氯胺酮作为阿片类药物的辅助剂治疗癌痛
摘要
研究背景
这是一项首次发表于2003年、上次更新于2012年的系统综述的更新。
氯胺酮是一种常用的麻醉剂,当阿片类药物单独使用或联合其他镇痛药物被证明无效时,亚麻醉剂量氯胺酮也被用于联合阿片类药物治疗难治性癌痛。氯胺酮已知会引起拟精神病的(包括致幻的)、泌尿系统的和肝脏的不良反应。
研究目的
确定氯胺酮辅助阿片类药物治疗成人难治性癌痛的有效性和不良反应。
检索策略
在本次更新中,我们检索至2016年12月前的MEDLINE(OVID)。我们还检索了至2017年1月前的CENTRAL(CRSO)、Embase(OVID)和两个临床试验注册中心。
纳入排除标准
本综述所考虑纳入的干预措施是在现有阿片类药物治疗的基础上,加入氯胺酮。二者皆无任何给药途径和给药剂量的限制。对照组为安慰剂或阳性对照。我们纳入完成试验的组内受试者至少为10人的研究。
资料收集与分析
两位综述作者独立评估了检索结果,并评估了“偏倚风险”的级别。我们旨在提取关于患者报告的疼痛强度、研究期间阿片类药物总用量、紧急用药、不良事件、患者满意/偏好度、功能和痛苦的资料。我们还评估了试验中退出(脱落)的受试者。我们使用GRADE(Grading of Recommendations Assessment, Development and Evaluation)评估了证据强度的级别。
主要结果
通过更新检索我们发现了一项新研究(185名受试者),并将其纳入系统评价。我们在此次更新中共纳入了三项研究。
原始系统综述中纳入的两项研究均为采用交叉设计的小型研究,分别涉及20名和10名受试者。涉及20名受试者的研究评估了与单独鞘内注射吗啡相比,在鞘内注射吗啡的同时联合鞘内注射氯胺酮的情况。另一项涉及10名受试者的研究则评估了与安慰剂相比,两种不同剂量下静脉推注氯胺酮对正在进行的吗啡治疗的影响。两项研究都表明,阿片类药物在加入氯胺酮联用后治疗难治性癌痛可降低疼痛强度和减少吗啡用量。通过更新检索而发现的新研究采用了平行设计,涉及185名受试者。这项安慰剂对照研究评估了使用不同阿片类药物的受试者皮下快速滴定氯胺酮至最高剂量(500mg)的情况。各组间患者报告疼痛强度无差异。
由于临床异质性,同时合并三项试验的数据是不合适的。
评估鞘内给药的研究未报告有关氯胺酮的不良反应。而在使用静脉推注给药的研究中,氯胺酮导致10名受试者中4名产生幻觉。在快速剂量递增/皮下高剂量注射氯胺酮研究中,与安慰剂相比,氯胺酮的不良事件发生率几乎是其两倍,最常见的不良事件是施针部位的刺激和认知障碍。该试验还报告了两项与氯胺酮有关的严重不良事件(缓慢性心律失常和心脏骤停)。
对于以上三项研究,总体上存在不确定偏倚风险。由于所有比较中受试者数量较少带来的不准确性和研究本身限制,我们通过GRADE判断证据的质量非常低。
作者结论
目前的证据不足以评估氯胺酮作为阿片类药辅助剂缓解难治性癌痛的益处和危害。由于证据的质量非常低,这意味着它不能对可能效应提供任何指导,并且效应差异很大的可能性很高。氯胺酮快速递增至高剂量(500mg)似乎未表现出临床益处,还可能产生严重不良事件。需要更多随机对照试验(RCTs)来评估当前使用的特定低剂量氯胺酮临床方案。
PICOs
简语概要
阿片类药物联合氯胺酮治疗阿片耐受性癌痛
概要
用低剂量氯胺酮联合强镇痛剂(如吗啡)以缓解癌痛的利弊尚未确定。高剂量氯胺酮似乎未表现出明显疗效,且可能伴有严重的副作用。
研究背景
这项系统综述首次发表于2003年,上次更新于2012年。
吗啡类药物(阿片类)经常用于治疗中重度癌痛,但在某些情况下,这些药物是无效的。氯胺酮作为一种麻醉剂,在姑息治疗中,当单独使用阿片类药物无效时,低剂量氯胺酮可提高镇痛效果。
研究特征
在2016年12月和2017年1月,我们检索了氯胺酮联合吗啡类药物治疗癌痛的相关临床试验。
我们发现了一项新的研究,以及原来系统综述中包含的两项研究。这三项研究存在相当大的异质性,主要来源于不同剂量的氯胺酮、不同的给药途径和不同的治疗持续时间,因此无法将这些研究结果进行合并。
关键结果
两项小样本研究表明,氯胺酮联合吗啡可缓解癌痛和减少吗啡用量。第三项研究表明,高剂量氯胺酮联合不同阿片类药物并未表现出任何临床获益。提高氯胺酮剂量会引起一些副作用,例如幻觉。这项研究研究还报告了两例可能与高剂量氯胺酮相关的严重不良事件。尽管三项研究中,两项都报告了疼痛减轻,但这可能是由于研究规模小而造成的。
证据质量
我们将证据质量评估为四个级别:极低,低,中等或高。极低质量的证据意味着我们对研究结果非常不确定。高质量的证据意味着我们对研究结果非常有把握。这些研究的证据质量极低。一些研究的设计存在问题,并且现有研究数据不足以回答部分我们所提出的问题。
Authors' conclusions
Background
This is an update of a previously published review in the Cochrane Database of Systematic Reviews (2003, Issue 1 (Bell 2003)), and updated in 2012 (Bell 2012b) on ketamine as an adjuvant to opioids for cancer pain.
