Secondary prevention of recurrent venous thromboembolism after initial oral anticoagulation therapy in patients with unprovoked venous thromboembolism
Abstract
Background
Currently, little evidence is available on the length and type of anticoagulation used for extended treatment for prevention of recurrent venous thromboembolism (VTE) in patients with unprovoked VTE who have completed initial oral anticoagulation therapy.
Objectives
To compare the efficacy and safety of available oral therapeutic options (aspirin, warfarin, direct oral anticoagulants (DOACs)) for extended thromboprophylaxis in adults with a first unprovoked VTE, to prevent VTE recurrence after completion of an acceptable initial oral anticoagulant treatment period, as defined in individual studies.
Search methods
For this review, the Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (March 2017) as well as the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2). We also searched trials registries (March 2017) and reference lists of relevant articles.
Selection criteria
We included randomised controlled trials in which patients with a first, symptomatic, objectively confirmed, unprovoked VTE, who had been initially treated with anticoagulants, were randomised to extended prophylaxis (vitamin K antagonists (VKAs), antiplatelet agents, or DOACs) versus no prophylaxis or placebo. We also included trials that compared one type of extended prophylaxis versus another type of extended prophylaxis.
Data collection and analysis
Two review authors independently selected studies, assessed quality, and extracted data. We resolved disagreements by discussion.
Main results
Six studies with a combined total of 3436 participants met the inclusion criteria. Five studies compared extended prophylaxis versus placebo: three compared warfarin versus placebo, and two compared aspirin versus placebo. One study compared one type of extended prophylaxis (rivaroxaban) versus another type of extended prophylaxis (aspirin). For extended prophylaxis versus placebo, we downgraded the quality of the evidence for recurrent VTE and all‐cause mortality to moderate owing to concerns arising from risks of selection and performance bias in individual studies. For all other outcomes in this review, we downgraded the quality of the evidence to low owing to concerns arising from risk of bias for the studies stated above, combined with concerns over imprecision. For extended prophylaxis versus other extended prophylaxis, we downgraded the quality of the evidence for recurrent VTE and major bleeding to moderate owing to concerns over imprecision. Risk of bias in the individual study was low.
Meta‐analysis showed that extended prophylaxis was no more effective than placebo in preventing VTE‐related mortality (odds ratio (OR) 0.98, 95% confidence interval (CI) 0.14 to 6.98; 1862 participants; 4 studies; P = 0.98; low‐quality evidence), recurrent VTE (OR 0.63, 95% CI 0.38 to 1.03; 2043 participants; 5 studies; P = 0.07; moderate‐quality evidence), major bleeding (OR 1.84, 95% CI 0.87 to 3.85; 2043 participants; 5 studies; P = 0.86; low‐quality evidence), all‐cause mortality (OR 1.00, 95% CI 0.63 to 1.57; 2043 participants; 5 studies; P = 0.99; moderate‐quality evidence), clinically relevant non‐major bleeding (OR 1.78, 95% CI 0.59 to 5.33; 1672 participants; 4 studies; P = 0.30; low‐quality evidence), stroke (OR 1.15, 95% CI 0.39 to 3.46; 1224 participants; 2 studies; P = 0.80; low‐quality evidence), or myocardial infarction (OR 1.00, 95% CI 0.35 to 2.87; 1495 participants; 3 studies; P = 1.00; low‐quality evidence).
One study showed that the novel oral anticoagulant rivaroxaban was associated with fewer recurrent VTEs than aspirin (OR 0.28, 95% CI 0.15 to 0.54; 1389 participants; P = 0.0001; moderate‐quality evidence). Data show no clear differences in the incidence of major bleeding between rivaroxaban and aspirin (OR 3.06, 95% CI 0.37 to 25.51; 1389 participants; P = 0.30; moderate‐quality evidence) nor in the incidence of clinically relevant non‐major bleeding (OR 0.84, 95% CI 0.37 to 1.94; 1389 participants; 1 study; P = 0.69; moderate‐quality evidence). Data on VTE‐related mortality, all‐cause mortality, stroke, and myocardial infarction were not yet available for participants with unprovoked VTE and will be incorporated in future versions of the review.
Authors' conclusions
Evidence is currently insufficient to permit definitive conclusions concerning the effectiveness and safety of extended thromboprophylaxis in prevention of recurrent VTE after initial oral anticoagulation therapy among participants with unprovoked VTE. Additional good‐quality large‐scale randomised controlled trials are required before firm conclusions can be reached.
PICOs
Plain language summary
The safety and effectiveness of extending anticoagulant treatment for people who have been treated for blood clots
Background
Venous thromboembolism (VTE) is a condition in which a blood clot forms in the deep veins of the leg or pelvis (deep vein thrombosis (DVT)), or the clot travels in the blood and blocks a blood vessel in the lungs (pulmonary embolism (PE)). People with a VTE are treated with an anticoagulant, which prevents formation of further clots. For patients with a VTE that has been caused by a certain risk factor (prolonged periods of immobility, cancer, pregnancy, oral contraceptives, hormone replacement therapy, trauma, or blood disorder), treatment can be safely discontinued after three months. However, for patients in whom the VTE has no known cause (unprovoked), the optimal length of treatment is unknown because evidence is limited. Doctors have to decide upon extended treatment based on benefit (i.e. prevention of VTE recurrence) and risk (i.e. of bleeding) associated with treatment. This review assessed whether extended treatment was safe and effective in preventing further clots in patients with an unprovoked VTE.
Study characteristics and key results
We found six studies with a combined total of 3436 patients (until March 2017). Five studies compared treatment with placebo, and one study compared one type of treatment with another. Three of the five studies that used a placebo used warfarin, and two used aspirin. Combining results of the five studies showed no clear difference in the rate of further clots between patients treated with an anticoagulant and those treated with a placebo, and no clear difference in the numbers of deaths, bleeding incidents, or adverse effects such as stroke or heart attack.
One study showed that oral treatment with the anticoagulant rivaroxaban was associated with fewer clots than aspirin. There was no evidence of a difference in major and non‐major bleeding events between rivaroxaban and aspirin. Data on deaths and deaths related to clots in the lungs, stroke, and heart attack were not yet available for participants relevant to this review and will be incorporated in a future version of the review.
