Primary care professionals providing non‐urgent care in hospital emergency departments
Abstract
Background
In many countries emergency departments (EDs) are facing an increase in demand for services, long waits, and severe crowding. One response to mitigate overcrowding has been to provide primary care services alongside or within hospital EDs for patients with non‐urgent problems. However, it is unknown how this impacts the quality of patient care and the utilisation of hospital resources, or if it is cost‐effective. This is the first update of the original Cochrane Review published in 2012.
Objectives
To assess the effects of locating primary care professionals in hospital EDs to provide care for patients with non‐urgent health problems, compared with care provided by regularly scheduled emergency physicians (EPs).
Search methods
We searched the Cochrane Central Register of Controlled Trials (the Cochrane Library; 2017, Issue 4), MEDLINE, Embase, CINAHL, PsycINFO, and King's Fund, from inception until 10 May 2017. We searched ClinicalTrials.gov and the WHO ICTRP for registered clinical trials, and screened reference lists of included papers and relevant systematic reviews.
Selection criteria
Randomised trials, non‐randomised trials, controlled before‐after studies, and interrupted time series studies that evaluated the effectiveness of introducing primary care professionals to hospital EDs attending to patients with non‐urgent conditions, as compared to the care provided by regularly scheduled EPs.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We identified four trials (one randomised trial and three non‐randomised trials), one of which is newly identified in this update, involving a total of 11,463 patients, 16 general practitioners (GPs), 9 emergency nurse practitioners (NPs), and 69 EPs. These studies evaluated the effects of introducing GPs or emergency NPs to provide care to patients with non‐urgent problems in the ED, as compared to EPs for outcomes such as resource use. The studies were conducted in Ireland, the UK, and Australia, and had an overall high or unclear risk of bias. The outcomes investigated were similar across studies, and there was considerable variation in the triage system used, the level of expertise and experience of the medical practitioners, and type of hospital (urban teaching, suburban community hospital). Main sources of funding were national or regional health authorities and a medical research funding body.
There was high heterogeneity across studies, which precluded pooling data. It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment or total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, or consultations or referrals to hospital‐based specialist (3 studies; 11,203 participants), as well as costs (2 studies; 9325 participants), as we assessed the evidence as being of very low‐certainty for all outcomes.
No data were reported on adverse events (such as ED returns and mortality).
Authors' conclusions
We assessed the evidence from the four included studies as of very low‐certainty overall, as the results are inconsistent and safety has not been examined. The evidence is insufficient to draw conclusions for practice or policy regarding the effectiveness and safety of care provided to non‐urgent patients by GPs and NPs versus EPs in the ED to mitigate problems of overcrowding, wait times, and patient flow.
PICOs
Plain language summary
Primary care professionals providing non‐urgent care in hospital emergency departments
What is the aim of this review?
The aim of this Cochrane Review was to find out whether placing primary care professionals, such as general practitioners, in the hospital emergency department (ED) to provide care for patients with non‐urgent health problems can decrease resource use and costs. We searched for and analysed published and unpublished studies and found four relevant studies. This is the first update of a previously published Cochrane Review.
Key messages
We cannot be sure whether placing primary care professionals in the ED to provide care for patients with non‐urgent problems is as effective or safe as regularly scheduled emergency physician care, as we found little evidence with inconsistent results, which we assessed as of very low certainty. Safety has not been examined.
What was studied in the review?
In many countries, EDs are under a lot of pressure due to high patient attendance, resulting in long waits. One way of solving this problem may be to place primary care professionals in EDs to provide care for patients who do not have problems assessed as urgent at arrival. It has been suggested that this would make emergency physicians more available to provide care to more serious cases, thus decreasing resource use and costs.
What are the main results of the review?
This review included one randomised and three non‐randomised studies, involving a total of 11,463 patients, 16 general practitioners, nine emergency nurse practitioners, and 69 emergency physicians. Studies were conducted in Ireland, the UK, and Australia, with money given by national or regional health authorities and a medical research funding body. We could not combine the results due to differences among the studies. Because the evidence we found was of very low certainty, we cannot be certain if the intervention makes any difference to waiting times or total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, consultations or referrals to hospital‐based specialists (3 studies; 11,203 participants), as well as costs (2 studies; 9325 participants). None of the included studies provided data on adverse events.
How up‐to‐date is this review?
We searched for studies published up to May 2017.
Authors' conclusions
Summary of findings
| Primary care professionals compared with ordinary emergency department physicians for patients with minor injuries and illnesses who attend hospital emergency departments | ||||
| Patient or population: patients with minor injuries and illnesses Settings: hospital emergency departments (Ireland, UK, Australia) Intervention: primary care professionals Comparison: ordinary emergency department physicians | ||||
| Outcomes | Relative effect | No. of participants | Certainty of the evidence | Comments |
| Time from arrival to clinical assessment and treatment | MD 2.1 minutes (95% CI ‐4.9 to 9.2) | 260 (1 study) | ⊕⊝⊝⊝1,2 very low | Expressed in minutes Follow‐up not reported. |
| Total length of ED stay | MD ‐3.2 minutes (95% CI ‐20.2 to 13.8) | 260 (1 study) | ⊕⊝⊝⊝1,2 very low | Expressed in minutes Follow‐up not reported. |
| Admission to hospital | RR ranged from 0.33 to 1.11 | 11,203 | ⊕⊝⊝⊝ | Percentage of patients admitted to hospital from ED |
| Diagnostic tests | RR ranged from 0.35 to 0.96 (laboratory investigations) RR ranged from 0.47 to 1.07 (imaging results) | 11,203 | ⊕⊝⊝⊝ | Percentage of patients for whom any blood investigation or imaging results were ordered |
| Treatments given | RR ranged from 0.95 to 1.45 (any prescription) | 11,203 | ⊕⊝⊝⊝ | Percentage of patients given medication or prescription |
| Consultations or referrals to hospital‐based specialists | RR ranged from 0.5 to 1.21 | 11,203 | ⊕⊝⊝⊝ | Percentage of patients referred to consultants In Dale 1995, patients referred to on‐call teams were excluded. |
| Costs | Cost reduction associated with the intervention ranged from GBP 60,876 to IEP 95,125. | 9325 (2 studies) | ⊕⊝⊝⊝4,6 | Cost in GBP excludes hospital admissions; it is unclear whether cost in IEP includes or excludes hospital admissions. |
| Adverse events | ‐ | ‐ | ‐ | We did not find any study reporting on adverse events. |
| CI: confidence interval; ED: emergency department; MD: mean difference; RR: risk ratio GRADE Working Group grades of evidence | ||||
| 1We downgraded the evidence due to indirectness. | ||||
Background
Description of the condition
Emergency departments (EDs) are designed to provide “rapid, high quality, continuously accessible, unscheduled care” for a wide range of acute illnesses and injuries (Ieraci 2000). Many large–volume and urban hospitals in high‐income countries now face rising costs and a crisis in ED overcrowding, a situation in which the demand for services cannot be met in a timely fashion. The cause of ED overcrowding is multifactorial, and can be broken down into input, throughput, and output factors (Asplin 2003). Input factors are those that affect the demand for ED services; throughput factors involve within‐ED management and determine patients' length of ED stay; and output factors involve the efficiency with which patients are discharged or transferred out of the ED for continuing care elsewhere (Asplin 2003).