Description of the condition
Studies report that moderate to severe pain is common in patients with advanced cancer (Are 2017). Cancer pain that is refractory to standard treatment occurs in 10% to 20% of these patients (Afsharimani 2015). Cancer pain is often of mixed aetiology and may have nociceptive, neuropathic and inflammatory components. Neuropathic pain which results from tumour infiltration in nerve plexi and damage of nerve tissue can be especially difficult to treat (Fallon 2013). Opioids (for example, morphine, fentanyl, hydromorphone, oxycodone, codeine) are frequently prescribed for the relief of moderate and severe cancer pain. However, not all cancer pain is sufficiently relieved by opioids alone.
Description of the intervention
The usual indication for using ketamine as an adjuvant to opioid in cancer pain is for pain which is unresponsive to opioids and adjuvant analgesics, for example in the case of refractory neuropathic pain or opioid tolerance. Clinical reports indicate that, when added to opioids, low subanaesthetic doses of ketamine may give improved analgesia (Sosnowski 1993; Fine 1999; Bell 1999). The practice of using ketamine as an adjuvant to opioids in the treatment of cancer pain that does not respond to opioids alone, or to opioids in combination with adjuvant analgesic drugs, is discussed in several pain and palliative care textbooks (Stannard 2005; Twycross 2009; Cherny 2015). Ketamine is not licensed for this purpose and this is an update of the first systematic review undertaken to establish the evidence base for this practice.
Ketamine hydrochloride has been used as a general anaesthetic agent for over 30 years, and is commonly given intravenously or intramuscularly for surgical anaesthesia (Fisher 2000). Ketamine causes dissociative anaesthesia and also has analgesic effects (Grahame‐Smith 2002); because it increases sympathetic nervous system activity, it is a useful anaesthetic for high‐risk patients who require a high degree of sympathetic activity to maintain cardiovascular function. However, the benefits are tempered by the high incidence of hallucinations and other transient psychomimetic sequelae when ketamine is used for anaesthesia in adults (BNF 2012). More recently, urological toxicity and hepatic toxicity have been described as adverse effects of ketamine (Bell 2012a).
In the 1980s ketamine was discovered to have N‐methyl‐D‐aspartate (NMDA) receptor antagonist properties and acts by blocking excitatory glutamate receptors in the central nervous system. There is an association between nociceptive activity involving the NMDA receptor and hyperalgesia/allodynia, and reduced opioid sensitivity (Dickenson 1994). The NMDA receptor plays a role in the development of opioid tolerance (Trujillo 1991; Mao 1995; Mayer 1995). Currently, there is much focus on ketamine for the treatment of major depression. A recent paper reports that ketamine metabolites exert antidepressant actions independent of NMDA receptor inhibition (Zanos 2016).
Evidence from experimental animal models, human volunteer studies and small clinical trials indicates that subanaesthetic doses of ketamine alleviate various chronic and neuropathic pain syndromes (Fisher 2000). Ketamine has anti‐inflammatory effects and may have an effect in inflammatory pain (Dale 2012; Sawynok 2014). However, the clinical use of ketamine at subanaesthetic dose levels has also been restricted by unpleasant adverse effects, typically sedation, nausea, disagreeable psychological disturbances or hallucinations (Willetts 1990).
Racemic ketamine is a mixture of two stereoisomers: R(‐) and S(+). More recently, S‐ketamine has been introduced. S(+) ketamine produces longer hypnosis than the (‐) isomer, and causes a greater rise in blood pressure and heart rate, less locomotor activity, and a shorter recovery time, and it is postulated to have twice the analgesic efficacy of racemic ketamine. S(+) ketamine is also thought to have a safer adverse effect profile (Grahame‐Smith 2002). The majority of published clinical studies in postoperative and chronic pain have used racemic ketamine. For a review on the pharmacokinetics of ketamine see Peltoniemi 2016. The oral bioavailability of ketamine is low and the drug undergoes fast cytochrome P450 (CYP) mediated N‐demethylation to norketamine. Approximately 80% of ketamine undergoes N‐demethylation to norketamine by CYP3A and CYP2B6 enzymes (Kharasch 1992; Yanagihara 2001; Hijazi 2002), with a smaller amount being metabolised to 4‐and 6‐hydroxyketamines (Woolf 1987). Compared to ketamine, norketamine is an approximately three to five times weaker NMDA receptor antagonist (Leung 1986; Ebert 1997). Being metabolised by CYP3A enzymes, ketamine may have significant interactions with opioids and other drugs. Studies in rodents indicate important interactions between ketamine and opioids. Edwards 2002 reported that in mice, distribution of ketamine into the brain was increased by low plasma concentrations of alfentanil. Recently Lilius 2015 found that ketamine co‐administration attenuates morphine tolerance and leads to increased brain concentrations of both drugs in the rat.
Ketamine has multiple routes of administration and is commonly given as an adjuvant to pre‐existing opioid treatment. A number of systematic reviews report that ketamine is effective in acute postoperative pain and reduces morphine requirements (Bell 2006; Laskowski 2011; Assouline 2016).