Quality of the evidence
The quality of the evidence provided by studies included in this review ranged from low to moderate because a small number of studies with few events were included.
This review found that trials are too few to show whether extended treatment is safe and effective in preventing further blood clots after three months' treatment. Further good‐quality and large‐scale studies are required.
Authors' conclusions
Summary of findings
| Extended prophylaxis compared to placebo in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with extended prophylaxis | |||||
| VTE‐related mortalitya | Study population | OR 0.98 | 1862 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of VTE‐related mortality. | |
| 2 per 1000 | 2 per 1000 | |||||
| Recurrent VTEc | Study population | OR 0.63 | 2043 | ⊕⊕⊕⊝ | ||
| 170 per 1000 | 114 per 1000 | |||||
| Major bleedinge | Study population | OR 1.84 | 2043 | ⊕⊕⊝⊝ | ||
| 11 per 1000 | 20 per 1000 | |||||
| All‐cause mortalityg | Study population | OR 1.00 | 2043 | ⊕⊕⊕⊝ | ||
| 38 per 1000 | 38 per 1000 | |||||
| Clinically relevant non‐major bleedingh | Study population | OR 1.78 | 1672 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of clinically relevant non‐major bleeding. | |
| 6 per 1000 | 11 per 1000 | |||||
| Strokei | Study population | OR 1.15 | 1224 | ⊕⊕⊕⊝ | ||
| 10 per 1000 | 11 per 1000 | |||||
| Myocardial infarction | Study population | OR 1.00 | 1495 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
| VTE extended prophylaxis compared to another VTE extended prophylaxis in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with aspirin | Risk with rivaroxaban | |||||
| VTE‐related mortalitya | See comments. | See comments. | See comments. | See comments. | Data on VTE‐related mortality are yet available for participants with unprovoked VTE. | |
| Recurrent VTEb | Study population | OR 0.28 | 1389 | ⊕⊕⊕⊝ | ||
| 56 per 1000 | 16 per 1000 | |||||
| Major bleedingd | Study population | OR 3.06 | 1389 | ⊕⊕⊕⊝ | ||
| 2 per 1000 | 7 per 1000 | |||||
| All‐cause mortalitye | See comments. | See comments. | See comments. | See comments. | Data on all‐cause mortality are not yet available for participants with unprovoked VTE. | |
| Clinically relevant non‐major bleedingf | 19 per 1000 | 16 per 1000 (7 to 37) | OR 0.84 (0.37 to 1.94) | 1389 | ⊕⊕⊕⊝ | |
| Strokeg | See comments. | See comments. | See comments. | See comments. | Data on stroke are not yet available for participants with unprovoked VTE. | |
| Myocardial infarction | See comments. | See comments. | See comments. | See comments. | Data on myocardial infarction are not yet available for participants with unprovoked VTE. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
Background
Description of the condition
Venous thromboembolism (VTE), a disease comprising deep vein thrombosis (DVT), pulmonary embolism (PE), or both, is a common (1 to 2 per 1000 person‐years) (Oger 2000; Spencer 2006), preventable, and treatable condition. However, it is a potentially fatal disease with a short‐term mortality rate of approximately 25% (Kyrle 2005; Murin 2002; NÆSS 2007). Risk of recurrence during the first few weeks after the acute phase of VTE is high and remains about 10% per year for non‐provoked VTE with a case‐fatality rate of 3.6% to 12% (Carrier 2010; Douketis 2007; Kyrle 2010). The implications of recurrent VTE include not just mortality but also considerable morbidity with post‐thrombotic syndrome (Leizorovicz 1998). The likelihood of recurrence varies among patients and is influenced by several factors including the presence or absence of trigger risk factors, characteristics of the index event (proximal DVT or PE compared to distal DVT), and patients' clinical features (male sex, post‐thrombotic syndrome, overweight, age). Although risk factors for recurrence have been identified, and indeed predictive rules determining duration of anticoagulation have been developed (Vienna Score, Men Do, and Her 2 Do from Canada and Italy), these have not been universally adopted in clinical practice (Eichinger 2010; Rodger 2008; Tosetto 2012).
VTE can be provoked by triggers, such as a cast, surgery, immobilisation, or recent trauma, or unprovoked with no apparent trigger. Patients with VTE are treated initially with therapeutic parenteral anticoagulants (usually subcutaneous low molecular weight heparin (LWMH)) and with a vitamin K antagonist (VKA) (i.e. warfarin). Parenteral therapy is continued until the international normalised ratio (INR) is ≥ 2 for at least 24 hours, after which parenteral therapy is stopped. All patients are treated with anticoagulants for at least three months; after this time, it is recommended to extend treatment for unprovoked VTE if bleeding risk is low to moderate (Kearon 2012).
Recently, direct oral anticoagulants (DOACs) such as direct factor Xa inhibitors (apixaban, rivaroxaban, and edoxaban) and direct thrombin inhibitors (dabigatran) have emerged as a treatment option in the acute phase of VTE, with similar (or better) efficacy as VKAs, but with potentially lower bleeding rates and easier administration (Agnelli 2013; Bauersachs 2010; Hokusai‐VTE 2013; Schulman 2009).
Description of the intervention
Provoked VTE has a low annual risk of recurrence; therefore oral anticoagulation can be safely discontinued after three months of therapy (Baglin 2003; Heit 2000; Prandoni 1996; Research Committee British Thoracic Society 1992; Segal 2007). Treatment with anticoagulants can significantly reduce the recurrence of unprovoked VTE, but the risk of bleeding is increased during such treatment. The optimal duration of extended treatment for unprovoked VTE is unknown because data in the literature are limited. Physicians have to decide upon extended treatment based on benefit (i.e. prevention of VTE recurrence) and risk (i.e. of bleeding) due to anticoagulation treatment (Carrier 2010). The risk of recurrence increases once anticoagulation is stopped, whether a short or prolonged period of treatment has been recommended for the patient (Boutitie 2011).
For many decades, and according to the American College of Chest Physicians guidelines (Kearon 2012), VKA (i.e. warfarin) has been the drug of choice for long‐term treatment of VTE. Warfarin is not an ideal drug because it has a narrow therapeutic window and multiple food and drug interactions. Close monitoring and dose adjustment are required to avoid risk of VTE recurrence or bleeding, or both (Weitz 2005).