One of the many possible explanations for overcrowding is the use of EDs for conditions triaged as non‐urgent, an input factor that contributes to increased demand for ED services. Use of the ED for non‐urgent problems that could be cared for in other settings has been described since the 1970s (Lees 1976), and is often labelled by health professionals as 'inappropriate use' (Liggins 1993). The term 'inappropriate use' is complicated by different definitions in the literature and by the fact that even patients with non‐urgent triage can require advanced imaging, consultations, and hospitalisations (Dong 2007). Inappropriate ED use can result in increased health service costs, contribute to overcrowding, and compromise care for true emergencies (Derlet 2000; Jepson 2001; Siddiqui 2002). Inappropriate ED use may also lead to suboptimal care of non‐urgent cases, which are managed hastily and without the benefit of comprehensive, continuous care that could be received in a primary care setting (Carret 2009). The introduction of general practitioners (GPs) and nurse practitioners (NPs) may provide more comprehensive and cost‐ and resource‐effective care for patients with non‐urgent problems in the ED. General practitioners and NPs may also reduce wait times and patient's length of ED stay (by seeing non‐urgent patients quickly and liberating emergency physicians (EPs) to see patients with more urgent problems), thus addressing some throughput and output factors that contribute to overcrowding.
It has been reported that between 6.7% and 89% of ED visits are for non‐urgent problems that could have been looked after in less specialised settings (Carret 2009; Lowy 1994; Murphy 1998; Thompson 2013). This large variation can be explained by a number of factors. First, there is a lack of consistency in the definition of ‘inappropriate use’ (Murphy 1998). Studies may use one or some combination of the following criteria to define inappropriate ED use: number of hours' wait without risk of death; need for tests or treatment; need for hospitalisations; possibility of treatment at other levels of care; hours of observation required; or self perceived urgency (Carret 2009). Second, different triage tools are used across the world, and definitions of non‐urgent triage also vary. Other reasons for the large variation in reported inappropriate use include regional differences in health services, sample population demographics, and the use of different professional groups to determine appropriate use. Inappropriate ED use has been shown to vary across age groups, time of day and day of week, type of disease, region, and socioeconomic status (Bezzina 2005; Carret 2009).
Description of the intervention
Research suggests that patients behave rationally, believing that emergency care is appropriate based on their perception of illness severity, health service availability, and ease of accessibility (Burns 2017; Carret 2009; Parboosingh 1987; Rieffe 1999; Walsh 1995). Moreover, many patients attempt to obtain care in other settings only to end up in the ED after referral there, through advice from others, or lack of access to other timely health care. One response to inappropriate ED use has thus been to provide primary care and community services to which patients can be directed alongside or within hospital EDs. An unpublished report estimates that approximately half of UK hospitals have primary care staff operating within or alongside the ED (Carson 2010). These interventions reflect a trend toward the provision of more comprehensive services in the hospital ED, and aim to provide appropriate services for patients with non‐urgent problems. The co‐location of a primary care out‐of‐hours facility in every ED is a joint recommendation by the College of Emergency Medicine, the Royal College of Physicians, the Royal College of Surgeons, the Royal College of Paediatrics and Child Health, and the NHS Confederation (College of Emergency Medicine 2014).
How the intervention might work
There are different models by which primary care can be introduced to the ED, including primary care services (Carson 2010):
-
within the ED, whereby patients enter the ED and are triaged into separate streams (broadly speaking urgent versus non‐urgent); the non‐urgent stream is staffed by primary care practitioners;
-
alongside the ED, whereby primary care is available on‐site, next to the ED, and patients either self select or are redirected from the ED towards the primary care service;
-
at the front of the ED screening or filtering patients, whereby primary care practitioners are involved in the triage of patients presenting to the ED and may also use the see‐and‐treat model of care for non‐urgent cases or redirect non‐urgent patients;
-
fully integrated and providing care jointly with ED staff on the full range of primary care and higher acuity emergency cases.
This review focussed on the first two models.
If GPs and NPs provide more efficient and less resource‐intense care than their EP colleagues when managing non‐urgent problems, ED time and resources might be more efficiently targeted towards urgent and potentially life‐threatening cases.
Why it is important to do this review
Overcrowding in EDs occurs throughout the world, and factors associated with crowding vary widely based on country, region, and health systems. The introduction of primary care services within or alongside hospital EDs is one response to this problem; however, it is not known if this intervention results in better care for patients with non‐urgent problems, if it liberates hospital and ED resources to provide better care for more urgent medical problems, if it is a safe strategy, or if it is cost‐effective.
A report commissioned by the UK Department of Health in 2009 examined the impact of introducing primary care services to the ED and concluded that "there is a paucity of evidence on which to base policy and local system design" (Carson 2010). This review strove to establish and identify gaps in the current evidence base for interventions that have introduced primary care professionals into the ED. This is the first update of the original Cochrane Review (Khangura 2012).
Objectives
To assess the effects of locating primary care professionals in hospital EDs to provide care for patients with non‐urgent health problems, compared with care provided by regularly scheduled EPs.
Methods
Criteria for considering studies for this review
Types of studies
We considered individual and cluster randomised trials (RTs), non‐randomised trials, controlled before‐after studies (CBA), and interrupted time series (ITS), which met the quality criteria used by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (EPOC 2017a). Controlled before‐after studies studies were eligible if (1) the pre‐ and postintervention periods were the same in both groups, and (2) if they included a minimum of two intervention and two control sites. We considered ITS studies that reported a clearly defined time point for the intervention and a minimum of three data points both before and after the intervention.
We decided to also include studies that evaluated resource use and cost and that were either conducted concurrently to, or based upon data from, effectiveness studies that met the eligibility criteria above.