How the intervention might work
By blocking activity at the NMDA receptor, ketamine may reduce neuropathic‐related cancer pain. Blocking NMDA receptor activity may reduce opioid tolerance thus increasing/restoring the analgesic effect of opioid. Ketamine has anti‐inflammatory effects and may be beneficial in inflammatory cancer pain.
Why it is important to do this review
This is an update of a Cochrane review first published in 2003, and previously updated in 2012. Ketamine is routinely used in the palliative care setting for the treatment of refractory cancer pain. Earlier versions of this review found limited and heterogenous data, and there was insufficient evidence to be able to make any conclusions. In recent years the standards used to assess evidence in pain trials have changed substantially, for example there is now particular attention being paid to participant withdrawal from trials, and statistical imputation following withdrawal, which can substantially alter estimates of efficacy. The most important change is the move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%) (PaPaS 2012). This update assesses the current evidence using the new criteria for what constitutes reliable evidence in pain trials.
Objectives
To determine the effectiveness and adverse effects of ketamine as an adjuvant to opioids for refractory cancer pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomised controlled trials (RCTs)
-
Double‐blind studies
-
Placebo‐ or active‐controlled trials, both with or without cross‐over, in in‐patient and out‐patient settings
We excluded studies with a group size of fewer than 10 participants who completed the study.
Types of participants
The population addressed by the review included adult patients (aged 18 or over) with cancer and pain despite being currently treated by an opioid agonist (e.g. morphine, fentanyl, oxycodone), in any dose and by any route. We excluded studies including patients who were on an established NMDA‐receptor antagonist treatment before the study began. We did not consider volunteer studies.
Types of interventions
The intervention considered by this review was the addition of ketamine, given by any route of administration, in any dose, to pre‐existing opioid treatment given by any route and in any dose.
Types of outcome measures
Primary outcomes
The primary outcome measure was patient‐reported pain intensity (e.g. visual analogue scales (VAS) and verbal rating scales).
Secondary outcomes
Secondary outcome measures were:
-
total opioid consumption over the study period;
-
rescue medication;
-
adverse events;
-
measures of patient satisfaction/ preference;
-
function;
-
distress.
Search methods for identification of studies
Electronic searches
We searched the following databases for this update:
-
CENTRAL (CRSO) April 2012 to January 2017;
-
MEDLINE (OVID) May 2012 to December 2016;
-
Embase (OVID) May 2012 to 2017 week 1.
Please see Appendix 1 and Appendix 2 for the searches conducted for the original review in 2003. For the searches conducted for the update in 2012 please see Appendix 3, Appendix 4 and Appendix 5. For searches conducted for the current update please see Appendix 6.
Searching other resources
We also searched two clinical trial registers (https://clinicaltrials.gov/) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) to identify additional published or unpublished data.
Language
We did not restrict searches or inclusion by language.
Data collection and analysis
Selection of studies
Two review authors (RB and EK) independently assessed the titles and abstracts from each of the electronic databases searched for relevance. We retrieved potentially relevant trial reports in full and three review authors (RB, CE, EK) assessed them for inclusion in the review.
Data extraction and management
We designed a data extraction form, and two review authors (RFB, EK) independently collected the following data items if available.
-
Publication details.
-
Patient population, number of participants, age, condition.
-
Description of the intervention(s) and control.
-
Outcomes: pain intensity, total opioid consumption, rescue medication, measures of patient satisfaction/preference, distress and function.
-
Adverse events (major and minor).
-
Quality (evaluated using the Oxford Quality Scale (Jadad 1996)).
-
Validity (evaluated using the Oxford Pain Validity Scale (OPVS) (Smith 2000)).
This information is recorded in the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors (RFB, EK) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion with a third author (CE). We completed 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan (RevMan 2014).
We assessed the following for each included study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We assessed the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, such as identical tablets matched in appearance and smell, or a double‐dummy technique); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved).
-
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study has a clear statement that outcome assessors were unaware of treatment allocation, and ideally describes how this was achieved); unclear risk of bias (study states that outcome assessors were blind to treatment allocation but lacks a clear statement on how it was achieved).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants did not complete the study or used ‘baseline observation carried forward’ analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
-
Selective reporting (reporting bias). We assessed the risk of reporting bias as: low risk of bias (all intended outcomes reported); unclear risk of bias (any anomaly in reporting, such as participants contributing more than one set of data, or some outcomes not participant‐reported); high risk of bias (prespecified outcome of interest not reported).
-
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
For dichotomous outcomes we planned to calculate the risk ratio (RR) and the corresponding 95% confidence interval (CI) and P value. We planned to calculate the number needed to treat for an additional beneficial outcome (NNTB)/number needed to treat for an additional harmful outcome (NNTH) as the reciprocal of the absolute risk difference (McQuay 1998). For continuous outcomes, we planned to calculate the mean difference (MD) and its corresponding 95% CI when means and standard deviations (SD) were available. If such information was unavailable we planned to use the methods described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions to calculate standardised mean differences (SMD), from for example, F ratios, t values, Chi2 values and correlation coefficients (Higgins 2011). In cases where continuous measures were used to assess the same outcomes using different scales, we would have pooled these data using Hedges' g to estimate the SMD. When effect sizes could not be pooled, we planned to report study level effects narratively.
Unit of analysis issues
We only included studies that randomised the individual participant.
Dealing with missing data
We assessed missing data in the included studies. Where possible, we investigated and reported the reasons and numbers of those dropping out of each included study. For dichotomous outcomes, we planned to perform an intention‐to‐treat (ITT) analysis. If there was missing participant information, we recorded this and commented in the individual study's 'Risk of bias' table. Participants with missing data would be assigned to a 'zero improvement category'.
