Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration
Information
- DOI:
- https://doi.org/10.1002/14651858.CD000253.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 30 July 2017see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Eyes and Vision Group
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
JE assessed studies for inclusion and exclusion, assessed risk of bias, extracted data, entered data and wrote the text of the review.
JL assessed studies for inclusion and exclusion, assessed risk of bias, extracted data, and reviewed and commented on the text of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base which funds part of Jennifer Evans's salary.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Declarations of interest
None known.
Acknowledgements
This work was undertaken in collaboration with the National Institute for Health and Care Excellence. The views expressed in this publication are those of the authors and not necessarily those of NICE.
We are grateful to:
-
the Systematic Review Training Unit at the Institute of Child Health, London for advice on the protocol for this review;
-
all the trialists who responded to requests for information;
-
peer reviewers Andrew Ness and Usha Chakravarthy for comments on an earlier version of this review.
-
Carol Mccletchie OBE who reviewed and commented on the plain language summary from the consumer perspective.
We thank Katherine Henshaw who was an author on the original review. The Cochrane Eyes and Vision editorial team prepared and executed the electronic searches for this review. We are grateful to Anupa Shah and Iris Gordon for their assistance with the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jul 30 | Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration | Review | Jennifer R Evans, John G Lawrenson | |
| 2012 Jun 13 | Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration | Review | Jennifer R Evans, John G Lawrenson | |
| 2008 Jan 23 | Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration | Review | Jennifer R Evans, Katherine S Henshaw | |
| 1999 Oct 25 | Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration | Review | Jennifer R Evans, Katherine S Henshaw | |
Differences between protocol and review
The original protocol was published in 1999 (Evans 1999). Since that time, there have been methodological improvements within Cochrane, and the methods have been updated to include assessment of risk of bias, 'Summary of findings' tables, GRADE assessment, and better consideration of unit of analysis issues.
For the 2017 update, we modified the outcome measures to ensure they were in line with those being used as part of the macular degeneration guidelines being prepared by NICE (NICE 2016). In previous versions of this review the outcomes were:
-
number of participants developing AMD;
-
number of participants with visual loss due to AMD;
-
quality of life measures;
-
any adverse outcomes reported.
In previous versions of this review, we pooled all antioxidants into one analysis. This was not possible in the current version because of overlapping participants in two trials.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Dietary Supplements;
- Antioxidants [*administration & dosage, adverse effects];
- Ascorbic Acid [administration & dosage, adverse effects];
- Drug Combinations;
- Macular Degeneration [*prevention & control];
- Minerals [administration & dosage];
- Randomized Controlled Trials as Topic;
- Vitamin E [administration & dosage, adverse effects];
- Vitamins [*administration & dosage, adverse effects];
- alpha‐Tocopherol [administration & dosage];
- beta Carotene [administration & dosage];
Medical Subject Headings Check Words
Humans;
PICOs

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
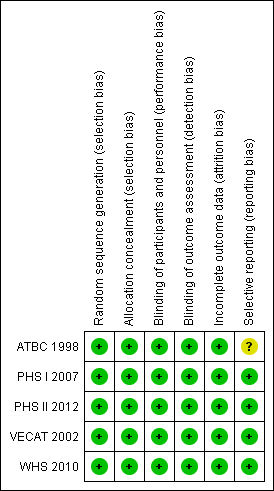
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Vitamin E versus placebo, Outcome 1 Any AMD.
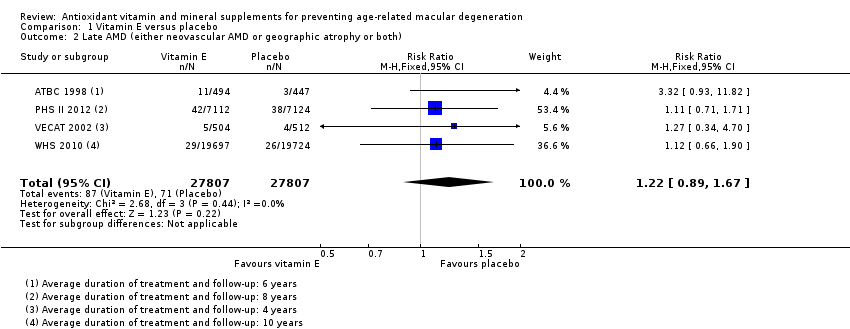
Comparison 1 Vitamin E versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both).