Antiplatelet therapy (i.e. acetylsalicylic acid (aspirin)) may have a role in long‐term prevention of recurrent VTE after initial VKA therapy for VTE. Two recently published studies reported a reduction in VTE recurrence and in major vascular events by a third (two‐ to three‐fold less than anticoagulants), with low annual risk of bleeding (0.3%) (Becattini 2012; Brighton 2012).
DOACs such as dabigatran, rivaroxaban, and apixaban have been studied for extended treatment of VTE after the initial anticoagulation period, and randomised controlled trials have been published in recent years (Agnelli 2013; Bauersachs 2010; Schulman 2013). When compared to placebo or warfarin, all DOACs demonstrated efficacy in reducing VTE recurrence, some with lower bleeding rates compared to warfarin (Agnelli 2013; Bauersachs 2010; Schulman 2013). When compared to placebo, data show increased bleeding rates with dabigatran and rivaroxaban but no increase in the rate of major bleeding with apixaban (Agnelli 2013; Bauersachs 2010; Schulman 2013).
Investigators have identified risk factors for recurrence of VTE and have developed predictive scores to determine the duration of anticoagulation (Eichinger 2010; Rodger 2008; Tosetto 2012). Overall, none of these models has been incorporated into clinical use because management studies and external prospective validation are still needed.
Why it is important to do this review
Prolonged treatment with anticoagulation prevents VTE recurrence. However, current standard treatment with a VKA is associated with bleeding complications. Thus, questions regarding duration of therapy for secondary prevention of VTE recurrence, and which is the best anticoagulant to achieve this goal, remain open. DOACs or antiplatelet agents may offer a simple and relatively safe alternative to VKAs for preventing secondary VTE in patients with unprovoked VTE. No Cochrane review has compared available oral therapeutic options (aspirin, warfarin, and DOACs). To date, best evidence to guide decision making for the optimal duration of extended prophylaxis or the type of oral anticoagulant for preventing secondary unprovoked VTE is lacking. To determine the best oral anticoagulant currently available for secondary prevention of VTE, we will perform a systematic review and meta‐analysis of all randomised controlled trials currently available in the literature. We intend to reach this decision by ascertaining the efficacy of oral anticoagulants in preventing VTE recurrence and their safety as regards bleeding complications.
Objectives
To compare the efficacy and safety of available oral therapeutic options (aspirin, warfarin, direct oral anticoagulants (DOACs)) for extended thromboprophylaxis in adults with a first unprovoked VTE, to prevent VTE recurrence after completion of an acceptable initial oral anticoagulant treatment period, as defined in individual studies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, irrespective of language, date of publication, and publication status. We excluded studies administering drugs that have been removed from the market (e.g. ximelagatran). We excluded studies on treatment of individuals with acute‐phase VTE as well as people with provoked VTE.
Types of participants
Adult participants (> 18 years) with their first symptomatic, objectively confirmed, unprovoked VTE after completion of an acceptable initial oral anticoagulant treatment period, as defined by individual studies.
Types of interventions
Oral therapeutic options for extended thromboprophylaxis include VKAs (including warfarin), antiplatelet agents (including aspirin), and DOACs (including dabigatran, rivaroxaban, edoxaban, and apixaban).
We considered two types of comparisons.
-
VTE extended prophylaxis (antiplatelets, DOACs, or VKAs) versus no prophylaxis or placebo.
-
VTE extended prophylaxis (antiplatelets, DOACs, or VKAs) versus any other VTE extended prophylaxis (antiplatelets, DOACs, or VKAs).
Extended prophylaxis is defined as treatment for participants with VTE who have completed at least three months and up to four years of anticoagulation therapy after initial treatment.
Types of outcome measures
Primary outcomes
-
VTE‐related mortality
-
Recurrence rate of symptomatic, objectively confirmed VTE (DVT or PE) during follow‐up
-
Major bleeding: A major bleeding episode is defined as clinically overt bleeding that is associated with at least one of a fall in haemoglobin levels of 20 g/L or more; transfusion of at least 2 units of packed red blood cells; involvement of the intracranial or retroperitoneal space or a body cavity; or death (International Society on Thrombosis and Haemostasis (ISTH) definition) (Schulman 2005); or as defined by the investigators in each trial
Secondary outcomes
-
All‐cause mortality
-
Clinically relevant non‐major bleeding: bleeding that is clinically overt but does not meet the definition of serious bleeding provided by the ISTH (Schulman 2005)
-
Stroke (both ischaemic and haemorrhagic) or transient ischaemic attack (TIA)
-
Serious adverse events, including acute myocardial infarction; acute coronary syndrome; or any life‐threatening or grade 3 to 4 non‐haematological event (such as hepatotoxicity or renal toxicity)
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
-
Cochrane Vascular Specialised Register (March 2017).
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The CIS maintains the Cochrane Vascular Specialised Register, which is constructed through weekly electronic searches of MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of databases, journals, and conference proceedings searched and the search strategies used are presented in the Specialised Register section of the Cochrane Vascular Module in the Cochrane Library (www.cochranelibrary.com).
The CIS also searched the following trial registries for details of ongoing and unpublished studies (March 2017).
-
CinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of these searches.
Searching other resources
We checked the citations of included trials and major reviews for additional studies.
When necessary, we contacted the first or corresponding author of included studies to obtain additional trial information.
Data collection and analysis
Selection of studies
Two review authors (LR, and AR or SEY) inspected the title and, when available, the abstract of each reference identified in the search and applied the inclusion criteria. When relevant articles were identified, we obtained the full article and two review authors independently inspected the article and applied the inclusion criteria. In the case of disagreement between the two review authors, the third review author independently applied the inclusion criteria. We documented the justification for excluding studies.
We included trials regardless of publication status and date and language of publication. We excluded trials regarding anticoagulants that are currently, or were previously, omitted from the market (e.g. ximelagatran). We also excluded trials that included participants with other indications or contraindications for treatment with anticoagulants or antiaggregants; or participants treated with other antiplatelet agents or non‐steroidal anti‐inflammatory drugs (NSAIDs).
Data extraction and management
Two review authors (LR, and AR or SEY) independently extracted the following data using data extraction forms.
-
Study design.
-
Length of follow‐up.
