaa 2002).
Where the comparison was of surgical technique the surgeon could not be blinded, Fasubaa 2002 and Bastani 2012 describe blinding of the anaesthetist assessing operative blood loss. The other studies do not mention any other forms of blinding.
For ethical reasons, David 1998 unblinded the anaesthetist administering tocolysis, however the woman, surgeon and neonatologist were blinded.
Incomplete outcome data
There was no loss of data and therefore all studies were assessed as low risk of attrition bias.
Selective reporting
All expected outcomes were reported in the included trials, with no concerns regarding selective outcome reporting.
Other potential sources of bias
No other potential sources of bias was identified in any of the studies included.
Effects of interventions
Tocolysis versus no tocolysis
We identified one study involving a three‐way comparison between nitroglycerin (comparing two different doses 0.25 mg and 0.5 mg), and placebo administered at the time of caesarean section to facilitate fetal extraction (David 1998).
Primary outcomes
Infant birth trauma was not reported in the David 1998 trial.
Secondary outcomes
There were no differences identified between nitroglycerin and placebo for the occurrence of any maternal side‐effect (one study; 97 women; risk ratio (RR) not estimable), Analysis 1.1. There were no other prespecified maternal or infant outcomes reported that could be included in this review. The trial reported the ease of delivery of the baby at caesarean section, and mean infant Apgar scores, but the method of reporting in the published paper precluded inclusion in the results of this review.
Reverse breech versus head push for obstructed labour
Four studies compared reverse breech extraction with vaginal head pushing to assist with delivery of the deeply impacted fetal head in obstructed labour (Bastani 2012; Fasubaa 2002; Frass 2011; Veisi 2012).
Primary outcomes
Of the fetal outcomes assessed, Fasubaa 2002, Bastani 2012 and Veisi 2012 reported infant birth trauma; with no difference between the two interventions (three studies; 239 women; RR 1.55, 95% confidence interval (CI) 0.42 to 5.73), Analysis 2.1.
Secondary outcomes
Two trials (Fasubaa 2002; Frass 2011) reported admission to neonatal intensive care unit (NICU) or special care nursery (SCN), with significantly fewer babies admitted in the reverse breech extraction group (two studies, 226 babies, average RR 0.53, 95% CI 0.23 to 1.22, Tau² 0.27, I² 74%), Analysis 2.8. Two trials (Bastani 2012; Veisi 2012) reported no admission to NICU but did not describe admission to SCN. There was no difference in the average Apgar score between the groups (three studies, 239 babies, mean difference (MD) 0.36, 95% CI ‐0.64 to 1.36; Tau = 0.77, I² = 98%), Analysis 2.13.
For maternal outcomes, all trials reported uterine incision extension and three trials (Bastani 2012; Fasubaa 2002; Frass 2011) reported endometritis rates. For the woman, reverse breech was associated with a significantly lower rate of uterine incision extension when compared with head push (four studies, 357 women, average RR 0.23, 95% CI 0.13 to 0.40; Tau²= 0.10, I² = 31), Analysis 2.2, and reverse breech was also associated with less endometritis (three studies, 285 women, average RR 0.52, 95% CI 0.26 to 1.05;Tau² = 0.22, I² = 56%), Analysis 2.6. Operative time was described by all authors, with reverse breech associated with a significantly shorter duration of operation (four studies, 357 women, MD ‐14.99 minutes, 95% CI ‐27.67 to ‐2.30; Tau² = 164.16, I² = 99%), Analysis 2.11. Mean blood loss was also significantly less with reverse breech extraction (three studies, 298 women, MD ‐294.92 mL, 95% CI ‐493.25 to ‐96.59; Tau² = 28196.38, I² = 98%), Analysis 2.4.
Reverse breech for obstructed labour was not associated with any significant reduction in blood loss greater than 500 mL,blood transfusion, wound infection or mean hospital stay (Analysis 2.3; Analysis 2.5; Analysis 2.7; Analysis 2.12). No difference was observed between groups for early neonatal death (one study, 108 women, RR 0.54, 95% CI 0.23 to 1.24), Analysis 2.9. In one trial, mean fall in haemoglobin (Hb) was reduced in the reverse breech group (118 women, MD ‐0.44, 95% CI ‐0.72 to ‐0.16), Analysis 2.10.
The heterogeneity for mean blood loss (Analysis 2.4), operative time (Analysis 2.11), mean hospital stay (Analysis 2.12) and average Apgar score (Analysis 2.13) was extreme, the results being very variable and so should be interpreted with caution.
No study addressed long‐term outcomes for the infant or measures of satisfaction for the woman. No information was given as to cost‐analysis differences between the groups.
Elective use of instrument versus fundal pressure at elective caesarean section
Two studies compared instrumental with manual pressure at elective caesarean section (Bofill 2000; Owens 2003). Owens 2003 aimed to compare the amount of placental alkaline phosphatase released into the maternal circulation with the two techniques.