Comparison 1 Vitamin E versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately.

Comparison 2 Beta‐carotene versus placebo, Outcome 1 Any AMD.
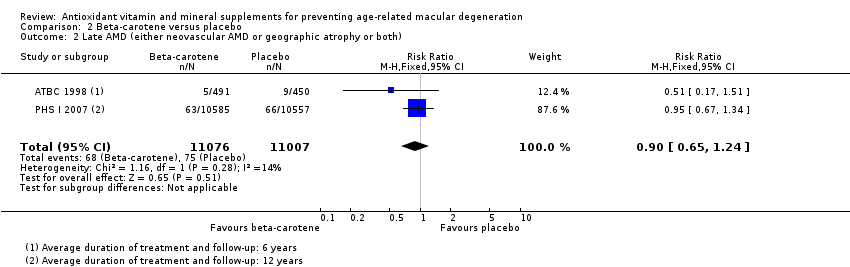
Comparison 2 Beta‐carotene versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both).
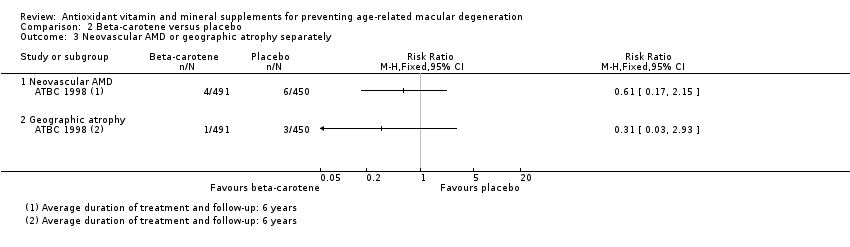
Comparison 2 Beta‐carotene versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately.
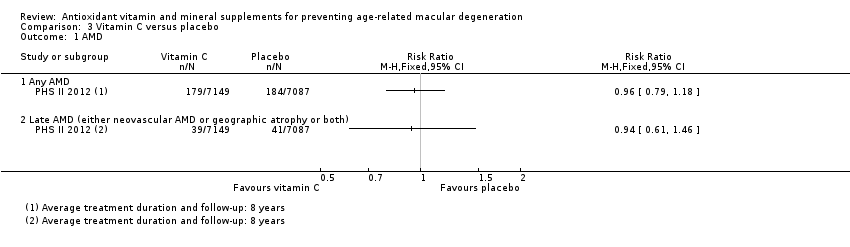
Comparison 3 Vitamin C versus placebo, Outcome 1 AMD.

Comparison 4 Multivitamin versus placebo, Outcome 1 AMD.