We paid particular attention to methods used for imputation of missing data due to withdrawals for adverse events and lack of efficacy. Where data were missing for substantial numbers of participants (greater than 10%), we would have rated the study as high risk of bias.
Assessment of heterogeneity
We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. Statistical heterogeneity would have been assessed visually (L'Abbé 1987).
Assessment of reporting biases
We looked for the original trial protocols of the included studies and compared the results to these when they were found. When no protocol was available, we compared the reported outcomes against the Methods section of the paper to look for selective reporting of outcomes.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result for pain clinically irrelevant (usually taken to mean an NNTB of 10 or higher) (Moore 2008). In the event, there were insufficient data for statistical analysis.
Data synthesis
Quality of the evidence
We planned to combine data in a series of meta‐analyses on both primary and secondary outcomes.
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to rank the quality of the evidence using the GRADE profiler Guideline Development Tool software (GRADEpro GDT 2015), and the guidelines provided in Chapter 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence:
-
high: we are very confident that the true effect lies close to that of the estimate of the effect;
-
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
-
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
-
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We decreased the grade rating by one (‐ 1) or two (‐ 2) if we identified:
-
serious (‐ 1) or very serious (‐ 2) limitation to study quality;
-
important inconsistency (‐ 1);
-
some (‐ 1) or major (‐ 2) uncertainty about directness;
-
serious (‐1) or very serious (‐2) imprecise or sparse data;
-
high probability of reporting bias (‐ 1).
'Summary of findings' table
We planned to include a 'Summary of findings' table as set out in the PaPaS author guide PaPaS 2012 and recommended in the Cochrane Handbook (Chapter 11, Higgins 2011) to present the main findings in a transparent and simple tabular format. However, we judged that a 'Summary of findings' table with only three very different studies would be unhelpful.
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses.
Sensitivity analysis
Had there been sufficient data available, we would have examined the robustness of meta‐analyses by conducting a sensitivity analysis.
Results
Description of studies
The original review included one study which compared intrathecal ketamine + intrathecal morphine with intrathecal morphine alone (Yang 1996), and one study which compared intravenous ketamine bolus with intravenous bolus of placebo as a supplement to ongoing morphine therapy (Mercadante 2000). We found one new study for this update which compared subcutaneous infusion of ketamine at three dose levels (100 mg, 300 mg, or 500 mg) with placebo in participants with ongoing treatment with opioids (Hardy 2012).
Results of the search
The updated searches of the three databases (see Electronic searches) retrieved 271 records. Our searches of the trials registers did not identify further studies. Our screening of the reference lists of the included publications did not reveal additional RCTs. We therefore had a total of 271 records.
Once duplicates had been removed, we had a total of 216 records. We excluded 215 records based on titles and abstracts. We obtained the full text of the remaining record and this study was included (Hardy 2012). For a further description of our screening process, see the study flow diagram (Figure 1).

Study flow diagram.
We identified three ongoing studies and added these records to Characteristics of studies awaiting classification. Two additional trials with status 'completed' do not appear to have been published and possibly represent double registration of the same trial. These are described under Characteristics of ongoing studies.
Protocols for the studies by Yang 1996 and Mercadante 2000 were not available. The protocol for the study by Hardy 2012 was retrieved.
Included studies
Study design
Two included studies (Yang 1996; Mercadante 2000) had a cross‐over design. The trial conducted in Taiwan by Yang 1996 compared ketamine and morphine with morphine alone. The time period over which the intervention was assessed was not stated in the trial report, but there is an implication that the study was conducted over a period of days. (Attempts to contact the author to confirm the trial duration were not successful). The trial conducted in Italy by Mercadante 2000 was a placebo‐controlled trial and was conducted over a three‐hour period. The most recent trial Hardy 2012 was a multisite, dose‐escalation, double‐blind, randomised, placebo‐controlled parallel group study with a duration of five days.
Study population
Yang 1996: Twenty hospitalised participants (10 men and 10 women) aged 22 to 69 years with cancer pain of variable severity treated with opioids. The primary cancer sites were stomach, cervix, liver, lung, colon, pancreas.
Mercadante 2000: Ten participants (seven men and three women) aged 21 to 69 years who had pain unrelieved by their dose of morphine, and a Karnofsky status of 50 or more. The primary cancer sites were: bladder, rectum, lung, histiocytoma and uterus. In this study, the pain was classified as being "neuropathic" or having a "neuropathic component".
Hardy 2012: Hospitalised palliative care participants aged 18 or older, with refractory chronic nociceptive or neuropathic pain secondary to cancer or its treatment (Brief Pain Inventory (BPI) average pain score ≥ 3 despite ongoing treatment with opioids and co‐analgesics at predefined dose levels). One hundred and eighty participants were randomly assigned, two were deleted from the analysis, 93 were allocated to ketamine and 92 to placebo. Ninety‐one received ketamine and 90 received placebo. One hundred and forty‐nine were defined as having completed the trial, although only 39 participants in the ketamine arm and 35 participants in the placebo arm received either ketamine or placebo for the full five‐day period.
Intervention
Yang 1996 assessed intrathecal ketamine 1.0 mg twice daily as adjuvant to intrathecal morphine, compared with intrathecal morphine alone. The morphine dose was titrated until participants' pain relief had been stable for 48 hours, then the participants were randomly crossed over (no washout period) to morphine plus ketamine or continued on morphine (control), administered intrathecally twice a day.