-
Dates of study.
-
Location and setting of study.
-
Characteristics of participants: number, age, sex, ethnic group.
-
Previous DVT or PE.
-
Type of VTE at initial diagnosis (DVT, PE, etc.).
-
Creatinine clearance.
-
Methodological quality (risk of bias).
-
Characteristics of interventions (name of drug, dose, duration of treatment).
-
Description of primary and secondary outcomes (as defined above).
We resolved disagreements by discussion. If disagreement persisted, the third review author extracted the data independently. We discussed data extraction, documented disagreements and their resolution, and, when necessary, contacted study authors to request clarification. If this was unsuccessful, we reported disagreements.
Assessment of risk of bias in included studies
Two review authors (LR, AR or SEY) independently assessed trials for methodological quality. We described and assessed allocation concealment, sequence generation, blinding, incomplete outcome data, and selective outcome reporting individually, according to Cochrane's tool for assessing bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. If disagreement persisted, the third review author assessed trials for methodological quality.
Measures of treatment effect
We estimated odds ratios (ORs) with 95% confidence intervals (CIs) for dichotomous data using the Mantel–Haenszel method.
Unit of analysis issues
The unit of analysis was the individual participant.
We did not include cross‐over trials and cluster‐randomised trials.
Dealing with missing data
When data were missing, we attempted to contact study authors to request the missing information.
We planned to impute missing dichotomous data for participants who were lost to follow‐up after randomisation, assuming a poor outcome (worse‐case scenario) for missing individuals. However, as few participants were lost to follow‐up in the studies included in this review, it was not possible to do this.
We planned to perform a sensitivity analysis of the primary outcome while excluding trials in which more than 20% of participants were lost to follow‐up.
Assessment of heterogeneity
We assessed heterogeneity (the degree of difference between results of different trials) using the Chi2 test of heterogeneity and the I2 statistic for inconsistency, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We defined statistically significant heterogeneity as P value < 0.10, or an I2 statistic greater than 50%. An I2 < 25% was considered to show low‐level heterogeneity, and 25% to 50% moderate‐level heterogeneity.
Assessment of reporting biases
We planned to assess publication bias by preparing funnel plots if a sufficient number of studies (10 or more) were available in the meta‐analyses. Funnel plot asymmetry can be used for many reasons, and we planned to consult the Cochrane Handbook for Systematic Reviews of Interventions to aid interpretation of these results (Sterne 2011).
Data synthesis
We entered the data into RevMan 5 (RevMan 2014) and undertook analysis according to recommended guidelines (Higgins 2011). We pooled ORs with 95% CIs for dichotomous data. We reported exclusions after randomisation and used a fixed‐effect model (Mantel‐Haenszel method) unless significant heterogeneity (P < 0.10) was detected, in which case we used a random‐effects model (DerSimonian 1986). If it was not possible to pool data, we described results in the text.
Subgroup analysis and investigation of heterogeneity
We explored potential sources of heterogeneity by stratifying participants using the subgroups given below.
-
Types of comparison intervention (type of drug).
-
Age (≤ 65 years, > 65 years).
-
Sex.
-
Renal function.
We also planned to perform a subgroup analysis according to the duration of initial therapy in the acute phase of VTE.
We formally assessed differences between subgroups by using the Chi2 test.
Sensitivity analysis
We planned to perform sensitivity analyses to examine effects of different trials and their methods, as described in Chapter 9 (Section 9.7) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); therefore, we conducted a sensitivity analysis while excluding studies at high risk of bias due to inadequate allocation concealment.
We also planned to perform a sensitivity analysis of the primary outcome while excluding trials in which more than 20% of participants were lost to follow‐up.
When we identified heterogeneity, we performed a post hoc sensitivity analysis to investigate effects of the type of baseline VTE.
'Summary of findings'
We presented the main findings of the review in a 'Summary of findings' table, reporting the quality of evidence (according to Atkins 2004), the magnitude of effect of interventions examined, and the sum of available data on VTE‐related mortality; recurrence rate of symptomatic, objectively confirmed VTE (DVT or PE) during follow‐up; and major bleeding events. We calculated assumed control intervention risks from the mean numbers of events in the control groups of selected studies for each outcome. We used GRADEpro software to assist in preparation of the 'Summary of findings' table (GRADEproGDT 2015).
Results
Description of studies
Results of the search
See Figure 1.
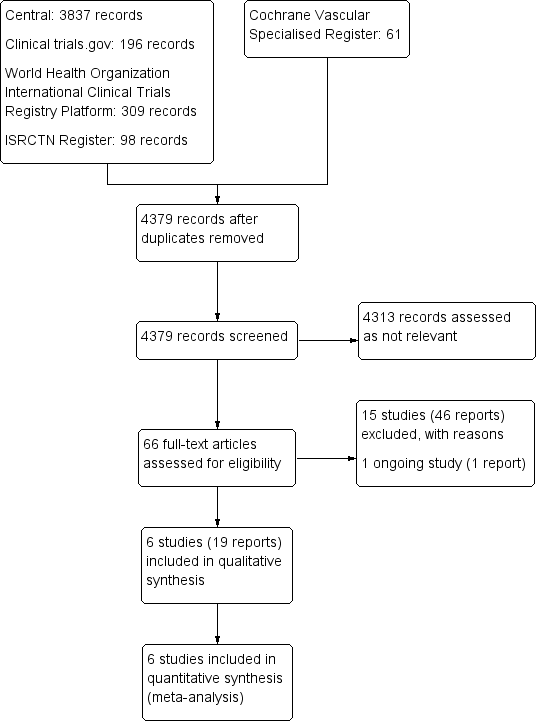
Study flow diagram.
Included studies
We included in the review six completed studies with a total of 3436 participants (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE). For detailed descriptions, see Characteristics of included studies.
ASPIRE TRIAL was a randomised, double‐blind study in which 822 participants with a first‐ever unprovoked VTE who had completed at least three months of anticoagulant therapy were randomised to receive aspirin at a dose of 100 mg or placebo for a period of two years. Outcomes included recurrent VTE, major cardiovascular events (stroke, myocardial infarction (MI), cardiovascular death), death from any cause, and bleeding (major and clinically relevant non‐major).