| Vitamin E versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with vitamin E | |||||
| Any AMD | 150 per 1000 | 146 per 1000 | RR 0.97 | 55,614 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up ranged from 4 years to 10 years |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 6 per 1000 | RR 1.22 | 55,614 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up ranged from 4 years to 10 years |
| Neovascular AMD | 3 per 1000 | 11 per 1000 | RR 3.62 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Geographic atrophy | 2 per 1000 | 6 per 1000 | RR 2.71 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects (AE) | ‐ | ‐ | ‐ | ‐ | ⊕⊕⊝⊝ | Two trials reported similar numbers of AEs in vitamin E and placebo group. Another trial reported excess of haemorrhagic strokes in vitamin E group (39 vs 23 events, hazard ratio 1.74, 95% CI 1.04 to 2.91). |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of vitamin E used in studies were: 50 mg/day, 400 IU/alternate days, 600 IU/alternate days, and 500 IU/day **The risk in the placebo group is the median risk in the placebo groups in the included studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. 2 Downgraded one level for indirectness (only one trial in male smokers) and downgraded two levels for imprecision as very few cases (10 neovascular AMD, 4 geographic atrophy) 3 Downgraded one level for imprecision due to wide confidence intervals and lower confidence near 1 and downgraded one level for inconsistency as effect only reported by one trial. | ||||||
| Beta‐carotene versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with beta‐carotene | |||||
| Any AMD | 150 per 1000 | 150 per 1000 | RR 1.00 | 22,083 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up was 6 years in one study and 12 years in the other study |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 5 per 1000 | RR 0.90 | 22,083 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 6 years in one study and 12 years in the other study |
| Neovascular AMD | 3 per 1000 | 2 per 1000 | RR 0.61 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Geographic atrophy | 2 per 1000 | 1 per 1000 | RR 0.31 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ⊕⊕⊕⊕ | Beta‐carotene associated with increased risk of lung cancer in people who smoke. | |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of beta‐carotene used was 20 mg/day in one study and 50 mg/alternate days in the other study. **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. 2 Downgraded one level for indirectness (only one trial in male smokers) and downgraded two levels for imprecision as very few cases (10 neovascular AMD, 4 geographic atrophy) | ||||||
| Vitamin C versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with vitamin C | |||||
| Any AMD | 150 per 1000 | 144 per 1000 | RR 0.96 | 14,236 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up was 8 years |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 5 per 1000 | RR 0.94 | 14,236 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 8 years |
| Neovascular AMD | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Geographic atrophy | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | None reported | |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of vitamin C used was 500 mg/day. **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. | ||||||
| Multivitamin versus placebo for preventing AMD | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with multivitamin | |||||
| Any AMD | 150 per 1000 | 182 per 1000 | RR 1.21 | 14,233 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 11 years |
| Late AMD | 5 per 1000 | 6 per 1000 | RR 1.22 | 14,233 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 11 years |
| Neovascular AMD | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Geographic atrophy | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | ⊕⊕⊕⊝ | "Those taking the active versus placebo multivitamin were more likely to have skin rashes (2111 and 1973 men in corresponding active and placebo multivitamin groups; HR 1.08, 95% CI 1.01 to 1.15; P = 0.016)". PHS II |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Multivitamin used was Centrum Silver (zinc 15 mg, vitamin E 45 IU, vitamin C 60 mg, beta‐carotene 5000 IU vitamin A, 20% as beta carotene, folic acid 2.5 mg, vitamin B6 50 mg, vitamin B12 1 mg) **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision | ||||||
| Definition of AMD used in this review | Study | ||
| No AMD | 0 = no ARM | Did not self‐report or no signs listed below in medical records | ‐ |
| Any AMD | I = dry maculopathy, with hard drusen, pigmentary changes, or both II = soft macular drusen III = disciform degeneration IV = geographic atrophy. | Drusen, RPE hypo or hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, | Early AMD 1: Soft intermediate or soft distinct or soft indistinct or pigment changes (hyperpigmentation or hypopigmentation) Early AMD 2: Soft intermediate or soft distinct or soft indistinct and pigment changes (hyperpigmentation or hypopigmentation) Early AMD 3: Soft distinct or soft indistinct or pigment changes (hyperpigmentation or hypopigmentation) Early AMD 4: Soft distinct or soft indistinct and pigment changes (hyperpigmentation or hypopigmentation) Late AMD: Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars, central areolar zone of retinal pigment epithelial atrophy with visible choroidal vessels, at least 175 µm in diameter |
| Late AMD | III = disciform degeneration IV = geographic atrophy. | Geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar | Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars, central areolar zone of retinal pigment epithelial atrophy with visible choroidal |
| Neovascular AMD | III = disciform degeneration | RPE detachment, subretinal neovascular membrane, or disciform scar | Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars |
| Geographic atrophy | IV = geographic atrophy. | Geographic atrophy | Central areolar zone of retinal pigment epithelial atrophy with visible choroidal |
| RPE: retinal pigment epithelial | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.06] |
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.67] |
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.24] |
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