Primary outcomes
Bofill 2000 reported birth trauma as an outcome but there were no instances of birth trauma in either comparison group, Analysis 3.1.
Secondary outcomes
No trial reported on any fetal outcomes. Maternal blood loss was assessed in both trials, but different reporting methods mean that results cannot be combined for the meta‐analysis. Bofill 2000 described mean fall in Hb; there was no difference between groups (one study, 44 women, MD 0.03, 95% CI ‐0.53 to 0.59) Analysis 3.3, while Owens 2003 described estimated blood loss, again with no difference between the interventions. Bofill 2000 reported uterine incision extension with no difference between interventions, (one study, 44 women, RR 0.70, 95% CI 0.13 to 3.73) Analysis 3.2.
Neither study described any of the other prespecified outcomes for this review.
It was not possible to conduct the planned subgroup analyses related to gestational age at caesarean section (less than 34 weeks' gestation or greater than 34 weeks' gestation); type of uterine incision at caesarean (lower segment transverse incision (with or without extension of the incision), midline uterine incision); type of tocolytic used; effect of labour (caesarean section prior to labour versus in labour); or multiple versus singleton gestation.
Discussion
Tocolysis versus no tocolysis
Tocolytic agents have been used extensively to induce uterine relaxation, and have been used anecdotally at the time of caesarean section, where difficulties with birth of the infant are anticipated or encountered. While uterine relaxation at caesarean section may be a beneficial effect as it relaxes the uterus to facilitate birth of the infant, it can also be followed by uterine atony (where the uterus does not contract after birth) causing subsequent postpartum haemorrhage. Any benefit to facilitate infant birth and reduction in birth trauma has to be balanced against the potential complications for the woman, including postpartum haemorrhage secondary to uterine atony.
There is currently insufficient information from randomised controlled trials assessing the role of tocolytic agents in facilitating infant birth at caesarean section and reducing the risk of infant birth trauma. The occurrence of maternal side‐effects and risk of postpartum blood loss was similarly not reported in the single trial identified and included in the review.
Further information is required to address the role of tocolysis to facilitate infant birth at caesarean section adequately, with attention particularly to adequate reporting of maternal and infant health outcomes.
Reverse breech versus head push for obstructed labour
Four studies investigated caesarean section for obstructed labour, comparing reverse breech extraction with vaginal push. Three studies (Bastani 2012; Fasubaa 2002; Veisi 2012) addressed our primary outcome of infant birth trauma, and found no difference between the techniques. However other outcome assessments reported by both authors were sufficiently homogeneous to allow analysis, they demonstrated that reverse breech extraction may be superior for mother and baby. For the mother, the risk of endometritis and uterine incision extension was significantly decreased with reverse breech extraction. For baby, likelihood of admission to special care nursery (SCN) or neonatal intensive care unit (NICU) was significantly less with reverse breech extraction. Neither study addressed Apgar score less than four at five minutes. Fasubaa 2002, Bastani 2012 and Veisi 2012 did report average Apgar at five minutes, there was no difference between the comparison groups. There was no difference in early neonatal death rate or stillbirth rate.
There was significant heterogeneity between the trials for the outcomes mean blood loss, operative time and mean hospital stay, making comparison difficult. However the operation duration was significantly shorter for reverse breech extraction, which may correspond with ease of delivery and therefore the amount of tissue trauma and therefore significantly less blood loss. Given the heterogeneity we cannot define the amount of difference in blood loss, operative time or hospital stay however. The reason for the extreme heterogeneity is not apparent from the text in the published studies. Possible reasons for difference in operative time may be related to different surgical technique, difference in duration of stay may be due to different hospital policies or financial restriction on length of stay, rather than medical need.
Two studies (Fasubaa 2002; Frass 2011) were conducted in low‐income settings (Yemen and Nigeria, respectively), and both authors commented that obstructed labour is still common in their country. Two studies were undertaken in Iran (Bastani 2012; Veisi 2012), both having less study participant than the former two trials. These results therefore may not be applicable to obstetric units in middle‐income or high‐income settings. Further research is required to confirm these results in low‐income settings, potentially including patients where a vaginal instrumental delivery was unsuccessfully attempted prior to caesarean section.
