Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates
Information
- DOI:
- https://doi.org/10.1002/14651858.CD001969.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 04 January 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Neonatal Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Vibhuti Shah (VS): performance of literature search, abstraction and analysis of data, writing of the original and updated reviews.
Arne Ohlsson (AO): performance of literature search, abstraction and analysis of data and editing of the original and updated reviews.
Henry L Halliday (HLH): editing of this update of the review.
Michael Dunn (MD): editing of this update of the review.
The searches for the 2011 update were completed by VS and AO. The searches in 2016 were conducted by Yolanda Brosseau at Cochrane Neonatal in Burlington, Vermont. This update was reviewed and approved by VS, AO, MD and HH.
Sources of support
Internal sources
-
Mount Sinai Hospital, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C.
Declarations of interest
Vibhuti Shah: no conflict of interest to declare
Arne Ohlsson: no conflict of interest to declare
Henry Halliday is the co‐author of one included trial (Bassler 2015). He acts as a consultant for Chiesi Farmaceutici, Parma, Italy and is also joint Editor‐in‐Chief of the journal Neonatology.
Michael Dunn: no conflict of interest to declare
Acknowledgements
We thank the following for providing information regarding their studies: Dr D Bassler, Department of Neonatology, University Hospital Zurich, University of Zurich, Switzerland; Dr C Cole, Department of Pediatrics, Floating Hospital for Children, New England Medical Center, Boston, USA; Dr T F Fok, Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, People's Republic of China; Dr U Merz, Children's Hospital, Neonatal Intensive Care Unit, Aachen University of Technology, Aachen, Germany; and Dr WSC Yong, Jessop Hospital for Women, Sheffield, UK.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 04 | Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates | Review | Vibhuti S Shah, Arne Ohlsson, Henry L Halliday, Michael Dunn | |
| 2012 May 16 | Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | Review | Vibhuti S Shah, Arne Ohlsson, Henry L Halliday, Michael Dunn | |
| 2007 Oct 17 | Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | Review | Vibhuti S Shah, Arne Ohlsson, Henry L Halliday, Michael Dunn | |