EINSTEIN CHOICE was a multi‐centre, randomised, double‐blind, active controlled, event‐driven study that compared two once‐daily doses of rivaroxaban (20 and 10 mg) versus aspirin (100 mg) once daily for prevention of recurrent VTE in 3365 participants who had completed 6 to 12 months of anticoagulant therapy for their index acute VTE event. For analysis in this review, we included only participants given a diagnosis of an unprovoked VTE as the index event (1389 participants). Primary outcomes included recurrent VTE and major bleeding. Secondary outcomes included MI, stroke, clinically relevant non‐major bleeding, and all‐cause mortality. Data on secondary outcomes and VTE mortality for participants with unprovoked VTE were not available in the main publication of the study, nor in the supplementary material. We contacted study authors to ask for these data, but they could not provide this information before completion of this review. Once the data are acquired, we will add them to future updates of this review.
PADIS‐PE STUDY was a randomised, double‐blind study in which 374 participants with a first‐ever unprovoked VTE who had completed at least six uninterrupted months of VKA therapy were randomised to receive warfarin or placebo for 18 months. Outcomes included recurrent VTE, VTE‐related mortality, bleeding (major and clinically relevant non‐major), and death from causes other than VTE.
WARFASA was a randomised, double‐blind study in which 403 participants with a first‐ever unprovoked VTE who had completed 6 to 18 months of anticoagulant therapy were randomised to receive aspirin 100 mg daily or placebo for a duration of two years. Outcomes included recurrent VTE, VTE‐related mortality, bleeding (major and clinically relevant non‐major), stroke, and MI.
WODIT PE was an open‐label RCT in which 181 participants with a first episode of PE who had been treated for three months were randomly assigned to extended prophylaxis or to no further anticoagulation. Participants were divided into two groups: those who had an idiopathic PE, and those who had a PE associated with a transient risk factor, including cancer, known thrombophilia, recent trauma, surgery or childbirth, prolonged immobilisation > 7 days, oral contraceptive use, or pregnancy. In total, the study included 326 participants, but for the purpose of this review, we included only 181 participants with idiopathic PE who were randomised to continue warfarin therapy for nine months or to discontinue anticoagulation. Outcomes included recurrence of symptomatic, objectively confirmed VTE, death, and major bleeding.
WODIT DVT was similar to WODIT PE, except that the study included 267 participants with an unprovoked DVT.
Excluded studies
See Characteristics of excluded studies.
In total, we excluded 15 studies from this review. We excluded seven studies because they included participants with known risk factors for VTE, including recent surgery or trauma, immobilisation, oestrogen therapy, active cancer or puerperium, and thrombophilia or other hypercoagulable states associated with increased risk of VTE (EINSTEIN‐Extension; Eischer 2009; Gibson 2017; Kearon 1999; PREVENT; RE‐MEDY; RE‐SONATE). We excluded three studies because they looked at initial anticoagulant treatment of acute VTE rather than extended prophylaxis (EINSTEIN STUDY; Nakamura 2015; RE‐COVER). We excluded two studies that included participants with a previous VTE (AMPLIFY EXT; Bleker 2016); one study that tested sulodexide, an LMWH (SURVET); and another study that tested idraparinux, which is an injectable rather than an oral anticoagulant (VAN GOGH). Last, we excluded one study because the primary outcome was the size of thrombotic masses, which is not relevant to our review (Vitovec 2009).
Ongoing studies
See Characteristics of ongoing studies.
We included one study as an ongoing study (NCT00740493). The PADIS‐TVP study is a randomised, double‐blind study in which 104 participants with a first‐ever unprovoked, proximal DVT who had completed at least six uninterrupted months of VKA therapy were randomised to receive warfarin or placebo for 18 months. Outcomes included symptomatic recurrent VTE and bleeding and death from causes other than VTE.
Risk of bias in included studies
For details on methodological quality of included studies, see Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of the six included studies reported that random sequences were generated by central computerised Internet‐based systems; therefore, we judged risk of selection bias in these studies as low (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA). The remaining two studies did not provide information on how the randomisation sequence was generated; we therefore judged the risk of selection bias to be unclear (WODIT DVT; WODIT PE).
For concealment of treatment allocation, five studies reported that this was done centrally; we therefore judged them to be at low risk (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WODIT DVT; WODIT PE). One study reported that participants were randomised according to the consecutive box number assigned to the study centre (WARFASA). As participants or investigators enrolling participants could possibly foresee assignments, we judged the risk of selection bias in this study to be high.
Blinding
Two studies were open trials comparing warfarin with a placebo (WODIT DVT; WODIT PE). Blinding could have been performed by using an identical‐looking placebo and sham INRs. Therefore we judged the risk of performance bias in these two studies as high. The remaining four studies blinded participants and personnel to treatment; therefore we deemed the risk of performance bias to be low (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA).
All six studies blinded outcome assessors to treatment; therefore we judged these studies to be at low risk of detection bias (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE).
Incomplete outcome data
All six included studies accounted for missing data; we therefore judged them to be at low risk of attrition bias (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE).
Selective reporting
All six of the included studies reported prespecified primary and secondary outcomes; therefore we judged them to be at low risk of reporting bias (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE).
Other potential sources of bias
All six included studies appeared to be free from other sources of bias (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE).
Effects of interventions
See: Summary of findings for the main comparison Extended prophylaxis compared to placebo in patients with unprovoked venous thromboembolism; Summary of findings 2 VTE extended prophylaxis compared to another VTE extended prophylaxis in patients with unprovoked venous thromboembolism
We identified five studies investigating prophylaxis versus placebo and one study comparing one type of extended prophylaxis versus another type of extended prophylaxis.
VTE extended prophylaxis versus placebo or no prophylaxis
VTE‐related mortality
Four studies measured the incidence of VTE‐related mortality between extended VTE prophylaxis and placebo (ASPIRE TRIAL; PADIS‐PE STUDY; WARFASA; WODIT DVT). Overall, data showed no clear differences in VTE‐related mortality between extended VTE prophylaxis and placebo (OR 0.98, 95% CI 0.14 to 6.98; 1862 participants; I2 = 0%). However, in the two studies comparing warfarin to placebo, no VTE‐related deaths occurred (PADIS‐PE STUDY; WODIT DVT). Subgroup analysis according to type of comparison intervention therefore was not applicable (Analysis 1.1).