Novel methods for fetal disimpaction are reported in the literature (Papanikolaou 2009; Seal 2013); these may be associated with less maternal and infant trauma than either vaginal push or reverse breech extraction. If data are published these may be included in the next review update.
Elective use of instrument versus fundal pressure at elective caesarean section
Two studies addressed the use of an instrument (either vacuum or forceps) at elective caesarean section, though only one author (Bofill 2000) conducted their study to investigate the safety and ease of delivery with an instrument compared with fundal pressure. Owens 2003 instead investigated the amount of transplacental microtransfusion from mother to infant with fundal pressure compared with an instrument, and thus did not address the prespecified outcomes of this review. There was no difference in birth trauma in the one study that addressed this outcome (Bofill 2000); there was no difference in infant outcomes where described, and no difference in blood loss, uterine incision extension or other maternal outcomes.
Further research is required to clarify the safety of using an instrument (either forceps or vacuum) instead of fundal pressure at elective caesarean section. Given the relatively low rate of birth trauma and other adverse outcomes at elective caesarean section, a large number of patients would need to be recruited to adequately power such a study.
Summary of main results
This review aimed to address three clinical situations at 'difficult' caesarean section: 1) use of tocolysis compared with placebo, 2) reverse breech compared with head push from below for the deeply impacted fetal head, and 3) use of an instrument compared with manual delivery at elective caesarean section.
-
One trial addressing the use of tocolysis at caesarean section was included for review, unfortunately infant outcomes were not addressed in the study. There was no significant difference in maternal side‐effects between the tocolysis and placebo group, and other maternal outcomes were not reported, therefore limiting analysis.
-
At caesarean section for obstructed labour with a deeply impacted fetal head, reverse breech extraction appears to be significantly safer for mother and baby than head push from below. Maternal outcomes demonstrating significant improvement in the reverse breech extraction group include less endometritis, decreased blood loss and decreased rates of wound extension. For babies, there was no significant difference in rates of the primary outcome, birth trauma, but neonatal intensive care unit (NICU) and SCN admissions were decreased with reverse breech extraction. These results suggest that reverse breech extraction may be better for both mother and baby than head push from below.
-
Use of an instrument (either forceps or vacuum) at elective caesarean section compared with manual delivery did not significantly alter maternal or infant outcomes, however there was only trial suitable for inclusion that addressed these outcomes.
Overall completeness and applicability of evidence
Evidence for all three interventions is limited, mostly due to the small numbers of participants in the included trials. In particular, tocolysis and instrument for elective caesarean each had only one trial with data describing relevant outcomes, therefore most of the results were not statistically significant.
The four trials addressing delivery of the deeply impacted fetal head in the context of obstructed labour were based in Nigeria, Iran and Yemen, therefore due to differences in practice and populations, generalisability may be limited.
Quality of the evidence
The risk of bias in trials was variable, with some trials not adequately describing allocation or randomisation. Blinding of assessors was frequently not adequately described. Whilst blinding of the surgeon may not be possible when studying different surgical techniques, there was inadequate description of blinding of other assessors including anaesthetists, paediatricians and the woman.
Potential biases in the review process
We attempted to minimise bias in the review process by conducting a comprehensive search of the literature and ensuring that study assessment, data extraction and data entry was conducted by two review authors.
Agreements and disagreements with other studies or reviews
One systematic review was found that addressed the issue of reverse breech extraction compared with head push at caesarean after obstructed labour (Berhan 2014). The authors also noted reduction in uterine incision extension, blood loss, operation time and admission to NICU with pull compared with push techniques.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Tocolysis versus placebo, Outcome 1 Maternal side‐effects (not prespecified).