Types of participants
-
Patients who present to hospital EDs with illness or injury conditions suitable for primary care. Primary care‐suitable problems are those that are non‐urgent, self referred, and unlikely to require admission (Bezzina 2005). Furthermore, these problems do not require the specialised services of an ED, such as resuscitative facilities, urgent intervention, rapid and/or complex diagnostic work‐up and could be equally managed in an outpatient primary care setting (Bezzina 2005). Given that what is ‘primary care suitable’ may vary by region, we used the definitions applied in individual studies. We excluded studies comparing triage nurse ordering (Rowe 2011), nurse practitioners for specific problems, or triage liaison physicians to standard care for patients with non‐urgent problems suitable for primary care (Holroyd 2007; Rowe 2011).
-
Primary care professionals working in hospital EDs. Primary care refers to the health services and health professionals that are the patient’s first point of contact; thus defined it can include GPs, NPs, EPs, optometrists, and dentists. In the context of this review, primary care professionals include any licensed member of an accredited health specialty who normally works in a non‐specialised, outpatient setting to provide continuous “comprehensive care in the sense that only rare or unusual manifestations of ill health are referred elsewhere, and coordination of care such that all facets of care (wherever received) are integrated" (Starfield 1994; Starfield 2001).
-
Hospital physicians, including residents, senior house officers (SHOs), hospital interns, registrars and consultants (attendings), who work primarily in emergency medicine.
We excluded studies involving dentists and optometrists.
Types of interventions
We included interventions in hospital EDs in which patients who presented with non‐urgent problems were cared for by primary care professionals instead of regularly scheduled EPs. The control group received standard ED care from assigned EPs.
We included all interventions for analysis independent of variations in the type of primary care professional, time of day the patients presented to the ED, or triage criteria used to determine ‘non‐urgent problems'.
A variant of the intervention is where primary care services (e.g. out‐of‐hours GP services) have been established alongside, but not within, a hospital ED. We included these interventions if the newly introduced primary care service and existing hospital ED worked co‐operatively to provide care.
We excluded interventions:
-
at non‐hospital urgent‐care centres;
-
in EDs that employed primary care professionals prior to the intervention;
-
which diverted patients into 'fast track' areas of the ED;
-
where primary care professionals triaged patients in the ED; and
-
where primary care professionals cared for both urgent and non‐urgent patients alongside EPs.
Types of outcome measures
Main outcomes
-
Time from arrival to clinical assessment and treatment for:
-
patients with non‐urgent problems;
-
patients with urgent problems.
-
-
Total length of ED stay (from time of triage/registration to time of admission or discharge)
-
Admission to hospital
Other outcomes
-
Diagnostic tests (overall number, cost)
-
Treatments (e.g. counselling, prescriptions, procedures)
-
Consultations or referrals to hospital‐based specialists
-
Arrangement of follow‐up care
-
Subsequent utilisation of primary care/re‐attendance to the ED
-
Patient education for self management or appropriate service use
-
Cost comparison of:
-
diagnostic tests/investigations;
-
treatment;
-
referrals.
-
-
Health outcomes:
-
mortality;
-
self reported health status;
-
adverse events (return visits to the ED or readmissions).
-
Search methods for identification of studies
Electronic searches
We searched the following electronic databases on 10 May 2017:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 4) in the Cochrane Library;
-
MEDLINE Ovid (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Versions) (1946 onwards);
-
Embase Ovid (1974 to 10 May 2017);
-
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 onwards);
-
PsycINFO Ovid (1967 to May Week 1 2017);
-
Science Citation Index (Web of Knowledge) (citation search for included studies only conducted 11 January 2016).
In addition, we searched:
-
NHS Economic Evaluation Database (NEED) (www.crd.york.ac.uk/crdweb/);
-
King's Fund Library Database (kingsfund.koha‐ptfs.eu/).
Search strategies are comprised of keywords and controlled vocabulary terms. We applied no language or time limits. Development of the final search strategy was done with the assistance of the EPOC Information Specialist. We included studies regardless of publication status or language of publication. Detailed search strategies are included in Appendix 1.
Searching other resources
We searched the following clinical trials registries on 10 May 2017:
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/);
-
ClinicalTrials.gov, US National Institutes of Health (clinicaltrials.gov).
One review author (DGB) searched the reference lists of included studies and relevant systematic reviews.
Data collection and analysis
Selection of studies
One review author (DGB) downloaded all titles and abstracts retrieved by the electronic searches to Covidence reference management platform (Covidence 2018), removing duplicates and excluding studies that clearly did not meet the inclusion criteria. One review author (DGB) examined the remaining references and obtained the full text of relevant references. Two review authors (DGB and JKK) independently assessed the eligibility of the full‐text studies. Any disagreements were resolved by discussion.
Data extraction and management
Two review authors (JKK and DGB) independently undertook data extraction using a modified version of the EPOC data extraction form (Appendix 2) (EPOC 2017b). We extracted the following study characteristics.
-
Methods: study design, number of study centres and location, study setting, withdrawals, date of study, follow‐up.
-
Participants: number, mean age, age range, sex, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria, other relevant characteristics.
-
Interventions: intervention components, comparison, fidelity assessment.
-
Outcomes: main and other outcomes specified and collected, time points reported.
-
Notes: funding for trial, notable conflicts of interest of trial authors, ethical approval.
Any disagreements were resolved by discussion between review authors.
Assessment of risk of bias in included studies
Two review authors (JKK and DGB) assessed eligible studies for their risk of bias, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, Higgins 2011, and the EPOC Risk of Bias Criteria for non‐randomised studies (EPOC 2017c), which included:
-
sequence generation;
-
concealment of allocation;
-
similar baseline outcome measurements;
-
similar baseline characteristics (for providers and patients);
-
incomplete outcome data;
-
blinding of participants, personnel, and outcome assessors;
-
selective reporting of outcomes;
-
protection against contamination; and
-
other sources of bias.
We classified individual studies by risk of bias for each of these criteria as low, unclear, or high risk of bias. Any disagreements were resolved by discussion. Since we identified four studies, we did not assess whether variations in the certainty of the evidence could explain differences in study results.
Measures of treatment effect
We reported postintervention risk ratios (RR) or mean difference (MD) for intervention versus control groups with associated 95% confidence intervals (CI). Postintervention RR were based on raw number of events, adjusted or variable depending on how they were reported. No pre‐intervention data were reported in the included studies. We were not able to combine data due to high levels of statistical heterogeneity, explained by a variety of study designs, interventions, and outcomes. Data are presented in forest plots without a summary estimate, and as a narrative summary.