Mercadante 2000 assessed two doses of ketamine (0.25 mg/kg and 0.5 mg/kg) administered intravenously as a bolus as adjuvant to ongoing morphine therapy, compared with saline. Patients were randomly assigned to receive in turn either 0.25 mg/kg or 0.5 mg/kg ketamine or saline, with a two‐day washout period between each intervention/control.
Hardy 2012 assessed either placebo (normal saline) or ketamine at three dose levels (100 mg, 300 mg, or 500 mg) as a subcutaneous infusion in a five‐day schedule, starting at the first dose level (100 mg/24 hours), as a supplement to ongoing opioid therapy. If 80% of the study drug had been delivered, and average pain improved by ≥ 2 BPI units, with no more than four doses of breakthrough medication, the dose remained the same. If not, the dose was increased to the next level.
Morphine was the only opioid participants received in the studies by Yang 1996 and Mercadante 2000. The route of administration of morphine in the study by Yang 1996 was intrathecal, while morphine was given by varied routes of administration (oral, intravenous or subcutaneous) in the trial by Mercadante 2000. The opioid was not standardised in the study by Hardy 2012 where participants used different opioids (morphine, oxycodone, hydromorphone, methadone, fentanyl, sufentanil, alfentanil) given by different routes of administration (oral, transdermal or parenteral).
It is assumed that racemic ketamine was used in all three studies.
Rescue medication
Yang 1996: In this trial a rescue dose of 5 mg morphine was administered intramuscularly as needed. Mercadante 2000 does not report the use of rescue medication. Hardy 2012 states that the participants had access to breakthrough analgesia and record the number of doses, but do not describe the rescue medication.
Outcomes
Yang 1996 measured patient‐reported pain intensity (zero to 10 numerical, 10 worst pain imaginable); pain frequency (four‐point verbal ordinal scale), group morphine dose, total titrated intrathecal morphine, total rescue medication, frequency of intrathecal titration. Mercadante 2000 measured patient‐reported pain intensity (zero to 10 numerical scale) at 30‐, 60‐, 90‐, 120‐, and 180‐minute intervals; and adverse events. Hardy 2012 defined the primary outcome as a positive response defined as a "clinically relevant improvement in pain" at the end of the ive‐day study period. A "clinically relevant improvement in pain" was defined as a reduction in BPI average pain score by ≥ 2 points from baseline in the absence of more than four breakthrough doses of analgesia over the previous 24 hours. Secondary outcomes included pain assessments at days two to five and adverse events.
See Characteristics of included studies tables.
Excluded studies
For this update we identified one eligible study (Hardy 2012), which was included. Overall, we excluded five studies. (For studies previously excluded see Characteristics of excluded studies).
Risk of bias in included studies
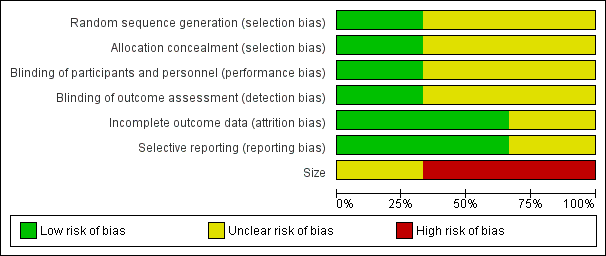
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
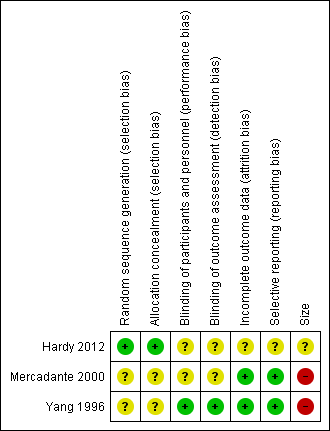
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The studies by Yang 1996 and Mercadante 2000 stated that patients were randomised to treatment and control groups, but in neither trial was the process of randomisation described (unclear risk of bias). In the trial by Hardy 2012 each site pharmacy used randomisation tables from an independent central registry. Stratification was by pain type (neuropathic or nociceptive) and randomisation was double‐blinded, allocated by blocks of four in a 1:1 ratio for each strata by site. We judged this study to be at low risk of bias.
Blinding
Performance bias
Participants, investigators and nurses were blinded using a double‐dummy technique in Yang 1996 and the drugs were prepared in identical syringes by a person not involved in the study and administered in the same volume in Mercadante 2000. The Hardy 2012 trial is described as double‐blinded. The blinding procedure was not described in the final paper, but was described in the study protocol ("All syringes will look identical in volume and colour").
There were no specific procedures to check for performance bias in any of the three included trials. In Yang 1996, one participant in the morphine phase and no participants in the combined morphine and ketamine phase reported psychotoxicity (hallucinations), whereas in Mercadante 2000 ketamine caused hallucinations in four of 10 participants, so the participants may have been able to tell which drug they had received. The study by Hardy 2012 involved rapid dose escalation of ketamine to high doses and blinding could have been compromised due to adverse effects from ketamine. We judged the study by Yang 1996 at low risk of performance bias and the studies by Mercadante 2000 and Hardy 2012 at unclear risk of performance bias.
Detection bias
We judged the study by Yang 1996 at low risk of detection bias and the studies by Mercadante 2000 and Hardy 2012 at unclear risk of detection bias.
Incomplete outcome data
All participants were accounted for in the trials by Yang 1996 and Mercadante 2000. Hardy 2012 reported an ITT analysis, but imputed missing data using last observation carried forward (LOCF). We judged this study at unclear risk of bias.