| 2000 Jan 24 | Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates | Review | Vibhuti S Shah, Arne Ohlsson, Henry HL Halliday, Michael S Dunn | |
Differences between protocol and review
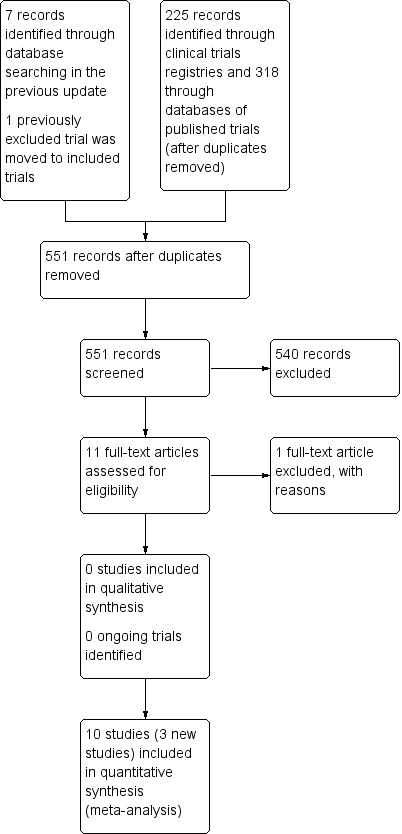
In a deviation from our protocol for this update we included all infants on any form of assisted ventilatory support (nasal CPAP/IMV or endotracheal IMV). Nakamura 2016 reported all outcomes as a combination of death and a complication of preterm birth. We wrote to the authors for clarifications regarding the outcomes on 20 May 2016 but as of 20 July 2016 we have not received a response. We report their findings separately in Analysis 1.23 to Analysis 1.32 as their data could not be incorporated in any of the meta‐analyses. This is a deviation from our protocol.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Infant, Very Low Birth Weight;
- *Respiration, Artificial;
- Administration, Inhalation;
- Anti‐Inflammatory Agents [administration & dosage, *therapeutic use];
- Chronic Disease;
- Glucocorticoids [administration & dosage, *therapeutic use];
- Infant, Premature;
- Infant, Premature, Diseases [mortality, *prevention & control];
- Lung Diseases [mortality, *prevention & control];
- Randomized Controlled Trials as Topic;
- Steroids [administration & dosage];
- Time Factors;
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICOs
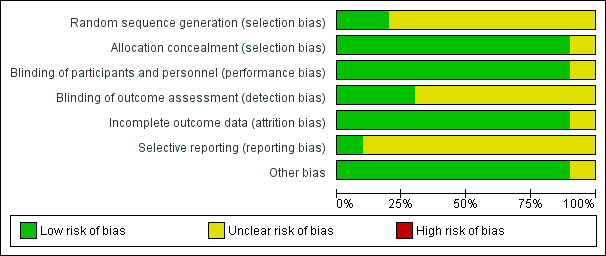
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
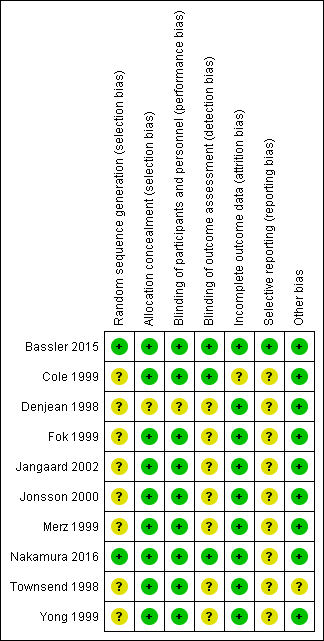
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.1 CLD at 36 weeks' PMA.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.6 Death by or CLD at 36 weeks' PMA.
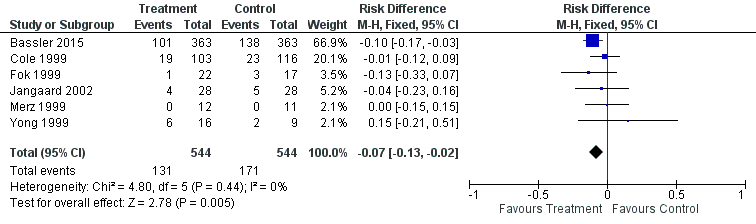
Forest plot of comparison: 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), outcome: 2.1 CLD at 36 weeks' PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 1 CLD at 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 2 CLD at 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 3 Death by 28 days of age.
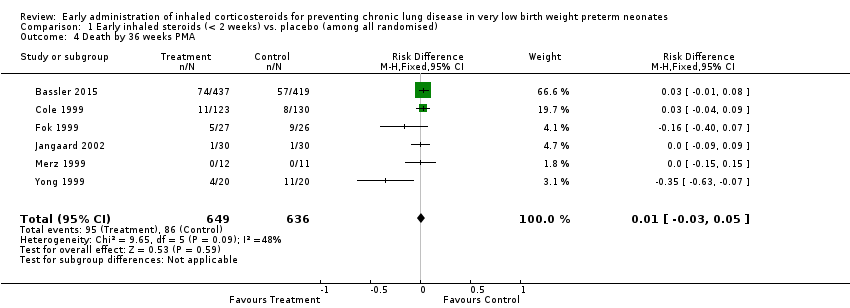
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 4 Death by 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 5 Death by or CLD at 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 6 Death by or CLD at 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 7 Survival to hospital discharge without CLD.
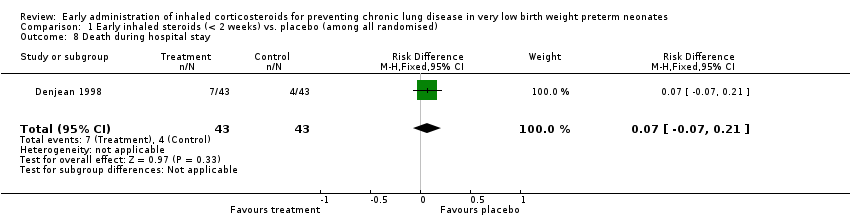
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 8 Death during hospital stay.