Recurrent VTE
Meta‐analysis of five studies with a total of 2043 participants showed no clear difference in recurrence between extended VTE prophylaxis and placebo (OR 0.63, 95% CI 0.38 to 1.03; 2043 participants; I2 = 66%). However, the level of statistical heterogeneity was high when these studies were combined. Subgroup analysis performed for warfarin showed no clear differences between participants treated with warfarin and those given a placebo or no further prophylaxis (OR 0.52, 95% CI 0.15 to 1.80; 819 participants; 3 studies; I2 = 83%; Analysis 1.2). Of the three warfarin studies, one study included participants with DVT only (WODIT DVT), one included participants with PE only (WODIT PE), and one included participants predominantly given a diagnosis of PE with a small proportion (8.4%) given a diagnosis of DVT (PADIS‐PE STUDY). When we excluded this study from the analysis, heterogeneity disappeared (I2 = 0%). Subgroup analysis comparing aspirin versus placebo showed that the rate of recurrent VTE was lower in patients treated with placebo (OR 0.68, 95% CI 0.50 to 0.92; 1224 participants; 2 studies; I2 = 0%; Analysis 1.2). Tests for subgroup differences showed no differences between the two types of comparison interventions (P = 0.68) (Analysis 1.2).
Major bleeding
Meta‐analysis of five studies showed no clear differences in the incidence of major bleeding between extended VTE prophylaxis and placebo (OR 1.84, 95% CI 0.87 to 3.85; 2043 participants; 5 studies; I2 = 0%). Subgroup analysis performed for two types of drugs found no association for warfarin (OR 2.81, 95% CI 0.89 to 8.92; 819 participants; 3 studies; I2 = 0%) nor for aspirin (OR 1.28, 95% CI 0.47 to 3.47; 1224 participants; 2 studies; I2 = 0%). Tests for subgroup differences showed no differences between the two types of comparison interventions (P = 0.31) (Analysis 1.3).
All‐cause mortality
Meta‐analysis of five studies showed no clear differences in the incidence of all‐cause mortality between extended VTE prophylaxis and placebo (OR 1.00, 95% CI 0.63 to 1.57; 2043 participants; I2 = 0%). Analysis performed for two types of comparison interventions (warfarin and aspirin) showed no association for warfarin (OR 1.07, 95% CI 0.53 to 2.17; 819 participants; 3 studies; I2 = 0%) nor for aspirin (OR 0.95, 95% CI 0.52 to 1.72; 1224 participants; 2 studies; I2 = 0%). Tests for subgroup differences showed no differences between the two types of comparison interventions (P = 0.79) (Analysis 1.4).
Clinically relevant non‐major bleeding
Four studies measured the incidence of clinically relevant non‐major bleeding between extended VTE prophylaxis and placebo (OR 1.78, 95% CI 0.59 to 5.33; 1672 participants; I2 = 0%). However, in the two studies comparing warfarin versus placebo, no clinically relevant non‐major bleeding events occurred (WODIT DVT; WODIT PE). Subgroup analysis performed for aspirin found no association (OR 1.78, 95% CI 0.59 to 5.33; 1224 participants; 2 studies; I2 = 0%) (Analysis 1.5).
Stroke
Meta‐analysis of two studies showed no evidence of a difference in the incidence of stroke between extended VTE prophylaxis with aspirin and placebo (OR 1.15, 95% CI 0.39 to 3.46; 1224 participants; I2 = 0%) (Analysis 1.6).
Serious adverse events
Myocardial infarction
Meta‐analysis of three studies showed no evidence of a difference in the incidence of myocardial infarction between extended VTE prophylaxis and placebo (OR 1.00, 95% CI 0.35 to 2.87; 1495 participants; 3 studies; I2 = 0%). Analysis performed for two types of comparison interventions (warfarin and aspirin) found no association for warfarin (OR 1.02, 95% CI 0.20 to 5.16; 271 participants; 1 study) nor for aspirin (OR 0.98, 95% CI 0.24 to 3.94; 1224 participants; 2 studies; I2 = 0%). Tests for subgroup differences showed no differences between the two types of comparison interventions (P = 0.97) (Analysis 1.7).
None of the included studies measured other serious adverse events such as acute coronary syndrome or any life‐threatening or grade 3 to 4 non‐haematological events (such as hepatotoxicity or renal toxicity).
VTE extended prophylaxis versus another VTE extended prophylaxis
VTE‐related mortality
The EINSTEIN CHOICE study measured fatal PE but reported it for the total number of study participants rather than separately for provoked and unprovoked VTE groups. We contacted study authors to ask for these data, but they could not provide this information before completion of this review. Once the data are acquired, we will add them to future updates of this review.
Recurrent VTE
One study measured the incidence of recurrent VTE between two types of extended prophylaxis and showed that rivaroxaban was associated with a lower VTE recurrence rate than aspirin (OR 0.28, 95% CI 0.15 to 0.54; 1389 participants) (EINSTEIN CHOICE).
Major bleeding
One study measured the incidence of major bleeding between two types of extended prophylaxis and showed no clear differences in the number of major bleeding events between rivaroxaban and aspirin (OR 3.06, 95% CI 0.37 to 25.51; 1389 participants) (EINSTEIN CHOICE).
All‐cause mortality
Although all‐cause mortality was a secondary outcome of the EINSTEIN CHOICE study, researchers did not present data in the study publication nor in the supplementary material for study participants with unprovoked VTE. We contacted study authors to ask for the data, and they stated that they would provide this information. However, the data were not available before publication of this review. Once the data have been received, we will include them in future updates of this review.
Clinically relevant non‐major bleeding
One study measured the incidence of clinically relevant non‐major bleeding and showed no clear differences in the number of non‐major bleeding events between rivaroxaban and aspirin (OR 0.84, 95% CI 0.37 to 1.94; 1389 participants) (EINSTEIN CHOICE).
Stroke
Although stroke was a secondary outcome of the EINSTEIN CHOICE study, study authors did not present data in the study publication nor in the supplementary material for study participants with unprovoked VTE. We contacted study authors to ask for the data, and they stated that they would provide this information. However, the data were not available before publication of this review. Once the data have been received, we will included them in future updates of this review.