Unit of analysis issues
We noted that the unit of analysis across all four included studies was the patients. In one study (Dale 1995), the unit of analysis (patients) did not correspond with the unit of allocation (type of physician). A correct analysis for this study adjusting for the unit of allocation would have reduced the precision of the study estimate (larger 95% CI); in the context of a meta‐analysis, this would have reduced the weight given to this study. As we attempted no pooling due to the heterogeneity observed, we decided not to attempt any further adjustment (which would have been based on assumptions of group correlation, as no data on this were reported in the study). We did not identify any ITS designs.
Assessment of heterogeneity
We assessed statistical heterogeneity using I2 and Chi2 tests. Given the limited number of included studies, we did not further explore quantitative assessment for potential sources of heterogeneity. We provided a qualitative assessment of potential sources of heterogeneity in the Discussion.
Data synthesis
High heterogeneity precluded pooling data for outcomes (I2 >= 85%). We have presented the main findings of this review as forest plots without summary estimates. We calculated and reported findings for each outcome as RRs. We could not calculate the relative percent change as planned, as no pre‐intervention data were available. We used Review Manager 5 for all data analyses (RevMan 2011).
'Summary of findings' table and GRADE
Two review authors (JKK and DGB) independently assessed the certainty of the evidence as high, moderate, low, or very low using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) for each of the following outcomes: time from arrival to clinical assessment and treatment, length of ED stay, admission to hospital, consultations or referrals to hospital‐based specialists, diagnostic tests, treatments given, cost, and adverse events (Guyatt 2008). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011), the EPOC worksheets (EPOC 2017d), and employed GRADEpro software (GRADEpro GDT). We resolved disagreements on certainty ratings by discussion and provided justification for decisions to down‐ or upgrade the ratings using footnotes in the table and made comments to aid readers' understanding of the review where necessary. We used plain language statements to report these findings in the review (EPOC 2017e). We created a 'Summary of findings' table for the main intervention comparison. We have presented the MD or range of the RR for each outcome across included studies, along with their 95% CI, in the 'Summary of findings' table instead of summary estimates.
Subgroup analysis and investigation of heterogeneity
We had planned the following subgroup analyses, but were unable to perform them due to insufficient data:
-
patients’ socioeconomic status;
-
level of primary care health professional training (years in practice or stage of training);
-
healthcare systems; and
-
patients' age (0 to 18, 18 to 65, > 65).
Sensitivity analysis
We had planned to conduct sensitivity analyses (using random‐effects versus fixed‐effect model and study quality); however, as we identified only four studies with high heterogeneity for inclusion, we did not pursue this.
Results
Description of studies
See Characteristics of included studies table and Characteristics of excluded studies table.
Results of the search
Bibliographic searches retrieved 4678 records, and screening references of relevant systematic reviews retrieved 16 additional references. Of these 4694 unique references, we short‐listed 124 for full‐text screening, of which 14 were further assessed. We found one eligible study for this update (Jennings 2015), which we added to the three studies identified by the previous version of the review (Khangura 2012). The review includes one randomised trial, Jennings 2015, and three non‐randomised trials (Dale 1995; Gibney 1999; Murphy 1996). See the flow diagram detailing the search results in Figure 1.

Study flow diagram.
Included studies
We identified four studies for inclusion in the review. The three non‐randomised studies evaluated the effectiveness of introducing GPs into the ED to provide care for patients with “non‐urgent” problems (Dale 1995; Gibney 1999; Murphy 1996). General practitioners working in the ED were supernumerary to the regularly scheduled EPs. These three studies were conducted in Ireland and the UK, where EPs are salaried. The randomised trial assessed the effectiveness of an emergency NP service model for patients who presented to the ED with pain but without immediately life‐threatening conditions. This study was conducted in Australia (Jennings 2015). The studies or the researchers were funded by the Australian National Health and Medical Research Council (Jennings 2015), the UK Department of Health (Murphy 1996), and the King's Fund and regional health authorities in the UK (Dale 1995). One study did not report sources of support (Gibney 1999). We identified no studies conducted in health systems where physicians are reimbursed on a fee‐for‐service basis.
All four trials were single‐site (i.e. one hospital) interventions, with study durations ranging between 7 and 15 months for three studies; one study did not report study duration (Jennings 2015).
Study design and intervention
Three trials were classified as non‐randomised because either (1) the allocation of patients to GPs or EPs was predictable, or (2) there was cross‐over of physicians allocated to primary care sessions (Dale 1995; Gibney 1999; Murphy 1996). The randomised trial was pragmatic, defined by the authors as a trial with limited control over the environment, a flexible intervention, and a heterogeneous sample (Jennings 2015).
Dale 1995 established three blocks of primary care sessions within the ED, to which a GP or an EP was allocated. All patients tagged as 'primary care suitable' during a particular session were seen by the same physician (either GP or EP). Murphy 1996 hired three GPs to work two four‐hour shifts each week alongside EPs, during which non‐urgent patients were allocated to either the GP or EP according to registration time. Gibney 1999 was conducted by the same team as Murphy 1996 and followed a similar design. In Jennings 2015, all eligible participants were randomly allocated to standard ED care, delivered by 17 emergency medicine registrars, or the intervention, staffed by nine emergency NPs. Further details can be found in the Characteristics of included studies table.
Classification of patients: triage methods and definition of non‐urgent patients
The methods to identify non‐urgent patients suitable for primary care differed across the included studies.
In Dale 1995, trained nurses triaged new attendees as either 'primary care' or 'accident and emergency', based on perceived need for care, rather than diagnosis or symptoms. 'Primary care' included self referred, non‐urgent problems that could be managed “in an average local general practice”. Patients referred by their GP, those requiring immediate resuscitation, or those likely to require hospital admission were excluded.
In Murphy 1996, patients were triaged by trained nurses according to the St James triage criteria, which classifies patients as:
-
life‐threatening;
-
urgent;
-
semi‐urgent; and
-
delay acceptable based on physiological criteria.
Patients in triage categories 3 and 4 were eligible for the study; however, those who were re‐attendees or who were referred by a GP were excluded.
Gibney 1999 used an unstructured triage system executed by untrained receptionists who categorised patients as 'urgent' or 'non‐urgent'. All ambulance patients were excluded from the 'non‐urgent' category. Further details of the criteria used to classify patients were not reported.