Selective reporting
There were no problems of selective reporting detected in the trials by Mercadante 2000 and Yang 1996. In the trial by Hardy 2012, assessing "the effect of ketamine on total opioid dose" was mentioned in the protocol as a "secondary objective", but was not reported. We judged this study at unclear risk of bias.
Other potential sources of bias
Size
The cross‐over studies by Yang 1996 and Mercadante 2000 had respectively 20 and 10 participants (fewer than 50 participants per treatment arm). We judged these trials at high risk of bias. The parallel group trial by Hardy 2012 had 185 participants (between 50 and 199 participants per treatment arm) and we judged it at unclear risk of bias.
Oxford quality assessment
Quality scores derived using the Oxford quality scale (Jadad 1996) were three for both Mercadante 2000 and Yang 1996, and four for Hardy 2012 out of a possible maximum of five points.
Using the method derived by Smith 2000, the three included studies (Yang 1996; Mercadante 2000; Hardy 2012) scored 13, 12 and 12, respectively on the Oxford Pain Validity Scale, a zero to 16‐point validity scale.
Effects of interventions
It was not possible to perform a quantitative meta‐analysis because of the small number of participants in two of the trials, lack of extractable data and general heterogeneity of the data. A description of the results from the three included trials is given below. None of the trials provided data on pain relief, patient satisfaction/preference, function or distress. For all outcomes we judged the quality of the evidence to be very low. We downgraded one level for serious risk of bias and two levels for very serious imprecision due to very small number of participants in two of the comparisons and small number of participants in the third comparison.
Patient‐reported pain intensity
Ketamine 1.0 mg twice daily (intrathecal)
One study (Yang 1996) assessed adjuvant ketamine 1.0 mg administered intrathecally. The trial duration is not specified, but it was conducted over several days. Pain intensity on a numerical rating scale zero to 10 was reduced from 7.95 ± 0.25 to 2.45 ± 0.17 after adjuvant treatment with ketamine.
Ketamine 0.25 mg/kg (intravenous)
One trial (Mercadante 2000) assessed pain intensity over three hours. Mean pain intensity scores showed a reduction in pain intensity after 30 minutes compared with saline solution; after 60 minutes the analgesic effect of ketamine began to diminish but continued to have an effect for a period of three hours.
Ketamine 0.5 mg/kg (intravenous)
One trial (Mercadante 2000) assessed pain intensity over three hours. Mean pain intensity scores showed a significant reduction after 30 minutes compared with saline solution. The analgesic effect of ketamine continued throughout the three‐hour period.
Ketamine dose escalation 100 mg, 300 mg, 500 mg (subcutaneous)
One study (Hardy 2012) assessed average BPI pain score on day six, following dose escalation of ketamine subcutaneous infusion in a five‐day schedule, starting at the first dose level (100 mg/24 hours). If 80% of the study drug had been delivered, and average pain improved by ≥ 2 BPI units, with no more than four breakthrough doses, the dose remained the same. If not, the dose was increased to the next level. There was no significant difference in patient‐reported pain intensity between the placebo and ketamine arms.
Total opioid consumption
Ketamine 1.0 mg twice daily (intrathecal)
Yang 1996 reported that on the last day of the morphine phase, participants required intrathecal morphine 0.38 mg/day ± 0.04 mg/day. On the last day of the combined ketamine and morphine (K+M) phase, intrathecal morphine requirements had decreased to 0.17 mg/day ± 0.02 mg/day. The total titrated dose of intrathecal morphine, total dose of intramuscular rescue morphine during the K+M phase was less than in the morphine phase.
Ketamine 0.25 mg/kg and 0.5 mg/ kg (intravenous)
Mercadante 2000 did not provide information on this outcome.
Ketamine dose escalation 100 mg, 300 mg, 500 mg (subcutaneous)
Hardy 2012 did not provide information on this outcome.
Rescue medication
Ketamine 1.0 mg twice daily (intrathecal)
Yang 1996 reported that the total dose of rescue morphine during the K+M phase was less than the morphine phase.
Ketamine 0.25 mg/kg and 0.5 mg/kg (intravenous)
Mercadante 2000 did not report the use of rescue medication, but stated in the text that the administration of ketamine allowed for "a reduction of opioid doses".
Ketamine dose escalation 100 mg, 300 mg, 500 mg (subcutaneous)
Hardy 2012 reported that there was no significant group difference in the median number of breakthrough analgesic doses given during the study.
Adverse events
Psychomimetic adverse events
Ketamine 1.0 mg twice daily (intrathecal)
One participant in the morphine only arm of the Yang 1996 study reported hallucinations. There were none reported in the ketamine arm.
Ketamine 0.25 mg/kg and 0.5 mg/kg (intravenous)
In the study by Mercadante 2000, ketamine injection produced hallucinations in four participants: three experienced hallucinations whilst receiving 0.25 mg/kg and 0.5 mg/kg ketamine, and one further participant experienced hallucinations when receiving ketamine 0.5 mg/kg. All were treated with diazepam 1 mg. In addition, two participants experienced light flashes, a 'buzzing' feeling in the head, and sensation of insobriety. Diazepam resolved these symptoms. No significant changes in the Mini‐Mental State Examination (MMSE) were observed.