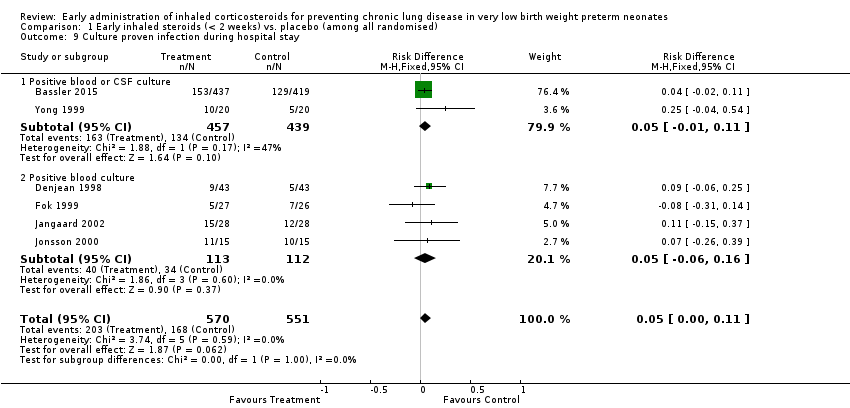
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 9 Culture proven infection during hospital stay.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 10 Hyperglycaemia.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 11 Hypertension.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 12 Gastrointesinal bleeding.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 13 Cataract.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 14 Intraventricular haemorrhage.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 15 Periventricular leukomalacia.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 16 Brain injury.
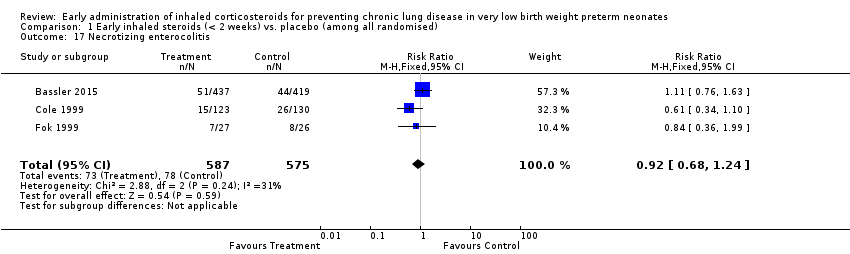
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 17 Necrotizing enterocolitis.
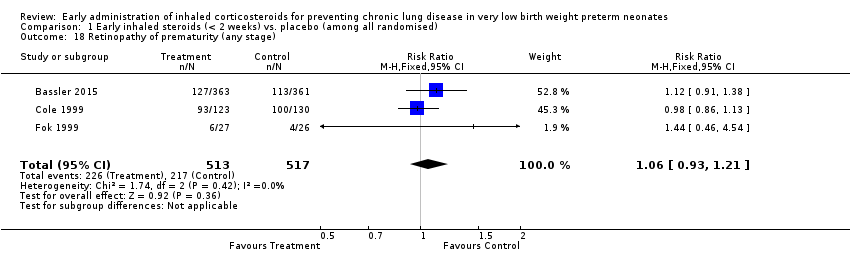
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 18 Retinopathy of prematurity (any stage).
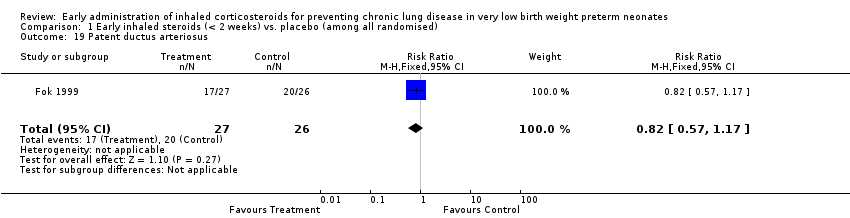
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 19 Patent ductus arteriosus.
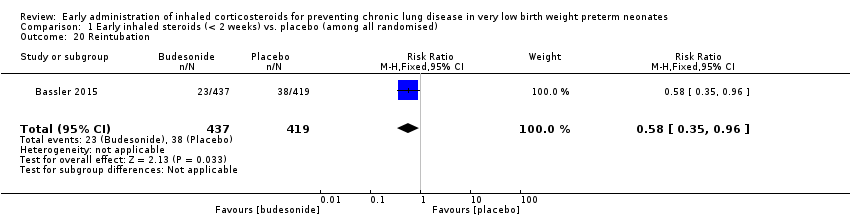
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 20 Reintubation.
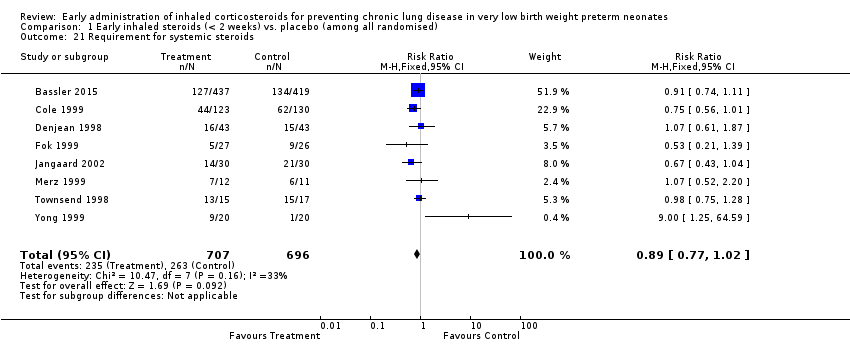
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 21 Requirement for systemic steroids.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 22 Failure to extubate within 14 days.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 23 Death or oxygen dependency at discharge.