In Jennings 2015, trained nurses triaged all patients presenting to the ED using the Australasian Triage Scale (ATS), which is an algorithm with five levels, where each level corresponds to the clinical urgency of the patient's symptoms and indicates the time frame within which the patient should be seen (Jennings 2015). All patients allocated an ATS category 2 to 5 (not immediately life‐threatening) were eligible for the study. Patients with neurovascular compromise, multiple injuries, altered conscious states, and Glasgow Coma Scale greater than 14 were excluded.
Participants and settings
Three of the studies were conducted at major urban teaching hospitals in England (Dale 1995), Ireland (Murphy 1996), and Australia (Jennings 2015). One study was conducted at a small district hospital catering to a mixed urban‐rural population in Ireland (Gibney 1999).
The four included studies involved a total of 11,463 patients, 16 GPs, nine emergency NPs, and 69 EPs (42 senior house officers (SHOs), 25 registrars, and two consultants). General practitioners' experience varied relative to EPs across studies. In Dale 1995, the time since registration was similar for GPs and EPs; in Murphy 1996, GPs had more experience than EPs (seven years versus six months since registration). The level and experience of practitioners in Gibney 1999 was not reported. In Jennings 2015, NPs had a maximum of four years autonomous prescribing experience, while registrars had at least three years of postgraduate experience.
Study populations were similar with respect to age and sex in Dale 1995, Murphy 1996, and Jennings 2015 (not reported in Gibney 1999).
Outcomes
Data were not available for all of the review outcomes outlined in our protocol, such as subsequent utilisation of primary care/re‐attendance to the ED, patient education for self management or appropriate service use (Abi‐Aad 2000). Two of the included studies reported admission to hospital (Gibney 1999; Murphy 1996), and one trial reported total length of ED stay and waiting time (Jennings 2015). Outcomes reported in all three non‐randomised trials were the number of patients: (a) undergoing investigations (laboratory, electrocardiographic, and X‐ray in Dale 1995; any blood or X‐ray in Murphy 1996 and Gibney 1999); (b) receiving prescriptions; and (c) being referred (to consultants in Dale 1995; unspecified referral in the other two papers).
Two of the four included studies provided economic evaluations of the cost‐effectiveness of introducing GPs to the ED, compared with the current standard of care/system with regular ED staff (Dale 1995; Murphy 1996).
Excluded studies
We excluded 20 studies (see Characteristics of excluded studies table). The main reason for exclusion was ineligible study design (7 studies). We excluded other studies due to ineligible intervention or participants.
Risk of bias in included studies
The risk of bias of included studies is described in the 'Risk of bias' table within the Characteristics of included studies table and summarised in Figure 2, Figure 3, and below. The main source of bias across studies related to non‐randomised methods of allocation.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In one of the included studies the method of sequence generation was random (Jennings 2015). We judged the remaining three studies to have high risk of selection bias due to non‐random allocation. We judged two included studies to be at high risk of bias for allocation concealment (Dale 1995; Gibney 1999), since triage nurses were not blinded to the grade and speciality of the physician providing care for 'non‐urgent ' patients at a particular session, which could have affected the triage and therefore also what type of patients the physician actually saw (i.e. more emergency‐type patients if an EP, and less so if a GP was providing the non‐urgent care). Murphy 1996 did not describe the allocation concealment, therefore we judged the risk of bias as unclear.
Baseline outcome measures
Jennings 2015 reported baseline outcome measures that were similar between groups and was judged to have a low risk of bias; the remaining studies did not and therefore had an unclear risk of bias.
Baseline provider characteristics
Dale 1995, Gibney 1999, and Jennings 2015 did not report any provider characteristics, therefore we judged the risk of bias as unclear. Murphy 1996 reported differences in age and work experience between GPs and EPs, with GPs being older and more experienced, resulting in a high risk of performance bias favouring GPs regarding the number of patients seen in a given time or the types of investigations ordered.
Baseline patient characteristics
In Dale 1995, there were differences in age, presenting complaints, and injury‐related diagnosis with type of doctor seen. Also, in Murphy 1996 there were differences between patients seen by GPs versus EPs for triage 3 (but not triage 4) patients. Hence, the risk of bias due to differences in patient characteristics was high for both of these studies.
We deemed the risk of bias for this item as unclear for Gibney 1999, and low for Jennings 2015, as there were little or no differences between patients.
None of the reported study outcomes adjusted for discrepancies in baseline characteristics.
Blinding
All studies used reliable, objective measures of outcome for investigating differences in processes of care (waiting time, length of ED stay, laboratory investigations, X‐rays, prescriptions, and admissions) between physician groups; risk of detection bias was low for these outcomes.
However, we judged detection bias for referrals as unclear in Murphy 1996 and Gibney 1999 due to a lack of clarity around the definition of referrals and uncertainty as to whether physicians were aware of study outcomes. We assessed Dale 1995 as at low risk of detection bias as physicians were unaware of study outcomes and referrals to outpatient clinics, community/general practice clinics, on‐call specialists teams and scheduled return visits to the ED were all included (Dale 1997).
Three studies provided self reported patient satisfaction and health status outcomes (Dale 1995; Jennings 2015; Murphy 1996); we judged risk of detection bias as unclear for these outcomes. Gibney 1999 did not present any self reported outcomes.
Performance bias was low in Dale 1995, as neither GPs, EPs, nor nurses were aware of study objectives or whether any particular primary care session was part of the study sample. The risk of performance bias for outcome assessment was also low for Jennings 2015. In Murphy 1996 and Gibney 1999 it was unclear if personnel were blinded to the study objectives or to the outcomes being assessed.
Incomplete outcome data
Dale 1995, Murphy 1996, and Jennings 2015 reported missing data (due to incomplete or missing records). The number of missing records was small relative to the overall sample size, hence we assessed the risk of bias due to incomplete outcome data as low for these three studies. The risk of bias due to incomplete outcome data was unclear in Gibney 1999 because of limited reporting of outcomes and no mention of missing data.
Selective reporting
We judged the risk of selective outcome reporting to be low in three studies (Dale 1995; Jennings 2015; Murphy 1996), where results for all outcomes mentioned in the methods section were reported. Gibney 1999 was a brief report, and was judged as at high risk for selective outcome reporting, as it is possible that the outcome data reported in the publication did not include all the outcomes measured in the study.
Other potential sources of bias
A potential source of bias in Dale 1995 and Murphy 1996 was the difference in number of hours worked by GPs versus EPs. General practitioners had limited numbers of shifts per week (range 6 to 9 hours per week across studies), while there were no restrictions on the number of shifts or hours worked by ED staff. This difference in ED work hours and experience could have created a performance bias affecting the number of patients seen, physicians' attitudes towards patients and their practice patterns when deciding on investigations, prescriptions, referrals, or admissions.