Comparison 2 Reverse breech versus head push, Outcome 1 Infant birth trauma.

Comparison 2 Reverse breech versus head push, Outcome 2 Extension of uterine incision.

Comparison 2 Reverse breech versus head push, Outcome 3 Blood loss > 500 mL.

Comparison 2 Reverse breech versus head push, Outcome 4 Mean blood loss.

Comparison 2 Reverse breech versus head push, Outcome 5 Blood transfusion.

Comparison 2 Reverse breech versus head push, Outcome 6 Endometritis.

Comparison 2 Reverse breech versus head push, Outcome 7 Wound infection.

Comparison 2 Reverse breech versus head push, Outcome 8 Admission to neonatal special care or intensive care unit.

Comparison 2 Reverse breech versus head push, Outcome 9 Early neonatal death rate.

Comparison 2 Reverse breech versus head push, Outcome 10 Mean fall in Hb (not prespecified).

Comparison 2 Reverse breech versus head push, Outcome 11 Operative time (min).

Comparison 2 Reverse breech versus head push, Outcome 12 Mean hospital stay (not prespecified).

Comparison 2 Reverse breech versus head push, Outcome 13 Average Apgar at 5 minutes (not prespecified).

Comparison 3 Elective instrument versus fundal pressure, Outcome 1 Infant birth trauma.

Comparison 3 Elective instrument versus fundal pressure, Outcome 2 Extension of uterine incision.

Comparison 3 Elective instrument versus fundal pressure, Outcome 3 Mean fall in Hb.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal side‐effects (not prespecified) Show forest plot | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infant birth trauma Show forest plot | 3 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.42, 5.73] |
| 2 Extension of uterine incision Show forest plot | 4 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.13, 0.40] |
| 3 Blood loss > 500 mL Show forest plot | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.18, 1.37] |
| 4 Mean blood loss Show forest plot | 3 | 298 | Mean Difference (IV, Random, 95% CI) | ‐294.92 [‐493.25, ‐96.59] |
| 5 Blood transfusion Show forest plot | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.66] |
| 6 Endometritis Show forest plot | 3 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.26, 1.05] |
| 7 Wound infection Show forest plot | 4 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.58, 1.59] |
| 8 Admission to neonatal special care or intensive care unit Show forest plot | 2 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.23, 1.22] |
| 9 Early neonatal death rate Show forest plot | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.23, 1.24] |
| 10 Mean fall in Hb (not prespecified) Show forest plot | 1 | 118 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.72, ‐0.16] |
| 11 Operative time (min) Show forest plot | 4 | 357 | Mean Difference (IV, Random, 95% CI) | ‐14.99 [‐27.67, ‐2.30] |
| 12 Mean hospital stay (not prespecified) Show forest plot | 3 | 285 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.75, 0.48] |
| 13 Average Apgar at 5 minutes (not prespecified) Show forest plot | 3 | 239 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.64, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infant birth trauma Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Extension of uterine incision Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.13, 3.73] |
| 3 Mean fall in Hb Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.53, 0.59] |