Serious adverse events
Although serious adverse events such as MI were a secondary outcome of the EINSTEIN CHOICE study, study authors did not present data in the study publication nor in the supplementary material for study participants with unprovoked VTE. We contacted study authors to ask for the data, and they stated that they would provide this information. However, the data were not available before publication of this review. Once the data have been received, we will include them in future updates of this review.
Subgroup analyses
We planned subgroup analyses by age (≤ 65 years, > 65 years), sex, renal function, and duration of initial therapy in the acute phase of VTE. However, we could not perform these subgroup analyses because participant‐level data were insufficient.
Sensitivity analyses
We performed sensitivity analyses according to the risk of bias item allocation concealment. One study did not adequately conceal treatment allocation; we therefore judged this study to be at high risk of selection bias (WARFASA). Excluding this study had no effect on the results for any outcomes. Extended prophylaxis was no more effective than placebo for prevention of VTE‐related mortality (OR 1.00, 95% CI 0.06 to 16.04), recurrent VTE (OR 0.62, 95% CI 0.31 to 1.24), major bleeding (OR 1.52, 95% CI 0.77 to 3.01), all‐cause mortality (OR 0.97, 95% CI 0.59 to 1.59), clinically relevant non‐major bleeding (OR 3.03, 95% CI 0.61 to 15.10), stroke (OR 0.80, 95% CI 0.21 to 2.99), nor serious adverse events (myocardial infarction) (OR 1.01, 95% CI 0.29 to 3.53).
We planned to perform a sensitivity analysis of the primary outcome while excluding trials in which more than 20% of participants were lost to follow‐up. However, none of the included studies had a 20% loss to follow‐up, so it was not possible to perform this analysis.
Discussion
Summary of main results
Venous thromboembolism (VTE) extended prophylaxis versus placebo or no prophylaxis
Our review included five studies with more than 2000 participants, and meta‐analysis found no evidence that extended prophylaxis is favourable over placebo in terms of preventing recurrent VTE, death, bleeding, and serious adverse events such as stroke and myocardial infarction. Subgroup analysis revealed that placebo may be favourable over aspirin for prevention of recurrent VTE.
VTE extended prophylaxis versus another VTE extended prophylaxis
One included study with more than 1300 participants with unprovoked VTE compared one type of extended VTE prophylaxis versus another. Results showed that rivaroxaban was associated with a lower VTE recurrence rate than aspirin and showed no clear differences in major bleeding or non‐major bleeding episodes between rivaroxaban and aspirin.
Overall completeness and applicability of evidence
At present, limited evidence is available to show whether oral therapeutic options such as aspirin, warfarin, and direct oral anticoagulants (DOACs) are effective and safe extended thromboprophylaxis for patients with a first unprovoked VTE who have completed at least three months of initial oral anticoagulation. Six studies met the inclusion criteria for this review (ASPIRE TRIAL; EINSTEIN CHOICE; PADIS‐PE STUDY; WARFASA; WODIT DVT; WODIT PE). Five studies compared prophylaxis versus placebo, and one study compared one type of prophylaxis versus another. Three studies compared warfarin, and two aspirin, versus placebo. All studies used similar concentrations of each drug and the standard International Society on Thrombosis and Haemostasis (ISTH) definition of major bleeding. As all trials had strict inclusion criteria, resulting in an overall participant population with almost identical conditions, statistical heterogeneity was low for all outcomes except recurrent VTE. However, the time frame for measuring outcomes ranged from 9 months to 37 months among studies. Some studies measured outcomes at the end of the treatment period, and others measured outcomes at the end of follow‐up. This could result in a situation where, if treatment is efficient, a difference is shown during and at the end of the treatment period but disappears during follow‐up. As such, the two groups would have similar overall event rates. Furthermore, the number of participants in each study was relatively small, and pooled analysis was based on a total of 3436 participants.
Data on VTE mortality and secondary outcomes for the EINSTEIN CHOICE study were not available in the main publication of this study, nor in the supplementary material provided for participants with unprovoked VTE. We contacted trial authors to request these data, but they could not provide this information before completion of this review. Once the data are acquired, we will add them to future updates of this review.
We planned subgroup analyses by age (≤ 65 years, > 65 years), sex, renal function, and duration of initial therapy for the acute phase of VTE. However, we could not perform these subgroup analyses because participant‐level data were insufficient.
Quality of the evidence
See summary of findings Table for the main comparison and summary of findings Table 2.
For the comparison extended prophylaxis versus placebo and for the outcomes recurrent VTE and all‐cause mortality, we downgraded the quality of the evidence to moderate owing to concerns arising from risk of bias in individual studies. One study was at risk of selection bias (WARFASA), and two studies were at risk of performance bias (WODIT DVT; WODIT PE). For all other outcomes in this review (VTE‐related mortality, major bleeding, clinically relevant non‐major bleeding, stroke, and serious adverse events), we downgraded the quality of the evidence to low because of concerns arising from risks of selection and performance bias for the studies stated above, combined with concerns over imprecision, as reflected by small numbers of outcome events and wide confidence intervals around the estimate of effect for these outcomes.
For the comparison extended prophylaxis versus extended prophylaxis and for the outcomes recurrent VTE, major bleeding, and clinically relevant non‐major bleeding, we downgraded the quality of the evidence to moderate owing to concerns over imprecision, as reflected by small numbers of outcome events and wide confidence intervals around the estimate of effect for these outcomes. The single study included under this comparison was at low risk of bias, so we did not further downgrade the quality of the evidence (EINSTEIN CHOICE).
Potential biases in the review process
The search was as comprehensive as possible, and we are confident that we have included all relevant studies. However, the possibility remains that we missed some relevant trials, particularly in the grey literature (e.g. conference proceedings). Two review authors independently performed study selection and data extraction to minimise bias in the review process. We performed data collection according to the process suggested by Cochrane. We also followed Cochrane processes as described by Higgins 2011 for assessing risk of bias.
The inclusion criteria for this review stipulate that participants must have completed at least three months' anticoagulation treatment before receiving extended prophylaxis. In one of the studies included in this review, 1% of participants had completed less than three months' anticoagulation treatment before randomisation (ASPIRE TRIAL). We attempted to contact the authors of this study to obtain outcome data excluding these participants, but we did not receive a reply. As this number was very small, it is unlikely to have affected the overall result. Furthermore, the number excluded from each group was 1% and was equally distributed between treatments. Therefore, we did not see the need to exclude this study from the review on this basis.