Ketamine dose escalation 100 mg, 300 mg, 500 mg (subcutaneous)
In the study by Hardy 2012, there was almost twice the incidence of adverse events in the ketamine arm compared with the placebo arm on day one and throughout the study. Psychomimetic adverse events were assessed daily using the Clinician‐Administered Dissociative States Scale (CADSS). CADSS scores were not reported but 17 cognitive disturbance events with grading worse than at baseline were recorded in the ketamine group and eight such events were recorded in the placebo group. Thirteen confusion events with grading worse than baseline were recorded in the ketamine group and nine such events were recorded in the placebo group. Psychomimetic toxicity was treated with haloperidol or midazolam at specified doses.
Other adverse events
Ketamine 1.0 mg twice daily (intrathecal)
On direct questioning, participants reported a number of adverse effects during the trial conducted by Yang 1996:
-
pruritis;
-
constipation;
-
urinary retention;
-
difficulty in urinating;
-
nausea and vomiting;
-
hallucinations;
-
respiratory depression.
However, these adverse events could not be attributed specifically to the study treatments as some were present prior to the commencement of the study.
Ketamine 0.25 mg/kg and 0.5 mg/kg (intravenous)
Information on the following adverse events were sought in the trial conducted by Mercadante 2000:
-
drowsiness;
-
nausea and vomiting;
-
dry mouth.
These adverse events were assessed on a scale from zero to three, where zero was 'not at all', and three was 'awful'. Participants treated with 0.25 mg/kg and 0.5 mg/kg ketamine reported increased drowsiness.
Ketamine dose escalation 100 mg, 300 mg, 500 mg (subcutaneous)
In the trial by Hardy 2012, adverse events were graded according to the National Institutes of Health Common Terminology Criteria for Adverse Events, version 3.0 (Cancer Therapy Evaluation Program Version 3). There was almost twice the incidence of adverse events in the ketamine arm compared with the placebo arm at the end of day one and throughout the study. The authors reported 31 episodes of injection site reactions, which were reported as nearly three times more likely than the placebo group. There were relatively few adverse events higher than grade three in severity (14 for ketamine; 16 for placebo). Seven serious adverse events were reported, two of which (bradyarrhythmia and cardiac arrest, both in participants receiving ketamine) were thought to be possibly related to the study drug.
Both the trial by Yang 1996 and the trial by Mercadante 2000 reported that the adverse events of ketamine were not serious.
Study withdrawals and dropouts
No study withdrawals or dropouts were reported in either trial by Yang 1996 or Mercadante 2000. In the study by Hardy 2012, 39 participants in the ketamine group and 55 participants in the placebo group withdrew from the trial. Sixteen participants in each group discontinued the study due to clinical deterioration, patient/ clinical request or change in therapy. Nineteen participants in the ketamine group and 37 participants in the placebo group discontinued due to treatment failure. Of these, 17 in the ketamine group and two in the placebo group discontinued due to toxicity.
Discussion
Summary of main results
There are three included studies in the current version of the review. Two small early studies Yang 1996 and Mercadante 2000 report reduction in pain intensity and reduction in morphine requirements. These two studies are of high risk of bias due to small sample size, and incomplete reporting. The new study from Hardy 2012 has unclear risk of bias due to size and incomplete reporting. Hardy 2012 reports no difference in their primary outcomes between groups. Overall, we cannot provide a reliable indication of the likely effect of ketamine, at any dose, as an adjuvant to opioids in cancer pain. Adverse events such as hallucinations and cognitive disturbance were reported for higher doses of ketamine. Two serious adverse events (bradyarrhythmia and cardiac arrest) reported in the trial examining rapid titration of ketamine to high dose were thought to be possibly related to the study drug.
There is large body of evidence demonstrating the efficacy of ketamine in acute postoperative pain. While this evidence cannot be directly extrapolated to other patient groups, it is important to note the complex pathophysiology of cancer pain and the difficulties of conducting clinical trials in palliative care. The authors of the study by Hardy 2012 should be commended for recruiting an impressive number of participants. The data from this trial could potentially provide further information on clinical questions such as whether ketamine has beneficial interactions with specific opioids, since both preclinical and clinical research have suggested that this is the case when ketamine is used as an adjuvant to morphine (Lilius 2015). To date, the fact that the participants in the Hardy 2012 trial used different opioids has not been addressed and the subgroup analyses based on type and dose of opioid have not been reported. We contacted the authors with a request for access to individual patient data, however the request was declined because such analyses were not stated in our original protocol for this review.
It is worth noting, also, that the dose escalation in the Hardy 2012 trial was very rapid, considering the pharmacokinetics of ketamine which has a short α half‐life (two to four minutes) and longer β half‐life (two to four hours) in humans (Peltoniemi 2016), and where steady state is achieved after five elimination half‐lives. The metabolite norketamine, which is also active has a much longer half‐life than ketamine, and very ill cancer patients would be likely to have a much poorer elimination than young healthy volunteers. Ketamine doses in the Hardy 2012 trial were higher than those used in the majority of ketamine regimens described in the literature. It is interesting that ketamine was found to have better effect in patients with high pain scores, however this was not mentioned in the abstract. The same finding is reported in a systematic review of 70 randomised controlled trials of intravenous ketamine for postoperative analgesia (Laskowski 2011). In clinical practice, ketamine is usually considered to be a third‐line drug which is reserved for patients with high pain intensity scores, despite adequate ongoing opioid therapy and co‐analgesics. Hardy 2012 included patients with a BPI pain intensity score of three at baseline and also patients being treated with comparatively low doses of opioid.
All three trials used pain intensity scores as the primary outcome. Percentage pain relief may be a more useful and reliable outcome measure (Dworkin 2008).