We assessed the risk of bias in Gibney 1999 as unclear due to lack of detailed information reported. We identified no other potential sources of bias for Jennings 2015, which we thus assessed as at low risk of bias.
Effects of interventions
Meta‐analysis for process outcomes (diagnostic investigations, admissions, and referrals) had very high statistical heterogeneity, with I2 values greater than 85%, and these analyses were not retained. See summary of findings Table for the main comparison and Table 1 for a summary of the results.
| (N = 4641) | (N = 4684) | (N = 1878) | |
| Laboratory investigations ordered | RR 0.22, 95% CI 0.14 to 0.33 | RR 0.35, 95% CI 0.29 to 0.42 | RR 0.96, 95% CI 0.76 to 1.2 |
| X‐rays ordered | RR 0.47, 95% CI 0.41 to 0.54 | RR 0.77, 95% CI 0.72 to 0.83 | RR 1.07, 95% CI 0.99 to 1.15 |
| Admissions | RR 0.33, 95% CI 0.19 to 0.58 | RR 0.45, 95% CI 0.36 to 0.56 | RR 1.11, 95% CI 0.70 to 1.76 |
| Referrals to specialists | RR 0.50, 95% CI 0.39 to 0.63 | RR 0.66, 95% CI 0.60 to 0.73 | RR 1.21, 95% CI 1.09 to 1.33 |
| Prescriptions | RR 0.95, 95% CI 0.88 to 1.03 | RR 1.45, 95% CI 1.35 to 1.56 | RR 1.12, 95% CI 1.01 to 1.23 |
CI: confidence interval; RR: risk ratio
Main outcomes
Time from arrival to clinical assessment and treatment
One study assessed time from arrival to clinical assessment and treatment, showing little or no difference between participants allocated to NPs or EP medical care (mean difference (MD) 2.1 minutes, 95% confidence interval (CI) ‐4.9 to 9.2) (Jennings 2015). It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment (1 study; 260 participants; very low‐certainty evidence).
Total length of ED stay
One study assessed total length of ED stay, showing little or no difference between participants allocated to NPs or EP for total length of ED stay (MD ‐3.2 minutes, 95% CI ‐20.2 to 13.8) (Jennings 2015). It is uncertain whether the intervention reduces total length of ED stay (1 study; 260 participants; very low‐certainty evidence).
Admission to hospital
General practitioners admitted fewer non‐urgent patients to hospital than EPs in two studies: risk ratio (RR) 0.33 (95% CI 0.19 to 0.58) in Dale 1995; and RR 0.45 (95% CI 0.36 to 0.56) in Murphy 1996. In Gibney 1999, there was little or no difference between the proportion of admissions made by each type of physician (RR 1.11, 95% CI 0.70 to 1.76; Analysis 1.1) (Figure 4). It is uncertain whether the intervention reduces admissions to hospital (3 studies; 11,203 participants; very low‐certainty evidence).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.1 Admissions.
Other outcomes
Diagnostic tests
Any investigations
Two studies reported the proportion of patients for whom any investigation was ordered (see Analysis 1.2; Figure 5) (Gibney 1999; Murphy 1996). The direction of effect in the two studies differed, with results in one study suggesting that GPs ordered fewer investigations than regularly scheduled EPs (RR 0.76, 95% CI 0.72 to 0.80) (Murphy 1996), and the second study reporting little or no difference between groups (RR 1.06, 95% CI 1.00 to 1.13) (Gibney 1999).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.2 All investigations.
Laboratory investigations
The results for laboratory investigations ordered (see Analysis 1.3; Figure 6) suggest that sessional GPs, defined as GPs who work as locum or salaried GPs, order fewer blood tests than regularly scheduled EPs, as the direction of effect across all studies was consistent. The size of the effect was similar in Dale 1995 (RR 0.22, 95% CI 0.14 to 0.33) and Murphy 1996 (RR 0.35, 95% CI 0.29 to 0.42). In Gibney 1999 this was less certain, as the effect size was smaller and confidence intervals crossed the line of no effect (RR 0.96, 95% CI 0.76 to 1.21). It is uncertain whether the intervention reduces laboratory investigations (3 studies; 11,203 participants; very low‐certainty evidence).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.3 Laboratory investigations.
Imaging results
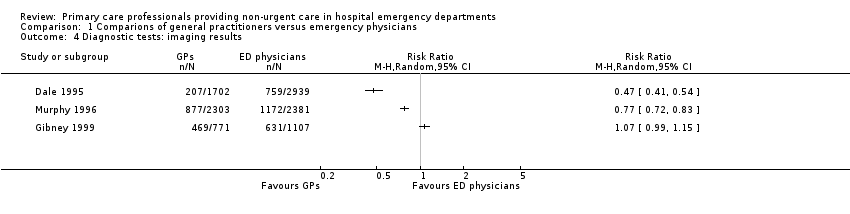
The results for imaging results ordered (see Analysis 1.4; Figure 7) showed that GPs ordered fewer X‐rays than EPs in two studies (RR 0.47, 95% CI 0.41 to 0.54 in Dale 1995; and RR 0.77, 95% CI 0.72 to 0.83 in Murphy 1996); however, data from Gibney 1999 did not support this, with a RR of 1.07, 95% CI 0.99 to 1.15. It is uncertain whether the intervention reduces the number of X‐rays ordered (3 studies; 11,203 participants; very low‐certainty evidence).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.4 Imaging results.
Treatments given
Any prescription (treatments)
As illustrated in Analysis 1.5 (Figure 8), there was little or no difference in prescribing behaviours between sessional GPs and regularly scheduled EPs in two studies: RR 0.95 (95% CI 0.88 to 1.03) in Dale 1995; and RR 1.12 (95% CI 1.01 to 1.23) in Gibney 1999. One study showed that GPs prescribed more than EPs (RR 1.45, 95% CI 1.35 to 1.56) (Murphy 1996). It is uncertain whether the intervention reduces treatments given (3 studies; 11,203 participants; very low‐certainty evidence).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.5 Any prescription.
Consultations or referrals to hospital‐based specialists
Two studies found that GPs made fewer referrals to hospital specialists or consultants: RR 0.50 (95% CI 0.39 to 0.63) in Dale 1995; and RR 0.66 (95% CI 0.60 to 0.73) in Murphy 1996. Gibney 1999 reported a greater number of referrals made by GPs than EPs (RR 1.21, 95% CI 1.09 to 1.33). See Analysis 1.6 (Figure 9). It is uncertain whether the intervention reduces consultations or referrals to hospital‐based specialists (3 studies; 11,203 participants; very low‐certainty evidence).