Similarly, our inclusion criteria required inclusion of participants aged 18 years and older, and two studies included participants aged 15 to 85 years (WODIT DVT; WODIT PE). Given that the mean age of participants in the trial was 67 (SD 12.4) years, it is likely that the trial included only a small number of participants under 18 years of age. Furthermore, as this was a randomised controlled trial, the number of participants under 18 years of age would be equally distributed between the two treatment groups, and this is unlikely to have any effect on the overall result.
Agreements and disagreements with other studies or reviews
Several researchers have studied DOACs such as dabigatran, rivaroxaban, and apixaban for extended treatment of patients with VTE after the initial anticoagulation period. Four randomised controlled trials reported that, when compared to placebo or warfarin, all DOACs demonstrated efficacy in reducing VTE recurrence, some with lower bleeding rates compared to warfarin (AMPLIFY EXT; EINSTEIN‐Extension;RE‐MEDY; RE‐SONATE). One of the studies included in our review compared rivaroxaban with aspirin and found a reduction in the VTE recurrence rate among patients treated with rivaroxaban compared with aspirin (EINSTEIN CHOICE). When compared to placebo, investigators found increased bleeding rates with dabigatran and rivaroxaban but no increase in the rate of major bleeding with apixaban (AMPLIFY EXT; EINSTEIN‐Extension; RE‐MEDY; RE‐SONATE). We excluded these four randomised controlled trials from the review, as some participants had a previous VTE (AMPLIFY EXT) or had a provoked VTE (EINSTEIN‐Extension; RE‐MEDY; RE‐SONATE), and we were unable to obtain data for participants with unprovoked VTE.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 1 VTE‐related mortality.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 2 Recurrent VTE.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 3 Major bleeding.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 4 All‐cause mortality.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 5 Clinically relevant non‐major bleeding.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 6 Stroke.

Comparison 1 Extended VTE prophylaxis versus placebo, Outcome 7 Serious adverse events (myocardial infarction).

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 1 Recurrent VTE.

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 2 Major bleeding.

Comparison 2 Extended VTE prophylaxis versus another extended VTE prophylaxis, Outcome 3 Clinically relevant non‐major bleeding.
| Extended prophylaxis compared to placebo in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with extended prophylaxis | |||||
| VTE‐related mortalitya | Study population | OR 0.98 | 1862 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of VTE‐related mortality. | |
| 2 per 1000 | 2 per 1000 | |||||
| Recurrent VTEc | Study population | OR 0.63 | 2043 | ⊕⊕⊕⊝ | ||
| 170 per 1000 | 114 per 1000 | |||||
| Major bleedinge | Study population | OR 1.84 | 2043 | ⊕⊕⊝⊝ | ||
| 11 per 1000 | 20 per 1000 | |||||
| All‐cause mortalityg | Study population | OR 1.00 | 2043 | ⊕⊕⊕⊝ | ||
| 38 per 1000 | 38 per 1000 | |||||
| Clinically relevant non‐major bleedingh | Study population | OR 1.78 | 1672 | ⊕⊕⊝⊝ | Two of the four studies reported no cases of clinically relevant non‐major bleeding. | |
| 6 per 1000 | 11 per 1000 | |||||
| Strokei | Study population | OR 1.15 | 1224 | ⊕⊕⊕⊝ | ||
| 10 per 1000 | 11 per 1000 | |||||
| Myocardial infarction | Study population | OR 1.00 | 1495 | ⊕⊕⊕⊝ | ||
| 9 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
| VTE extended prophylaxis compared to another VTE extended prophylaxis in patients with unprovoked venous thromboembolism | ||||||
| Patient or population: patients with unprovoked venous thromboembolism | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with aspirin | Risk with rivaroxaban | |||||
| VTE‐related mortalitya | See comments. | See comments. | See comments. | See comments. | Data on VTE‐related mortality are yet available for participants with unprovoked VTE. | |
| Recurrent VTEb | Study population | OR 0.28 | 1389 | ⊕⊕⊕⊝ | ||
| 56 per 1000 | 16 per 1000 | |||||
| Major bleedingd | Study population | OR 3.06 | 1389 | ⊕⊕⊕⊝ | ||
| 2 per 1000 | 7 per 1000 | |||||
| All‐cause mortalitye | See comments. | See comments. | See comments. | See comments. | Data on all‐cause mortality are not yet available for participants with unprovoked VTE. | |
| Clinically relevant non‐major bleedingf | 19 per 1000 | 16 per 1000 (7 to 37) | OR 0.84 (0.37 to 1.94) | 1389 | ⊕⊕⊕⊝ | |
| Strokeg | See comments. | See comments. | See comments. | See comments. | Data on stroke are not yet available for participants with unprovoked VTE. | |
| Myocardial infarction | See comments. | See comments. | See comments. | See comments. | Data on myocardial infarction are not yet available for participants with unprovoked VTE. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aVTE‐related mortality ‐ death due to fatal PE. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 VTE‐related mortality Show forest plot | 4 | 1862 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| 1.1 Warfarin vs placebo | 2 | 638 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.98] |
| 2 Recurrent VTE Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.38, 1.03] |
| 2.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.15, 1.80] |
| 2.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.92] |
| 3 Major bleeding Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.87, 3.85] |
| 3.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.89, 8.92] |
| 3.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.47, 3.47] |
| 4 All‐cause mortality Show forest plot | 5 | 2043 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.63, 1.57] |
| 4.1 Warfarin vs placebo | 3 | 819 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.53, 2.17] |
| 4.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 5 Clinically relevant non‐major bleeding Show forest plot | 4 | 1672 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| 5.1 Warfarin vs placebo | 2 | 448 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.59, 5.33] |
| 6 Stroke Show forest plot | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| 6.1 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.46] |
| 7 Serious adverse events (myocardial infarction) Show forest plot | 3 | 1495 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.35, 2.87] |
| 7.1 Warfarin vs placebo | 1 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.16] |
| 7.2 Aspirin vs placebo | 2 | 1224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent VTE Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Clinically relevant non‐major bleeding Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