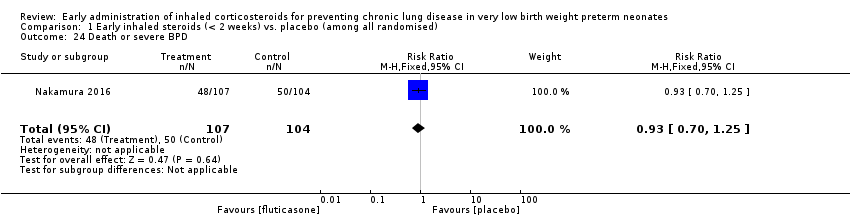
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 24 Death or severe BPD.
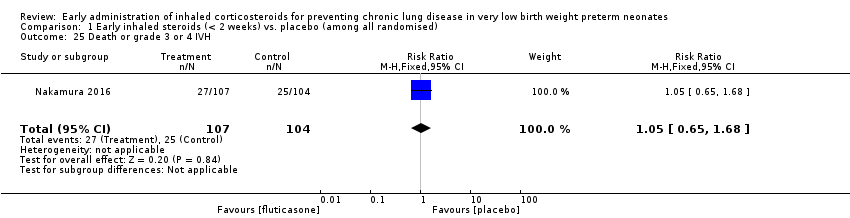
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 25 Death or grade 3 or 4 IVH.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 26 Death or PVL.
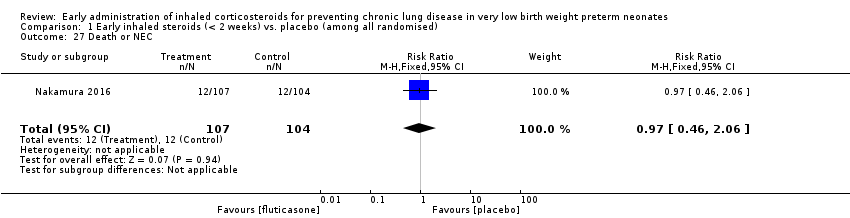
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 27 Death or NEC.
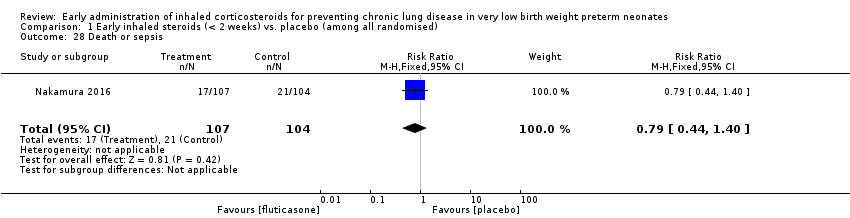
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 28 Death or sepsis.
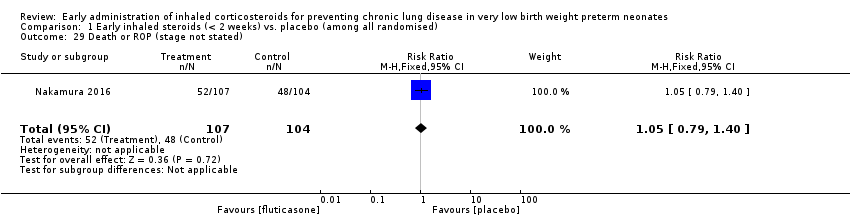
Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 29 Death or ROP (stage not stated).

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 30 Death or neurodevelopmental impairment at 18 months PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 31 Death or neurodevelopmental impairment at 3 years of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 32 Death or cerebral palsy at 3 years of age.