Other reports considered in the original review (2003)
Because of the paucity of data available from RCTs in the original review, we considered information presented in case studies and case series reports of ketamine for chronic cancer pain. In addition to the two RCTs included, the original review (2003) identified 32 case reports or open‐label, uncontrolled trials describing improvement of opioid analgesia with ketamine. We did not consider case studies and reports in the updated review.
Whilst the design of these studies and the issue of publication of positive outcomes preclude the inclusion of any data from these reports in this systematic review, the studies were discussed in the original review and are reported in this update in order to provide a more comprehensive review of the literature on this topic. Case reports cannot provide evidence for efficacy but may provide valuable information on adverse effects. They are, by definition, all of low quality.
The 32 reports described the use of ketamine to treat refractory cancer pain, frequently described as neuropathic pain. The total number of participants treated with ketamine in these reports was 246. The route of ketamine administration included oral, intramuscular bolus, subcutaneous bolus and infusion, intravenous bolus and infusion, epidural bolus, and intrathecal infusion. Ketamine doses ranged from 1 mg/kg/day subcutaneous infusion to 600 mg/day intravenously and 67.2 mg/day intrathecally. Treatment duration ranged from four hours to one year. Treatment was in most cases adjuvant to opioid and other drugs. Twenty‐eight reports described improved analgesia with ketamine. Where ketamine was administered as an adjuvant to opioids, the most commonly used opioid was morphine, but in some cases ketamine was given as an adjuvant to fentanyl (Ventura 1993; Bell 1999), hydromorphone (Fine 1999) or diamorphine (Garry 1996), or combinations of these. Ketamine was also used as sole analgesic in three reports (Parada 1971; Whizar‐Lugo 1987; Oshima 1990). Sixteen reports described dramatic relief of refractory cancer pain with ketamine: "complete cessation of pain" (Ventura 1993); "complete relief of pain" (Tarumi 2000); "disappearance of pain" (Parada 1971; Garry 1996); "no pain" (Fine 1999); "pain free" (Mitchell 1999),; "mostly pain free" (Lloyd‐Williams 2000); dramatic reduction in visual analogue scales (VAS) scores including VAS 100 reduced to zero (Bell 1999); average VAS score 8.3 reduced to one (Kanamaru 1990); average VAS score reduced from 5.9 +/‐ 2.0 to 0.3 +/‐ 0.8 (Ogawa 1994); VAS 7/10 reduced to 1/10 (Wood 1997); reduction of VAS 7/10 to below 2/10 (Lossignol 1999); "dramatic drop in VAS" (Lossignol 1992); "remarkable analgesia" (Fukuida 1981); "excellent analgesia" (Sosnowski 1993; Mercadante 1995).
The most commonly reported adverse events in this literature were sedation and hallucination. In general, adverse events were not reported as severe and only two studies reported patient withdrawal from treatment because of unacceptable "adverse cognitive effects" (Garry 1996), and pronounced sedation (Klahr 1997). One report described sedation which improved on tapering the opioid dose (Bell 1999). Other side effects described included evoked nystagmus (jerky eye movements) during treatment with intravenous ketamine (Lossignol 1999), and inflammation of syringe driver sites during subcutaneous treatment (Oshima 1990; Mitchell 1999). One report described generalised hyperalgesia and allodynia after abrupt termination of subcutaneous ketamine infusion (Mitchell 1999). One postmortem report described subpial vacuolar myelopathy in a participant who had received continuous intrathecal ketamine infusion (Karpinski 1997), while another described focal lymphocytic vasculitis close to the intrathecal catheter site (Stotz 1999). One report described maintenance of syringe driver sites with topical 0.1% hydrocortisone cream (Lloyd‐Williams 2000).
Overall completeness and applicability of evidence
The two small studies (30 participants) included in the original review provided insufficient data to enable any evidence‐based conclusions about the benefits and harms of adjuvant ketamine to be drawn. The larger trial by Hardy 2012 reported negative outcomes for a rapid titration, high‐dose ketamine regimen.
Quality of the evidence
The evidence from this review is limited to three very different studies which could not be combined. There are two very small studies undertaken in the 1990s of low‐dose ketamine, and one larger multi‐centre modern trial reported in 2012 on rapid titration of ketamine to high dose. Overall, the quality of the evidence base is very low and cannot provide a reliable indication of any likely effect across outcomes.
Potential biases in the review process
We are unaware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
A qualitative systematic review of ketamine for cancer pain in adults and children concluded that despite limited available data, there is evidence that ketamine may be a "viable option" for cancer pain that is poorly responsive to opioid therapy, that it appears to contribute to decreased opioid use and improved pain control (Bredlau 2013). The authors of this review specifically wanted to perform a comprehensive review of all available data. They included the same three RCTs included in our review and in addition, two trials which were excluded by our review (Lauretti 1999a; Lauretti 1999b) (see Characteristics of excluded studies). They also included six prospective, non‐randomised, uncontrolled trials and one retrospective case series of more than 10 participants.
A recent systematic review on adjuvant analgesics for cancer pain found that there is low‐grade evidence suggesting that ketamine as an adjuvant to opioid in cancer pain leads to pain reduction, but conclude that there is generally insufficient evidence on the effectiveness of NMDA receptor antagonists in cancer pain (van den Beuken‐van Everdingen 2017). This review considered our Cochrane update from 2012, the review by Bredlau 2013, and a RCT excluded from our last update (Salas 2012).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