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.6 Referrals.
Arrangement of follow‐up care
We did not find any study reporting on arrangement of follow‐up care.
Subsequent utilisation of primary care/re‐attendance to the ED
Murphy 1996 found little or no difference in ED re‐attendance rate by patients seen by GPs versus EPs, with 17% (95% CI 15.7% to 18.8%) of patients seen by a GP, and 18% (95% CI 16.3% to 19.5%) of patients seen by an EP re‐attending the ED for the same problem within 30 days of index visit.
Neither Dale 1995 nor Murphy 1996 reported differences in rates of general practice use across groups. In Murphy 1996, 25% (95% CI 17.9% to 31.1%) of study patients seen by a GP, and 22% (95% CI 13.7% to 30.4%) seen by an EP attended a general practice for the same complaint within 30 days of their index ED visit. The Dale 1995 study looked at general practice use in the 7 to 10 days following patients' index visit and reported that 20% (95% CI 14.9% to 25.1%), 18% (95% CI 13.3% to 22.5%), and 21% (95% CI 10.5% to 31.7%) of patients seen by GPs, SHOs, and registrars respectively consulted a GP or nurse practitioner in that time.
Patient education for self management or appropriate service use
We did not find any study reporting on patient education for self management or appropriate service use.
Costs
Dale 1995 reported that employing GPs to attend to primary care patients in the ED between 10 a.m. and 9 p.m. saved a total of GBP 60,876 at 1991 costs when admission costs were excluded, and GBP ˜150,000 when the cost of admissions was included.
Murphy 1996 provided a limited cost comparison for process variables used by GPs versus regularly scheduled EPs and estimated a total savings of IEP 95,125 by employing GPs. It is unclear whether this included the cost of admissions. It is uncertain whether the intervention reduces costs (2 studies; 9325 participants; very low‐certainty evidence).
Health outcomes
We did not find any study reporting on mortality or adverse events.
Only self reported outcome data on patient satisfaction and health status were available in two of the included studies. The type of physician seen made little or no difference for health status scores in Dale 1995 or Murphy 1996. In Dale 1995, self reported health status (n = 563) one week after attending the ED showed that the proportion of patients who were "recovered or improving" was 85.5% of GP patients versus 85.7% of EP patients. In Murphy 1996, 83.4% of patients seen by the GP in the ED were “cured” or “improved” compared to 87.4% of patients who saw ED staff one month after attending the ED.
A sub‐sample of patients were administered questionnaires in Dale 1995 (N = 565) and Murphy 1996 (N = 435 with 74% response rate). Dale 1995 reported high satisfaction ratings (> 71%) amongst the 565 people sampled, with little or no difference across GPs, SHOs, and registrars. Murphy 1996 also reported little or no difference in patient satisfaction between GPs or EPs.
Discussion
Summary of main results
This review included one randomised and three non‐randomised trials evaluating the effectiveness of employing emergency NPs, Jennings 2015, or sessional GPs, Dale 1995, Gibney 1999, Murphy 1996, in EDs to provide care for patients with non‐urgent problems. It is uncertain whether the intervention reduces time from arrival to clinical assessment and treatment, total length of ED stay (1 study; 260 participants), admissions to hospital, diagnostic tests, treatments given, or consultations or referrals to hospital‐based specialist (3 studies; 11,203 participants; very low‐certainty evidence), as well as costs (2 studies; 9325 participants; very low‐certainty evidence). No data were available on mortality or adverse events. Results were inconsistent across studies.
Overall completeness and applicability of evidence
The three non‐randomised studies were conducted in the UK or Ireland between 1993 and 1999, whereas the randomised trial was conducted in Australia in 2014, which may limit the generalisability of results to other countries. Data on the proportion of non‐urgent visits to the ED in these studies would be of interest, especially given the different financial structures in the UK and Ireland at the time the studies were conducted; these data were not available for comparison across all three studies conducted in the 1990s, plus the Australian study was conducted several years later and assessed the role of NPs, as opposed to GPs. In the UK’s national health system, GP and ED visits are available free of charge. The two studies conducted in Ireland, Murphy 1996 and Gibney 1999, were undertaken at a time when the Irish health system was a mix of public (˜85%) and private, in which approximately two‐thirds of patients paid a fee for GP and ED visits (Murphy 1996). Ireland has since adopted a publicly funded health system with the introduction of the Health Act in 2004 (Health Act 2004). Australia has an universal healthcare system that covers approximately 75% of GP costs and all ED costs for citizens who are covered by Medicare. The results of this review may not be applicable in countries with different healthcare structures.
Two major differences that make meaningful comparisons of EDs across studies and centres challenging are variations in: (1) the type of physicians who normally staff EDs; and (2) the triage definitions of 'urgent' and 'non‐urgent'. In major urban centres in many countries such as Canada and the USA, consultants in emergency medicine provide ED coverage every hour of every day. In contrast, the majority of the EPs in the included studies were senior house officers and registrars, who in North America would be considered trainee doctors and would not be categorised as EPs. Additionally, the lack of consensus on triage categories and definitions of non‐urgent primary care‐suitable problems make meaningful comparisons across studies difficult, since patients who classify as 'non‐urgent' at one centre may be triaged as 'urgent' at another.
The two largest included studies (each N > 4000) were conducted at major urban teaching centres (Dale 1995; Murphy 1996). Their results may not be applicable in other healthcare settings (e.g. rural or community hospitals), which are often staffed by GPs. Patient case‐mix may also vary between healthcare settings, which could help explain (in addition to the selection bias) why the results in Gibney 1999, which was conducted at a community hospital, differed consistently across outcomes from the two studies conducted at urban teaching hospitals (Dale 1995; Murphy 1996). There was also some debate on whether the NPs recruited by Jennings 2015 would qualify as primary care professionals, as although they catered to the primary care needs of patients who could not see a GP due to undersupply, they were integrated in a specialised tertiary ED setting.
Finally, the demographics of patients attending any ED are variable across centres, reflecting local socioeconomic factors, health status, and accessibility of primary care services. The type and number of non‐urgent problems that present to a particular centre will vary, and the results from these studies may not be applicable at EDs that cater to a patient population with a different set of non‐urgent problems.
