疫苗对普通感冒的作用
Abstract
研究背景
普通感冒是一种自限性的上呼吸道感染疾病,主要症状有鼻塞、流涕、喷嚏、咳嗽、不适感、咽痛和发热(通常<37.8℃)。比起病情严重性,普通感冒的全球广泛流行性更突出。由于普通感冒病毒的抗原变异性和难以区分的多种其他病毒,甚至还有细菌性感染,因此普通感冒的疫苗开发十分困难。预防健康人群感冒的干预措施的有效性和安全性均存在不确定性。这篇Cochrane系统综述于2011年第一次发表,2013年第一次更新,这次是第二次更新。
研究目的
本篇综述旨在评价疫苗对于健康人群普通感冒的预防临床效果和安全性。
检索策略
我们检索了Cochrane临床对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL,2016年9月),美国医学文献分析和联机检索系统(MEDLINE)(1948年至2016年9月),荷兰医学文摘数据库(EMBASE)(1974年至2016年9月),护理与医疗文献数据库(CINAHL)(1981年至2016年9月),以及LILACS(1982年到2016年9月)。还检索了3个临床注册中心网站的正在进行中的研究,截止时间为2017年2月。研究没有语言或时间限制。
标准/纳入排除标准
纳入研究为与安慰剂对比的任何病毒疫苗预防健康人群普通感冒的随机对照试验(RCTs)。
数据收集与分析
两位研究人员独立评价证据的方法学质量并提取试验资料。通过向第三人咨询或讨论的方式解决意见分歧的情况。
主要结果
结果本次更新没有纳入新的RCT。本综述只纳入了一项20世纪60年代的的高偏倚风险的RCT。这篇RCT纳入了2307名健康人,所有人的数据都进入分析。这项研究对照了腺病毒疫苗和安慰剂的效果。发现在普通感冒的发病率上没有统计学显著差异:疫苗组1139人中13例发生普通感冒(1.14%),安慰剂组1168人中14例(1.19%)(RR=0.95,95%CI=0.45 ‐ 2.02;P=0.90)。报告中没有发生由于活疫苗引起的不良反应。由于方法学质量局限性以及跨度较宽的95%可信区间,证据的质量较低。
作者结论
这篇Cochrane系统综述是基于一项低质量证据的研究。本综述认为没有确凿的结果支持与安慰剂对比,疫苗对健康人群换普通感冒的预防作用。目前还需要设计精良的、把握度充分的RCT去研究疫苗对健康人群预防普通感冒的作用。未来研究普通感冒的预防性医疗方式的试验应该评价不同病毒疫苗的情况。结局指标应该包括普通感冒发生率、疫苗安全性以及疫苗引起的死亡率。
PICOs
Plain language summary
疫苗预防普通感冒
系统综述问题
我们关注疫苗能否预防普通感冒
背景
普通感冒是由上呼吸道病毒感染引起,通常在病毒死亡时人们能感觉舒服。患有普通感冒的人会感到不适,流鼻涕,鼻塞,打喷嚏和咳嗽,伴随或不伴随咽痛,体温稍有升高。治疗的主要目的在于缓解症状。
普通感冒是全球范围内广泛传播的疾病。但由于涉及多种病毒,难以生产疫苗来预防感冒。同时,疫苗对健康人预防普通感冒的疗效未知。
检索时间
本次更新检索截止到2016年9月2日。
研究特征
本次更新未发现新研究。本综述纳入了一项1965年发表的随机对照试验。这项研究纳入了美国海军培训机构的2307名健康人,并评估了与假疫苗(安慰剂)相比,经过减毒的活腺病毒疫苗的疗效。
研究资金来源
这项研究由政府机构资助。
主要结果
接受两种疫苗的人群在普通感冒的发生率上没有差异。也没有发生由疫苗引起的不良反应。然而,由于研究纳入人数和患感冒的人数较少,且研究设计有缺陷,这项研究的结果的可信度不高。由于现有证据不能支持腺病毒疫苗预防普通人患普通感冒,未来可能需要更深入的研究来阐明疫苗能否预防普通感冒。
证据的质量
我们评估了纳入研究的证据质量,由于高偏倚风险以及纳入人数和患病人数少,证据质量低,导致证据结果不精确。
Authors' conclusions
Summary of findings
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: healthy people | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold | Study population | RR 0.95 | 2307 | ⊕⊕⊝⊝ | ||
| 12 per 1000 | 11 per 1000 | |||||
| Vaccine safety | The study stated that there were no adverse events related to the vaccine. | 2307 | ⊕⊕⊝⊝ | |||
| Mortality related to the vaccine ‐ not reported | See comments | See comments | See comments | See comments | See comments | The included study did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Adenovirus vaccine used for preventing the common cold. | ||||||
Background
Description of the condition
There are no standardised definitions for a common cold (see Appendix 1). The common cold is a spontaneously remitting infection of the upper respiratory tract, characterised by a runny nose, nasal congestion, and sneezing, and sometimes cough, malaise, sore throat, and fever (usually < 100º F). A temperature of 100º F (37.8º C) or higher for three to four days is typically associated with influenza and other respiratory diseases (Appendix 2) (DDCP 2010; Heikkinen 2003). While benign in nature, the common cold is the most frequent illness experienced in humans. Children experience six to 11 upper respiratory tract infections per year (Evans 1997; Leder 2003; Nelson 2000), and adults experience two to four episodes per year (Evans 1997; Grüber 2008; Harrison 2008). Because it causes frequent absences from school and work, the common cold has become a significant economic burden (Glezen 2000; Hall 2001; Henrickson 1994; Henrickson 2003); the cost in the United States is estimated at more than USD 60 billion each year (Poland 2009). Furthermore, bacterial complications can lead to morbidity and mortality (Thompson 2003; Wat 2004).