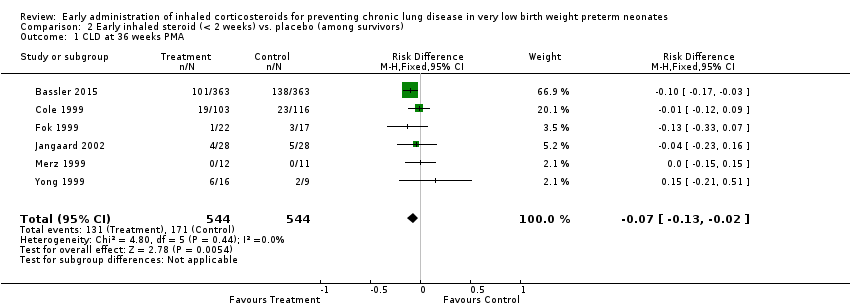
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 1 CLD at 36 weeks PMA.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 2 CLD at 28 days of age.
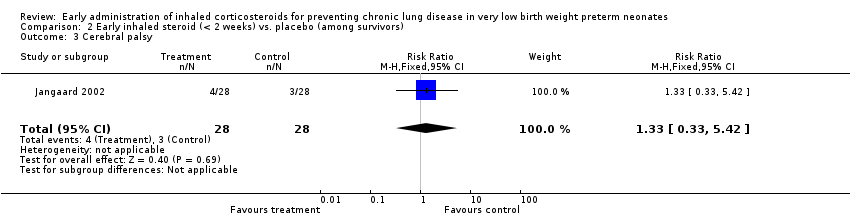
Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 3 Cerebral palsy.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 4 Mean developmental index on BSID‐II < 2 SD of the mean.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 5 Respiratory readmission.
| Early inhaled steroids (< 2 weeks) compared to placebo (among all randomised) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among all randomised) | Early inhaled steroids (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.97 | 429 | ⊕⊕⊕⊝ | ||
| 152 per 1000 | 148 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 112 per 1000 | |||||
| Death by, or CLD at, 36 weeks' PMA | Study population | RR 0.86 | 1285 | ⊕⊕⊕⊝ | ||
| 403 per 1000 | 346 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 301 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of sequence generation was unclear in all included studies except for the study by Bassler 2015. In the studies by Fok 1999, Jangaard 2002, Merz 1999 and Yong 1999 blinding of outcome assessment was unclear. Except for the study by Bassler 2015, none of the included studies were registered and we were unable to identify whether there was selective reporting or not. | ||||||
| Early inhaled steroid (< 2 weeks) compared to placebo (among survivors) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: Very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among survivors) | Early inhaled steroid (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.76 | 1088 | ⊕⊕⊕⊝ | ||
| 314 per 1000 | 239 per 1000 | |||||
| Moderate | ||||||
| 188 per 1000 | 143 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 5 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.52] |
| 2 CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| 3 Death by 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Death by 36 weeks PMA Show forest plot | 6 | 1285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 5 Death by or CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 6 Death by or CLD at 36 weeks PMA Show forest plot | 6 | 1285 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.75, 0.99] |
| 7 Survival to hospital discharge without CLD Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| 8 Death during hospital stay Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 9 Culture proven infection during hospital stay Show forest plot | 6 | 1121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| 9.1 Positive blood or CSF culture | 2 | 896 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 9.2 Positive blood culture | 4 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 10 Hyperglycaemia Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Hyperglycaemia | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.44] |
| 10.2 Hyperglycaemia requiring insulin treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Hypertension | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.99] |
| 11.2 Hypertension requiring treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.57] |
| 12 Gastrointesinal bleeding Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| 13 Cataract Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.56] |
| 14 Intraventricular haemorrhage Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| 15 Periventricular leukomalacia Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.46] |
| 16 Brain injury Show forest plot | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| 17 Necrotizing enterocolitis Show forest plot | 3 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 18 Retinopathy of prematurity (any stage) Show forest plot | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 19 Patent ductus arteriosus Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 20 Reintubation Show forest plot | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.96] |
| 21 Requirement for systemic steroids Show forest plot | 8 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 22 Failure to extubate within 14 days Show forest plot | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 23 Death or oxygen dependency at discharge Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| 24 Death or severe BPD Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| 25 Death or grade 3 or 4 IVH Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| 26 Death or PVL Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 27 Death or NEC Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.06] |
| 28 Death or sepsis Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| 29 Death or ROP (stage not stated) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 30 Death or neurodevelopmental impairment at 18 months PMA Show forest plot | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.70, 1.70] |
| 31 Death or neurodevelopmental impairment at 3 years of age Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 32 Death or cerebral palsy at 3 years of age Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 6 | 1088 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] |
| 2 CLD at 28 days of age Show forest plot | 5 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 3 Cerebral palsy Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.42] |
| 4 Mean developmental index on BSID‐II < 2 SD of the mean Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.17] |
| 5 Respiratory readmission Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.29] |