Certainty of the evidence
We identified few studies, which limits the applicability of the study findings given the wide variation in the functions of EDs and healthcare systems. The overall strength of the evidence was weak as assessed with the GRADE approach (Guyatt 2008), with very low certainty of evidence for all outcomes. This was primarily because three of the included studies were non‐randomised trials, and the only randomised trial was very small, with very serious imprecision. We recognise that randomised trials are costly and difficult to conduct in the busy, unpredictable setting of an ED without encumbering ED staff or limiting patient flow; however, innovative trial methods (e.g. cluster or step‐wedge designs) are possible alternatives. The non‐randomised studies included in this review were large (total N = 11,203) and pragmatically designed to limit risk of bias; however, due to the loss of randomisation arising from cross‐over of physicians in Dale 1995 and the predictable allocation of patients to EPs or GPs in Murphy 1996 and Gibney 1999, we were unable to classify them as low risk. We also downgraded the certainty of the evidence for imprecise or inconsistent effects, as illustrated by the high heterogeneity across studies (I2 > 86%). The high heterogeneity may have resulted from differences in study design (e.g. the method of allocation), triage criteria used, healthcare systems, medical practitioner experience, outcome measurements (e.g. laboratory investigations versus haematology and biochemistry), or how events were reported. Finally, reporting bias due to the limited information reported lowered the certainty of evidence of one study (Gibney 1999). Combining data for meta‐analysis for each outcome was not possible because of high heterogeneity across studies.
Potential biases in the review process
The search strategy was developed with experienced information technologists and was designed to maximise sensitivity (detection of relevant research) at the expense of specificity (excluding irrelevant research). We also handsearched conference abstracts from emergency medicine conferences from the last three years, which should have reduced the likelihood of missing relevant studies. Previous research in this field has demonstrated publication bias (positive results published more often than negative results), and the authors recognise that negative results likely exist (Ospina 2006). Another potential bias in systematic reviews is selection bias. Attempts were made to avoid selection bias through independent identification of studies for inclusion, data extraction, 'Risk of bias' assessment, and grading by two or more review authors.
Agreements and disagreements with other studies or reviews
Previous reviews of this topic also reported weak evidence, suggesting cost‐benefits of employing primary care professionals in the ED, and conflicting evidence on resource utilisation with respect to investigations, prescriptions issued, or referrals made (Carson 2010; Cooke 2004; Ramlakhan 2016; Roberts 1998; Turner 2015; Winters 2009). They often included retrospective and observational study designs. None of these reviews provided a formal 'Risk of bias' assessment of included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.1 Admissions.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.2 All investigations.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.3 Laboratory investigations.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.4 Imaging results.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.5 Any prescription.

Forest plot of comparison: 1 Comparisons of general practitioners versus emergency physicians, outcome: 1.6 Referrals.

Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 1 Admission to hospital.

Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 2 Diagnostic tests: all investigations.

Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 3 Diagnostic tests: laboratory investigations.
Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 4 Diagnostic tests: imaging results.

Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 5 Treatments given: any prescription.

Comparison 1 Comparions of general practitioners versus emergency physicians, Outcome 6 Consultations or referrals to hospital‐based specialists.
| Primary care professionals compared with ordinary emergency department physicians for patients with minor injuries and illnesses who attend hospital emergency departments | ||||
| Patient or population: patients with minor injuries and illnesses Settings: hospital emergency departments (Ireland, UK, Australia) Intervention: primary care professionals Comparison: ordinary emergency department physicians | ||||
| Outcomes | Relative effect | No. of participants | Certainty of the evidence | Comments |
| Time from arrival to clinical assessment and treatment | MD 2.1 minutes (95% CI ‐4.9 to 9.2) | 260 (1 study) | ⊕⊝⊝⊝1,2 very low | Expressed in minutes Follow‐up not reported. |
| Total length of ED stay | MD ‐3.2 minutes (95% CI ‐20.2 to 13.8) | 260 (1 study) | ⊕⊝⊝⊝1,2 very low | Expressed in minutes Follow‐up not reported. |
| Admission to hospital | RR ranged from 0.33 to 1.11 | 11,203 | ⊕⊝⊝⊝ | Percentage of patients admitted to hospital from ED |
| Diagnostic tests | RR ranged from 0.35 to 0.96 (laboratory investigations) RR ranged from 0.47 to 1.07 (imaging results) | 11,203 | ⊕⊝⊝⊝ | Percentage of patients for whom any blood investigation or imaging results were ordered |
| Treatments given | RR ranged from 0.95 to 1.45 (any prescription) | 11,203 | ⊕⊝⊝⊝ | Percentage of patients given medication or prescription |
| Consultations or referrals to hospital‐based specialists | RR ranged from 0.5 to 1.21 | 11,203 | ⊕⊝⊝⊝ | Percentage of patients referred to consultants In Dale 1995, patients referred to on‐call teams were excluded. |
| Costs | Cost reduction associated with the intervention ranged from GBP 60,876 to IEP 95,125. | 9325 (2 studies) | ⊕⊝⊝⊝4,6 | Cost in GBP excludes hospital admissions; it is unclear whether cost in IEP includes or excludes hospital admissions. |
| Adverse events | ‐ | ‐ | ‐ | We did not find any study reporting on adverse events. |
| CI: confidence interval; ED: emergency department; MD: mean difference; RR: risk ratio GRADE Working Group grades of evidence | ||||
| 1We downgraded the evidence due to indirectness. | ||||
| (N = 4641) | (N = 4684) | (N = 1878) | |
| Laboratory investigations ordered | RR 0.22, 95% CI 0.14 to 0.33 | RR 0.35, 95% CI 0.29 to 0.42 | RR 0.96, 95% CI 0.76 to 1.2 |
| X‐rays ordered | RR 0.47, 95% CI 0.41 to 0.54 | RR 0.77, 95% CI 0.72 to 0.83 | RR 1.07, 95% CI 0.99 to 1.15 |
| Admissions | RR 0.33, 95% CI 0.19 to 0.58 | RR 0.45, 95% CI 0.36 to 0.56 | RR 1.11, 95% CI 0.70 to 1.76 |
| Referrals to specialists | RR 0.50, 95% CI 0.39 to 0.63 | RR 0.66, 95% CI 0.60 to 0.73 | RR 1.21, 95% CI 1.09 to 1.33 |
| Prescriptions | RR 0.95, 95% CI 0.88 to 1.03 | RR 1.45, 95% CI 1.35 to 1.56 | RR 1.12, 95% CI 1.01 to 1.23 |
| CI: confidence interval; RR: risk ratio | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Admission to hospital Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Diagnostic tests: all investigations Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Diagnostic tests: laboratory investigations Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Diagnostic tests: imaging results Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Treatments given: any prescription Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Consultations or referrals to hospital‐based specialists Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