The aetiology of the common cold is diverse (Appendix 3) (Heikkinen 2003). Children, the elderly, and other age groups with comorbidities such as prematurity, chronic lung diseases (chronic obstructive pulmonary disease), congenital heart disease, and asthma are more prone to viral infections that cause the common cold, such as respiratory syncytial virus (RSV), rhinovirus, parainfluenza, coronavirus, and adenovirus (non‐polio) (Edlmayr 2009; Jackson 2008; Krasinski 1985; Peltola 2008). Human rhinovirus (HRV) is responsible for 50% to 80% of common colds and is an important cause of morbidity, reduced productivity, and inappropriate use of antibiotics and over‐the‐counter medications. In humans, the coronavirus (HCoV 229E) causes the common cold by infecting the upper respiratory tract. This is mainly encountered in children, and re‐infection occurs in adults (Eriksson 2006). The primary factors that contribute to the spread of this disease are poor hand hygiene, overcrowding, and captive populations (schools and daycare centres) (Harrison 2008).
Description of the intervention
Treatment of the common cold is symptomatic. Studies have shown that simple preventive measures are important but may be difficult to enforce practically (Jefferson 2011). Another method of prevention could be vaccination.
The development of vaccines for the common cold has been challenging because of multiple aetiologies, Poland 2009, and antigenic variability of the common cold viruses (Bembridge 1998; Hussell 1998). In the case of rhinovirus, there are over 167 different rhinoviral serotypes (Ren 2017). For this reason, it is difficult to create a vaccine that can give total protection. However, the future for vaccines for the common cold looks promising, considering the current knowledge of the full genomes of HRV serotypes (Palmemberg 2009). Immune responses are triggered whenever a person is infected with the same virus but with different antigenic molecules (Tobin 2008).
One of the most common causes of respiratory diseases are rhinoviruses. A recombinant vaccine has been reported, produced with rhinovirus‐derived VP1, a surface protein that is critically involved in the infection of respiratory cells, and a non‐allergenic peptide of the major grass pollen type allergen Ph1 p1 (Edlmayr 2009).
Adenovirus is a commonly recognised pathogen of the upper respiratory tract and has been particularly common in captive populations (Binn 2007). Adenovirus serotype 4 (Ad4) and serotype 7 vaccines were used during immunisation programmes starting in 1971. Unfortunately, their interruption triggered the re‐emergence of adenovirus‐produced diseases in crowded locations. An example of this reappearance was documented in United States military training sites in 1999, where Ad4 accounted for 98% of all diagnoses (Russell 2006).
Epidemiological and clinical studies have revealed important changes with regard to clinical adenovirus infection, including alterations in its antigenic presentation, geographical distribution, and virulence (Gray 2007). Adenoviral vaccines delivered orally have been used for decades to prevent respiratory illnesses. New studies have concluded that these vaccines are safe and have brought about a good immune response in the studied populations.
Respiratory syncytial virus causes approximately 5% of common colds in adults (Heikkinen 2003). The vaccine development for RSV has had some problems due to antigenic variability, especially in proteins F and G. People who were vaccinated with the formalin‐inactivated vaccine displayed X‐ray evidence of severe pneumonia and bronchiolitis due to pulmonary Arthus reaction and a process of immunopotentiation. This process was induced by a T helper (Th)‐2 and Th17 T cell responses with the enrolment of T cells, neutrophils, and eosinophils causing inflammation and tissue damage (Rey‐Jurado 2017). Where an effective vaccine can be offered, it should be administered to children younger than six months of age, when immune systems are still immature. For this reason another approach is the development of a vaccine for maternal immunisation as it has been demonstrated that RSV‐neutralising antibodies are transferred efficiently through the placenta from the pregnant woman to the newborn (Munoz 2003). Furthermore, a phase II clinical trial has found that a recombinant F nanoparticle vaccine formulation reduces the incidence of RSV infection when compared to placebo (the incidence was 11% versus 21%, respectively) in healthy women of childbearing age (Glenn 2016).
Vaccines for parainfluenza (HPIV3 cp45) are safe and immunogenic in seronegative children aged between six and 18 months (Belshe 2004a). The vaccine has also demonstrated less risk of transmission than others (wt HPIV3), making it possible to develop more randomised trials. Bovine parainfluenza virus vaccines are also being developed, which have been well‐tolerated, effective, and immunogenic in infants (Belshe 2004b).
How the intervention might work
Almost all vaccines work by inducing antibodies in the serum to interfere with microbial invasion of the bloodstream, or in the mucosa, and to block adherence of pathogens to epithelial cells (Pichichero 2009). To protect the body, antibodies must be efficient, neutralising agents or have opsonisation and phagocytosis properties. Correlates of protection after vaccination are sometimes absolute quantities, but are often relative. Most infections are prevented at a particular response level, but some could occur above that level because of a large challenge dose or deficient host factors. There may be more than one correlate of protection for a disease; authors refer to these as "co‐correlates". Either the effector or central memory may co‐correlate with protection. Cell‐mediated immunity may also operate as a correlate or co‐correlate of protection against disease, rather than against infection (Plotkin 2008). Some studies suggest that vaccines that mimic natural infection and take into account the structure of pathogens seem to be effective in inducing long‐term protective immunity (Kang 2009).
Why it is important to do this review
-
Common cold vaccines would reduce the prevalence of this disease in more than 25 million people with upper respiratory tract infections each year (Gonzales 2001).
-
The common cold results in an important economic burden with over 189 million missed school days, Roxas 2007, and 8 million to 20 million days of restricted activity (Adams 1999).
-
If randomised controlled trials demonstrate that there is a vaccine providing efficacy and safety to prevent the common cold, scientists could continue research in this area.
Objectives
To assess the clinical effectiveness and safety of vaccines for preventing the common cold in healthy people.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not apply limits with respect to follow‐up periods.
Types of participants
Healthy people aged between 6 months and 90 years.
Types of interventions
Any vaccine that prevents the common cold, which protects against RSV, rhinovirus, parainfluenza, or adenovirus (non‐polio), irrespective of dose, schedule, or administration route, versus placebo. We excluded trials on the prevention of influenza A and B because influenza and the common cold are two different diseases (Jefferson 2012). See Appendix 3 for details.
Types of outcome measures
Primary outcomes
-
Incidence of the common cold after vaccination, regardless of the causal agent determined by laboratory or clinical examination.
-
Vaccine safety, i.e. adverse events ("any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment") (Nebeker 2004); and adverse drug reactions ("a response to a drug which is noxious, uninitiated and which occurs at doses normally used in men for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions") (Nebeker 2004).
-
Mortality related to the vaccine.
Secondary outcomes
We did not consider secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 2 September 2016:
-
CENTRAL (the Cochrane Central Register of Controlled Trials), which contains the Cochrane Acute Respiratory Infections Specialised Register (CENTRAL; August 2016, Issue 8), in the Cochrane Library (searched 2 September); using the strategy in Appendix 4;
-
MEDLINE via Ovid (from 1948 to 2 September 2016) using the strategy in Appendix 5;
-
Embase via Elsevier (from 1974 to 2 September 2016) using the strategy in Appendix 6;
-
CINAHL via EBSCO (from 1981 to 2 September 2016) using the strategy in Appendix 7; and
-
LILACS via BIREME (from 1982 to 2 September 2016) using the strategy in Appendix 8.
We used the Cochrane Highly Sensitive Search Strategy to identify randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase, CINAHL, and LILACS.
We searched the following trial registries on 2 February 2017:
-
ISRCTN registry (www.isrctn.com);
-
ClinicalTrials.gov (clinicaltrials.gov/); and
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/).
We did not restrict the results by language, dates, or publication status (published, unpublished, in press, or in progress).
Searching other resources
We checked the reference lists of all relevant trials and identified reviews. We searched the following websites for trials on 2 February 2017:
-
US Food and Drug Administration (www.fda.gov);
-
European Medicines Agency (www.emea.europa.eu);
-
Medicines & Healthcare Products Regulatory Agency (www.mhra.gov.uk/index.htm);
-
Evidence in Health and Social Care (www.evidence.nhs.uk/).
Data collection and analysis
Selection of studies
Two review authors (MJMZ, JVAF) independently screened the titles and abstracts of studies identified as a result of the search for possible inclusion in the review. We retrieved full‐text reports of potentially relevant studies. Two review authors (MJMZ, JVAF) independently screened the full texts to identify studies for inclusion and identified and recorded reasons for exclusion of the ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (DSR) when needed. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of included studies table (Moher 2009). We imposed no language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that we had piloted on at least one study in the review. Two review authors (DSR, CVG) extracted study characteristics from the included studies. We extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (DSR, CVG) independently extracted outcome data from the included studies. We had planned to note in the Characteristics of included studies table if outcome data were not reported in a usable way. We had planned to resolve disagreements by consensus or by involving a third review author (RH). One review author (DSR) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that the data were entered correctly by comparing the data presented in the systematic review with the study report. A second review author (MJMZ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Three review authors (DSR, CVG, RH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (MJMZ). We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' table. Where necessary, we considered blinding separately for different key outcomes.
Measures of treatment effect
We calculated the risk ratio (RR) with 95% confidence intervals (CIs) for incidence of the common cold.
We entered outcome data for the study into a data table in Review Manager 5 to calculate the treatment effects (RevMan 2014). We used RR for dichotomous outcomes.
Unit of analysis issues
The unit of analysis was the participant. We collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
We had planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified only as an abstract). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
If numerical outcome data were missing, such as standard deviations or correlation coefficients, and could not be obtained from the authors, we planned to calculate them from other available statistics such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
However, we did not apply these approaches because this update included only one study.
Assessment of heterogeneity
We had planned use the I² statistic to measure heterogeneity among the trials in each analysis; however, this update did not include meta‐analysis. If in future updates we identify substantial heterogeneity, we will report this and explore possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We did not assess publication bias using a funnel plot because we included only one trial. For future updates, we will attempt to assess whether the review is subject to publication bias by using a funnel plot if 10 or more trials are included.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). For future updates, we will summarise findings using a fixed‐effect model according the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011).
GRADE and 'Summary of findings' tables
We created a 'Summary of findings' table using the following outcomes: incidence of the common cold, vaccine safety, and mortality. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the evidence as it relates to the study that contributed data (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade study quality using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
In subsequent updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
-
children and adults;
-
country of study; and
-
different responses in relation to different viral agents.
We will explore sources of heterogeneity in the assessment of the primary outcome measure by subgroup analyses and meta‐regression analyses. The meta‐regression analyses will assess the effect of methodological quality (high versus low), type of virus vaccines, and participant characteristics. We will only conduct meta‐regression if 10 or more RCTs are included.
Sensitivity analysis
For future updates, we plan to conduct sensitivity analyses comparing the results using all trials as follows.
-
Trials with high methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') (Higgins 2011).
-
Trials that performed intention‐to‐treat versus per‐protocol analyses.
We will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. We will exclude trials with a total attrition of more than 30% or where differences between the groups exceeded 10%, or both, from meta‐analysis, but will include them in the review.
Results
Description of studies
Results of the search
For this 2016 update we assessed 221 results from our electronic searches (Figure 1). We excluded 218 records based on assessment of title and abstract. We obtained three full‐text reports, which we excluded after full‐text assessment. Consequently, we did not include any new studies for this update. Only one study was included (Griffin 1970).
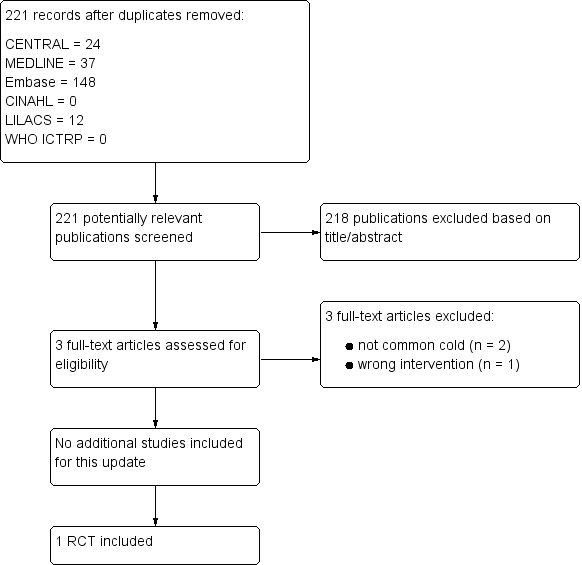
Study flow diagram
Included studies
This review included one RCT involving 2307 healthy people (Griffin 1970). See the Characteristics of included studies table.
Excluded studies
In the previous review we excluded 41 studies (Belshe 1982; Belshe 1992; Belshe 2004a; Belshe 2004b; Clements 1991; DeVincenzo 2010; Doggett 1963; Dudding 1972; Falsey 1996; Falsey 2008; Fulginiti 1969; Gomez 2009; Gonzalez 2000; Greenberg 2005; Hamory 1975; Karron 1995a; Karron 1995b; Karron 1997; Karron 2003; Karron 2005; Langley 2009; Lee 2001; Lee 2004; Lin 2007; Lyons 2008; Madhi 2006; Munoz 2003; Murphy 1994; Paradiso 1994; Piedra 1995; Pierce 1968; Power 2001; Ritchie 1958; Simoes 2001; Tang 2008; Top 1971; Tristram 1993; Watt 1990; Welliver 1994; Wilson 1960; Wright 1976). In this update, we excluded three new studies: two did not evaluate the common cold as an outcome (Glenn 2016; Karron 2015), and the third study involved an intervention that was not relevant to this review (probiotics) (Kumpu 2015).
See the Characteristics of excluded studies tables.
Risk of bias in included studies
Griffin 1970 had overall low methodological quality. See Figure 2 and Figure 3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages
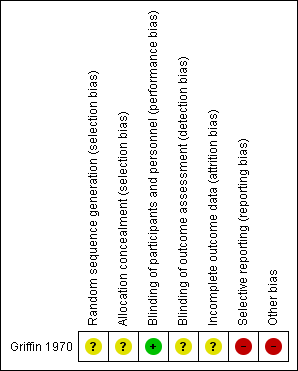
'Risk of bias' summary: review authors' judgements about each risk of bias item for the included study
Allocation
We assessed Griffin 1970 as at unclear risk of bias for random sequence generation and allocation concealment since the information provided was inadequate for judgement of this domain.
Blinding
We assessed Griffin 1970 as at low risk of bias for blinding of participants and personnel, but unclear for outcome assessor. Although a placebo was used, there could be a risk of detection bias because common cold does not require hospitalisation.
Incomplete outcome data
We assessed Griffin 1970 as at unclear risk of attrition bias because the information provided was insufficient to enable assessment of this domain.
Selective reporting
Griffin 1970 was at high risk of bias for selective reporting. The study protocol was not available, but it was clear that the published reports include all expected outcomes. However, some of the outcomes were described in a narrative fashion and did not specify incidence for each group.
Other potential sources of bias
Griffin 1970 had a high risk for other sources of bias. Participants' base characteristics were not described, and because there was no detailed information relating to assessment of selection bias, information was insufficient to evaluate if both groups were comparable.
Effects of interventions
Results were based on one RCT (Griffin 1970, N = 2307 healthy people), which we assessed as providing low‐quality evidence. See summary of findings Table for the main comparison.
Primary outcomes
1. Incidence of the common cold
Griffin 1970 (2307 participants, 27 events) showed that adenovirus vaccine was associated with a non‐statistically significant reduction in the incidence of the common cold compared with placebo (RR 0.95, 95% CI 0.45 to 2.02; P = 0.90; Analysis 1.1). We downgraded the quality of the evidence to low due to high risk of bias and imprecision; there were few events in each group, resulting in imprecision represented by a wide 95% confidence interval.
2. Vaccine safety
Griffin 1970 reported that there were no adverse events related to the live vaccine preparation. We downgraded the quality of the evidence to low due to high risk of bias and imprecision (0 events).
3. Mortality related to vaccine
Griffin 1970 did not assess this outcome.
Discussion
Summary of main results
We included one RCT that met our inclusion criteria. Critical appraisal of Griffin 1970 did not support the use of any virus vaccines for preventing the common cold in healthy people. We did not find significant differences in the incidence of the common cold in people treated with adenovirus vaccines compared with placebo. Griffin 1970 did not evaluate main clinical outcomes such as mortality related to the vaccine. This RCT reported that there were no adverse events related to the vaccine. The relative effect of any of the vaccines for viruses that cause the common cold remains unclear.
Overall completeness and applicability of evidence
The included trial did not detect statistically significant differences between the treatment groups (Griffin 1970).
When dealing with such neutral results, we need to keep in mind that 'absence of evidence' is not 'evidence of absence' (Altman 1995; Fermi Paradox 2012). The fact that this review did not detect any differences between the intervention groups does not imply that placebo and adenovirus vaccine have the same effect on preventing the common cold. The first possible explanation is failure to determine an appropriate sample size (Green 2002; Schulz 1995), in this case resulting in small differences in the incidence of the common cold and few events in the comparison groups. In a remarkable paper from 28 years ago, Freiman 1978 suggested that "many of the therapies labelled as 'no different from control' in trials using inadequate samples, have not received a fair test" and that "concern for the probability of missing an important therapeutic improvement because of small sample sizes deserves more attention in the planning of clinical trials". Moher 1998 emphasised that "most trials with negative results did not have large enough sample sizes to detect a 25% or a 50% relative difference". Moreover, it has been suggested that the most important therapies adopted in clinical practice have shown more modest benefits (Kirby 2002).
Quality of the evidence
The results for the primary outcomes 'incidence of the common cold' and 'vaccine safety' were based on low‐quality evidence due to imprecision (low number of events and wide confidence intervals) and methodological limitations. The random sequence generation, allocation, sample size, and base characteristics of participants were not reported. Furthermore, the study may be at high risk of detection bias, since the common cold syndrome rarely requires hospitalisation, and the effect of the intervention could not be adequately evaluated. The report of adverse events was not individualised for each group.
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases known as 'significance‐chasing' (Ioannidis 2010). This group includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. However, we did an exhaustive search that included many RCTs that did not evaluate common cold outcomes.
Agreements and disagreements with other studies or reviews
We found no other reviews or studies that investigated vaccines for the common cold. We excluded 11 non‐RCTs that evaluated vaccines for upper respiratory tract infections (Belshe 1982; Clements 1991; Doggett 1963; Dudding 1972; Fulginiti 1969; Hamory 1975; Karron 1997; Ritchie 1958; Watt 1990; Wilson 1960; Wright 1976). However, only one study evaluated the incidence of common cold (Ritchie 1958), while the others focused on immunologic outcomes. Ritchie 1958 prepared an "autologous vaccine" developed from the nasal secretions of 125 healthy volunteers, who were then inoculated with this product, while 75 served as a control. The results showed a lower incidence of common cold in the vaccine group than in the control group.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages

'Risk of bias' summary: review authors' judgements about each risk of bias item for the included study
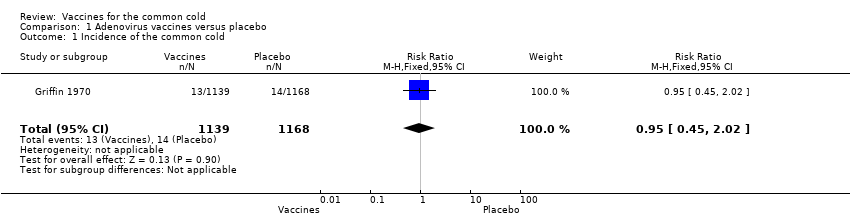
Comparison 1 Adenovirus vaccines versus placebo, Outcome 1 Incidence of the common cold.
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: healthy people | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold | Study population | RR 0.95 | 2307 | ⊕⊕⊝⊝ | ||
| 12 per 1000 | 11 per 1000 | |||||
| Vaccine safety | The study stated that there were no adverse events related to the vaccine. | 2307 | ⊕⊕⊝⊝ | |||
| Mortality related to the vaccine ‐ not reported | See comments | See comments | See comments | See comments | See comments | The included study did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Adenovirus vaccine used for preventing the common cold. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of the common cold Show forest plot | 1 | 2307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |

