Vaccines for the common cold
Information
- DOI:
- https://doi.org/10.1002/14651858.CD002190.pub5Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 18 May 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Acute Respiratory Infections Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
Conceiving the review: DSR
-
Designing the review: DSR, CVG, MLF, RH
-
Co‐ordinating the review: DSR
-
Data collection for the review: DSR
-
Screening search results: JVAF, MJMZ
-
Appraising quality of papers: DSR, CVG, RH, JVAF, MJMZ
-
Extracting data from papers: DSR
-
Writing to authors of papers for additional information: DSR
-
Obtaining and screening data on unpublished studies: JVAF, MJMZ
-
Data management for the review: DSR
-
Entering data into Review Manager 5: DSR, JVAF, MJMZ
-
Interpretation of data: All authors
-
Providing a methodological perspective: MJMZ
-
Providing a clinical perspective: CVG, MLF, RH
-
Writing the review: DSR, JVAF, MJMZ
-
All authors contributed to the improvement of this updated review and approved the final version of the review.
Sources of support
Internal sources
-
Universidad Tecnológica Equinoccial, Ecuador.
Methodological
-
Centro de Investigación en Salud Pública y Epidemiología Clínica (CISPEC). Facultad de Ciencias de la Salud Eugenio Espejo, Ecuador.
Methodological
-
Instituto Universitario Hospital Italiano, Argentina.
Methodological
External sources
-
Instituto de Salud Carlos III, Spain.
Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
Daniel Simancas‐Racines: None known.
Juan VA Franco: None known.
Claudia V Guerra: None known.
Maria L Felix: None known.
Ricardo Hidalgo: None known.
Maria José Martinez‐Zapata: None known.
Acknowledgements
The authors wish to thank Tom Jefferson and David Tyrell, who coauthored the first published version of this review; Anne Lyddiatt, Gulam Khandaker, Lisa Jackson, Mark Griffin, and Meenu Singh for commenting on the draft protocol; and Theresa Wrangham, John Jordan, Viviana Rodriguez, and Meenu Singh for commenting on the first draft review.
The authors wish to express their thanks to Liz Dooley, Managing Editor of the Cochrane Acute Respiratory Infections Group, for her comments, which improved the quality of this review.
Daniel Simancas‐Racines is a PhD candidate at the Department of Pediatrics, Gynecology and Obstetrics, and Preventive Medicine, Universitat Autònoma de Barcelona, Spain.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Dec 14 | Vaccines for the common cold | Review | Camila Montesinos-Guevara, Diana Buitrago-Garcia, Maria L Felix, Claudia V Guerra, Ricardo Hidalgo, Maria José Martinez-Zapata, Daniel Simancas-Racines | |
| 2017 May 18 | Vaccines for the common cold | Review | Daniel Simancas‐Racines, Juan VA Franco, Claudia V Guerra, Maria L Felix, Ricardo Hidalgo, Maria José Martinez‐Zapata | |
| 2013 Jun 12 | Vaccines for the common cold | Review | Daniel Simancas‐Racines, Claudia V Guerra, Ricardo Hidalgo | |
| 2011 Apr 13 | Vaccines for the common cold | Protocol | Maria L Felix, Claudia V Guerra, Miguel A Hinojosa, Clarita I Cabezas, Ricardo Hidalgo, Diana H Samaniego, Susana Nicola | |
Differences between protocol and review
Three new authors contributed to this update: Juan VA Franco, Maria L Felix, and Maria José Martinez‐Zapata.
We considered risk of bias as unclear for blinding in the previous version of this review. For this update, we reassessed this as low because the study used a placebo.
We added two additional primary outcomes, vaccine safety and mortality related to the vaccine, to summary of findings Table for the main comparison.
We did not search Scirus for this update since this service became unavailable in 2014.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child; Humans; Male;
PICOs

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages
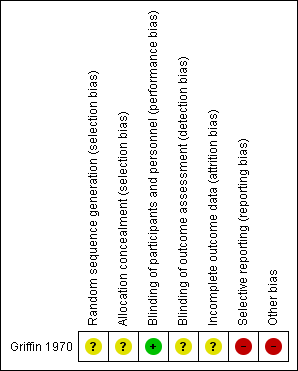
'Risk of bias' summary: review authors' judgements about each risk of bias item for the included study
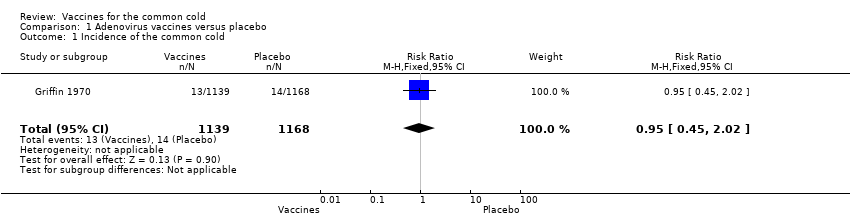
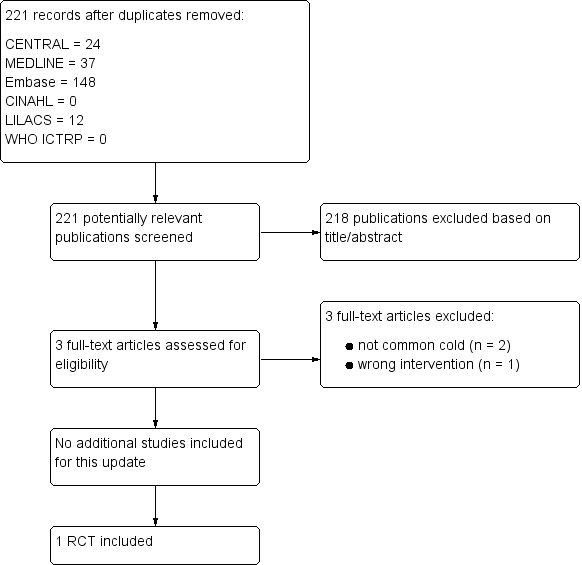
Comparison 1 Adenovirus vaccines versus placebo, Outcome 1 Incidence of the common cold.
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: healthy people | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold | Study population | RR 0.95 | 2307 | ⊕⊕⊝⊝ | ||
| 12 per 1000 | 11 per 1000 | |||||
| Vaccine safety | The study stated that there were no adverse events related to the vaccine. | 2307 | ⊕⊕⊝⊝ | |||
| Mortality related to the vaccine ‐ not reported | See comments | See comments | See comments | See comments | See comments | The included study did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Adenovirus vaccine used for preventing the common cold. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of the common cold Show forest plot | 1 | 2307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |