Herramientas creadas y distribuidas por los realizadores de guías para promover la adopción de las mismas
Resumen
Antecedentes
La adopción de las guías para la práctica clínica (GPC) es inconsistente, a pesar de su potencial de mejorar la calidad de la asistencia sanitaria y los resultados del paciente. Algunos realizadores de guías han considerado este problema mediante la creación de herramientas para promover la adopción más rápida de nuevas guías. Esta revisión se centra en la efectividad de las herramientas creadas y distribuidas por los realizadores de guías para mejorar la adopción de las GPC.
Objetivos
Evaluar la efectividad de las herramientas de implementación creadas y distribuidas por los realizadores de guías, que acompañan o siguen a la publicación de una GPC, para promover la adopción. Un objetivo secundario es determinar qué enfoques a la implementación de las guías son más efectivos.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane para una Práctica y Organización Sanitaria Efectivas (Cochrane Effective Practice and Organisation of Care Group, EPOC), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL); NHS Economic Evaluation Database, HTA Database; MEDLINE y MEDLINE In‐Process and other non‐indexed citations; Embase; PsycINFO; CINAHL; Dissertations and Theses, ProQuest; Index to Theses; Science Citation Index Expanded, ISI Web of Knowledge; Conference Proceedings Citation Index ‐ Science, ISI Web of Knowledge; Health Management Information Consortium (HMIC), y NHS Evidence hasta febrero 2016. También se hicieron búsquedas en registros de ensayos, en listas de referencias de estudios incluidos y en sitios web relevantes.
Criterios de selección
Se incluyeron ensayos controlados aleatorios (ECA) y ECA con asignación al azar por grupos, estudios controlados de antes y después (CAD) y estudios de series de tiempo interrumpido (STI) que evaluaban los efectos de las herramientas de implementación de guías creadas por realizadores reconocidos de guías para mejorar la adopción de sus propias guías. La guía podía estar dirigida a cualquier área clínica.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, extrajeron los datos y evaluaron el riesgo de sesgo de cada estudio incluido mediante los criterios Cochrane del "Riesgo de sesgo". La confianza en las pruebas se calificó mediante el enfoque recomendado por el grupo de trabajo de GRADE. Las afecciones clínicas estudiadas y las herramientas de implementación utilizadas fueron demasiado heterogéneas para combinar los datos en un metanálisis. Se informa la diferencia de riesgos absoluta (DRA) mediana y el rango intercuartil (RIC) para el resultado principal del cumplimiento de las guías.
Resultados principales
Se incluyeron cuatro ECA con asignación al azar por grupos realizados en los Países Bajos, Francia, los EE.UU. y Canadá. Estos estudios evaluaron los efectos de las herramientas creadas por los realizadores de guías nacionales para implementar sus GPC. Las herramientas de implementación evaluadas se dirigieron a los profesionales de la asistencia sanitaria; ninguna estuvo dirigida a las organizaciones de asistencia sanitaria o a los pacientes.
Un estudio usó dos talleres educacionales cortos adaptados a las barreras. En tres estudios la intervención constó de la provisión de material didáctico basado en artículos, formularios de pedido o recordatorios, o ambos. La afección clínica, el tipo de profesional de la asistencia sanitaria y el comportamiento considerados por la GPC variaron entre los estudios.
Dos de los cuatro estudios incluidos informaron datos sobre el cumplimiento de las guías por parte de los profesionales de asistencia sanitaria. Una herramienta para el cumplimiento de las guías creada por los realizadores de una guía probablemente da lugar a un mayor cumplimiento de las mismas; la DRA mediana (RIC) fue de 0,135 (0,115 y 0,159 para los dos estudios respectivamente) al momento de un seguimiento promedio de cuatro semanas (pruebas de certidumbre moderada), lo cual indica un cumplimiento mediano del 13,5% mayor de las guías en el grupo de intervención. La provisión de una herramienta para mejorar la implementación de una guía para los profesionales de la asistencia sanitaria puede dar lugar a poca o ninguna diferencia en los costos para el servicio de salud.
Conclusiones de los autores
Las herramientas de implementación creadas por realizadores reconocidos de guías probablemente dan lugar a la mejoría del cumplimiento por parte de los profesionales de la asistencia sanitaria de las guías en el tratamiento del lumbago no específico y el pedido de pruebas de función de la tiroides. Hay datos limitados sobre los costos relativos de la implementación de las intervenciones. No hay estudios que evalúen la efectividad de las intervenciones dirigidas a la organización de la atención (p.ej. herramientas de evaluación comparativa, plantillas de cálculo de costos, etc.), o de las intervenciones relacionadas con los medios masivos de comunicación. No fue posible establecer conclusiones acerca del segundo objetivo, la efectividad comparativa de las herramientas de implementación, debido al número pequeño de estudios, la heterogeneidad entre las intervenciones y los trastornos clínicos considerados.
PICOs
Resumen en términos sencillos
Efectividad de las herramientas creadas y distribuidas por los realizadores de guías para mejorar la adopción de las mismas
Antecedentes
Las guías para la práctica clínica (GPC) son recomendaciones basadas en pruebas para los profesionales de la asistencia sanitaria acerca de la atención de los pacientes con trastornos específicos. La adopción de las GPC por parte de los profesionales de la asistencia sanitaria es inconsistente, a pesar de su potencial para mejorar la calidad de la asistencia sanitaria y los resultados del paciente. Algunos realizadores de guías han considerado este problema mediante la creación de herramientas para promover la adopción de guías nuevas. Esta revisión se centra en la efectividad de las herramientas creadas y distribuidas por realizadores reconocidos de guías para mejorar la adopción de sus GPC.
Características de los estudios incluidos
Los investigadores de Cochrane realizaron búsquedas en la bibliografía hasta febrero de 2016 e identificaron cuatro estudios aleatorios que evaluaban los efectos de las herramientas desarrolladas por realizadores reconocidos de guías para implementar las mismas. Dichas guías fueron desarrolladas por los realizadores de guías de Francia, los Países Bajos y de los EE.UU. y Canadá. En los cuatro estudios las intervenciones se dirigieron al profesional sanitario. Ninguna de las herramientas se dirigió específicamente a la organización de la atención o al paciente. Las afecciones clínicas y el comportamiento de los profesionales de la asistencia sanitaria considerados en la GPC variaron entre los estudios, al igual que las herramientas utilizadas para mejorar la implementación de la guía.
Resultados clave
Dos de los cuatro estudios incluidos informaron sobre cuán adecuado es el cumplimiento de las recomendaciones de las guías por parte de los profesionales sanitarios al administrar atención a los pacientes, dependiendo de si recibieron una GPC con una herramienta dirigida a la mejoría del uso de la misma, o si recibieron la GPC solamente. Los resultados de esta revisión muestran que los profesionales de la asistencia sanitaria que recibieron una herramienta de implementación de las guías junto con la GPC sobre el tratamiento del lumbago no específico o el pedido de pruebas de la función de la tiroides probablemente cumplen más estrictamente las recomendaciones, en comparación con los que recibieron la GPC solamente. Una herramienta para la implementación de las guías, dirigida a mejorar el uso de las mismas, puede dar lugar a poca o ninguna diferencia en el costo para el servicio de salud.
Certeza de las pruebas incluidas
Las pruebas incluidas provinieron de ensayos controlados aleatorios, que se consideran el nivel más alto de pruebas. Sin embargo, debido a riesgo alto de sesgo en los estudios incluidos la confianza en el efecto sobre la observación de las recomendaciones de las guías fue moderada. La confianza en las pruebas para el costo‐eficacia fue baja, debido a que sólo un único estudio proporcionó pruebas sobre esta comparación.
Conclusiones de los autores
Summary of findings
| Clinical practice guideline (CPG) + implementation tool compared with CPG only for adherence to guidelines | ||||
| Patient or population: Healthcare professionals (physiotherapists, hospital physicians) providing care for people with different clinical conditions (patients with non specific low back pain, patients with symptoms indicating a need for a thyroid function test) Setting: Private physiotherapy clinics in the Netherlands, general hospitals in France Intervention: CPG + guideline implementation tool (e.g. training workshops, paper‐based materials and order forms, reminders, web‐based tools) | ||||
| Outcomes | Median ARD (Absolute risk difference) | No of Participants | Certainty of the evidence | Comments |
| Adherence to guidelines | Guideline tools provided to healthcare professionals as an aid to improve the use of a CPG probably lead to improved adherence with the CPG, as compared to guidelines only. Median ARD: 0.135 (0.115 to 0.15.9) at mean 4 weeks follow‐up | 68 physio‐ therapy practices; and 6 hospitals (2 C‐RCTs) | ⊕⊕⊕⊝ | 2 of the 4 included studies reported a proxy measure for adherence to guidelines, and results from these studies could therefore not be included in the ARD calculation |
| Costs | Guideline tools aimed at improving the use of guidelines may lead to little or no difference in healthcare costs | 68 physio‐ therapy clinics (1 C‐RCT) | ⊕⊕⊝⊝ low2 | 1 trial reported no difference in mean direct annual cost* per patient between intervention and control groups. 1 French paper belonging to 1 of the included trials (6 hospitals) and reporting on costs awaits translation |
| * Direct costs included costs of the dissemination of the guideline and healthcare resource use by the patient GRADE Working Group grades of evidence | ||||
| 1We downgraded the certainty of the evidence one step due to high risk of bias. 2We downgraded the certainty of the evidence two steps due to imprecision. | ||||
Antecedentes
La adopción de las guías para la práctica clínica (GPC) es inconsistente, a pesar de su potencial de mejorar la calidad de la asistencia sanitaria y los resultados del paciente (Grol 2003; Schuster 1998; Seddon 2001). Se han creado diversas herramientas para mejorar la adopción de las GPC, aunque no siempre por parte de los mismos realizadores de las guías. Esta revisión se centra en la efectividad de las herramientas creadas y distribuidas por los realizadores de guías para mejorar la adopción de las GPC. Estas herramientas están integradas a una guía, por ejemplo la adaptación de una recomendación de las guías para un grupo particular de usuarios, o acompañan la GPC, por ejemplo los módulos de aprendizaje interactivos desarrollados para apoyar el uso de una GPC.
Descripción de la afección
Las GPC tienen el potencial de mejorar la prestación de asistencia sanitaria y los resultados, aunque la adopción de las guías por parte de los profesionales de la asistencia sanitaria y los coordinadores de los sistemas de salud es inconsistente, y aún hay brechas entre la atención recomendada y la práctica clínica. Las revisiones sistemáticas anteriores han identificado un rango de intervenciones para apoyar la implementación de las guías (Grimshaw 2004). Sin embargo, la mayoría de las mismas se ha desarrollado de forma independiente de los realizadores de las guías. En respuesta a este hecho, algunos realizadores de guías han creado herramientas para mejorar la adopción de las GPC. Algunas de estas intervenciones se centran en la mejoría de las aptitudes necesarias para aplicar las pruebas a la práctica y otras procuran integrar el contenido de una GPC en un sistema de asistencia sanitaria local. El valor de estas herramientas ha sido cuestionado por el National Institute for Health and Care Excellence (NICE), (New Reference, Leng 2013 [com pers]) debido a que son una inversión adicional para el realizador de guías y las pruebas de la efectividad de este enfoque son inciertas.
Descripción de la intervención
Las intervenciones creadas y distribuidas por los realizadores de guías para mejorar el uso consistente de las GPC por parte de los profesionales de la salud y los coordinadores de sistemas de salud incluyen módulos de aprendizaje (que pueden acreditarse con puntos de la Continuing Medical Education [CME]), visitas de extensión educativas (p.ej. visitas académicas), herramientas de comunicación (p.ej. comunicados de prensa posteriores a la publicación de las GPC) o formato adaptado (p.ej. redacción de las recomendaciones adaptada al público destinatario o al sistema de salud). Los módulos de aprendizaje son un enfoque popular para apoyar el uso de GPC; p.ej. NICE ha desarrollado un rango de herramientas educacionales en línea (NICE 2012b) en colaboración con BMJ Learning, Nursing Times y e‐Learning for Health (por ejemplo la herramienta educacional en línea eVTE para reducir el riesgo de tromboembolia venosa (eVTE 2013)). El objetivo es permitir a los usuarios de las GPC ser más conscientes de las pruebas recientes como lo resumido en las guías relevantes de NICE y aplicar el conocimiento recién adquirido en la práctica y considerar cualquier barrera potencial. Los ejemplos de los realizadores de GPC que funcionan dentro de los sistemas de salud para mejorar la adopción de las GPC incluyen: NICE junto con el National Health Service (NHS) en Inglaterra y Gales mediante normas de calidad a los comisionados (NICE 2016); la Scottish Intercollegiate Guidelines Network (SIGN) que proporciona módulos de aprendizaje en grupos pequeños basados en la solución de problemas (SIGN 2012); el American College of Cardiology que proporciona un aplicativo clínico de guía y la ejecución del proyecto Guidelines in Practice (GAP) para proporcionar herramientas de implementación adaptadas y específicas de las guías (ACC 2016; Mehta 2002); la Veterans Health Administration que adapta sus GPC sobre la detección colorrectal a las organizaciones de salud locales; el sistema de asistencia sanitaria Kaiser Permanente que ha desarrollado e implementado el Proactive Officer Encounter Programme para proporcionar apoyo a las decisiones clínicas y aumentar la adopción de la propia y de otras GPC (Kanter 2010); y el National Prescribing Centre del Reino Unido que estableció las “comunidades de la práctica” (las NHS Medicines y las comunidades de la práctica de prescripción). Los datos de la NHS Evidence indican que un 92% (33/36) de los realizadores de guías que presentan las GPC para su acreditación por medio de NHS Evidence publican las herramientas de apoyo dirigidas a ayudar en la implementación de su guía (NHS 2012a). Muchos realizadores de guías están trabajando en la transformación de sus GPC narrativas a un formato electrónico, debido a que este procedimiento puede mejorar la adopción mediante la implementación de las GPC en los sistemas de apoyo a las decisiones por computadora (Peleg 2010).
De qué manera podría funcionar la intervención
Los realizadores de GPC que desarrollan intervenciones de implementación para apoyar su uso se han centrado en las necesidades de información de diferentes grupos de usuarios. Las intervenciones tienen como finalidad mejorar la conciencia sobre las GPC, fortaleciendo las aptitudes necesarias para comprender e implementar una GPC, y apoyar el uso de la misma dentro del contexto de un sistema de asistencia sanitaria local (Greenhalgh 2005). La adaptación de la implementación de las intervenciones para facilitar el cambio en la práctica (para promover una GPC) habitualmente incluye la identificación de los determinantes de la práctica de la asistencia sanitaria. Lo anterior puede incluir discusiones con los profesionales de la asistencia sanitaria acerca de las barreras potenciales y los sistemas que requieren un cambio (Baker 2015), la identificación de formas para facilitar el cambio y el diseño, la aplicación y evaluación de las intervenciones apropiadas (Wensing 2011). El instrumento de la Guideline Implementability Appraisal (GLIA) puede ser usado por los realizadores de las guías para identificar las barreras a la implementación durante la fase de diseño de una GPC y permitir las modificaciones antes de la publicación (Shiffman 2005). Por ejemplo, pueden crearse plantillas para los usuarios de las GPC para llenar con datos locales y evaluar la aplicabilidad y el impacto de una GPC. El proceso de adaptación también es importante para involucrar a los médicos en el proceso de implementación (Horbar 2004; Titler 2009). Los resultados de una encuesta reciente de más de 300 individuos del personal de servicio del NHS, que usan GPC para guiar la toma de decisiones, confirman la importancia de estos enfoques. La inteligencia de salud pública local, el asesoramiento de expertos y los ejemplos de la práctica adecuada parecen ser los tipos más codiciados de pruebas, y para que se utilice el conocimiento tiene que traducirse en un recurso práctico (Gkeredakis 2011). Finalmente, si un productor de guías tiene autoridad y trabaja dentro del sistema de salud, o se percibe que es influyente, la adopción de las GPC puede mejorar (Rogers 1995).
Otros determinantes de la implementación efectiva de todas las GPC son que se escriben de forma clara, son específicas para una población y contexto, son de fácil uso y que hay pruebas de investigación de su efectividad para el contexto de trabajo de un usuario particular (Titler 2001). La formulación de guías por lo general es realizada por un grupo multidisciplinario representativo a nivel nacional, que realiza una revisión sistemática para identificar y evaluar críticamente las pruebas y que aseguran que las recomendaciones de las guías estén vinculadas explícitamente a las pruebas de apoyo. Las opiniones de los expertos también se usan en las GPC cuando no hay pruebas de investigación disponibles. Los realizadores de las guías también pueden utilizar la herramienta AGREE mediante la cual puede evaluarse la calidad de una guía, lo cual permite a los usuarios decidir cuán bien se ha desarrollado una guía y si será aplicable al contexto en el que están trabajando (AGREE 2010).
El formato utilizado para comunicar el contenido de una GPC también puede influir en su adopción (Greenhalgh 2005; Rogers 1995). Aunque las GPC con frecuencia se escriben como documentos de texto (Peleg 2010), los estudios han revelado que los médicos generalmente no usan las guías escritas durante el proceso real de atención (Wang 2002). En cambio, se sugiere que el asesoramiento específico sobre el paciente, en particular si se proporciona durante los encuentros con el paciente, es más efectivo para cambiar del comportamiento de los médicos (Shea 1996). Por lo tanto, la implementación de las GPC en los sistemas de apoyo a las decisiones por computadora puede mejorar la aceptación y la aplicación de las guías en la práctica diaria, en particular si las acciones y observaciones de los trabajadores de la asistencia sanitaria son controlados y se genera asesoramiento cuando no se sigue una guía (Wang 2002). Un ejemplo de realizadores de guías que han proporcionado a los profesionales de la asistencia sanitaria el apoyo a las decisiones clínicas para aumentar la adopción de las GPC es el sistema de asistencia sanitaria Kaiser Permanente con su Proactive Officer Encounter Programme (Kanter 2010).
Gagliardi 2011 identificó ocho características de las GPC que son deseadas por los usuarios de las mismas, o que se asocian con su uso:
-
Facilidad de uso: la estructura de la GPC se ha modificado para facilitar el acceso, por ejemplo al proporcionar un resumen de una página de recomendaciones;
-
Adaptabilidad: la GPC está disponible en diferentes formatos para diferentes usuarios o finalidades, p.ej. formato impreso y electrónico, y hay versiones de las GPC disponibles para los pacientes y los cuidadores;
-
Validez: uso de un sistema estandarizado para calificar la calidad de las pruebas que apoyan cada recomendación, por ejemplo GRADE;
-
Aplicabilidad: la redacción de la recomendación de las GPC se ha adaptado para diferentes públicos destinatarios con objeto de apoyar la aplicación de la guía a las circunstancias locales; lo anterior puede incluir información clínica y contextual;
-
Transmisibilidad: información para complementar la GPC, p.ej. recursos educacionales para los pacientes e información para apoyar el compromiso del paciente;
-
Adaptación: agregado de información sobre los costos y los recursos, p.ej. las plantillas de cálculo de costos proporcionadas por NICE, e información sobre las competencias y el entrenamiento necesario para poner en práctica las recomendaciones;
-
Implementación: información sobre las barreras potenciales y las estrategias para facilitar la ejecución, p.ej. una evaluación clínica mediante una plantilla en el punto de atención;
-
Evaluación: medidas del rendimiento o los indicadores de la calidad para la auditoría y la vigilancia.
Por qué es importante realizar esta revisión
Las GPC pueden mejorar la prestación de asistencia sanitaria y los resultados, aunque la adopción de las guías por parte de los médicos y los gestores de asistencia sanitaria es inconsistente. Las Revisiones Cochrane anteriores han descrito la efectividad de un rango de intervenciones para apoyar la implementación de las guías (Akl 2013; Flodgren 2011; Flodgren 2013a; Forsetlund 2009; Giguère 2012; Grilli 2002; Jamtvedt 2006; O'Brien 2007; Shojania 2009). Sin embargo, la mayoría de las mismas se ha desarrollado de forma independiente de los realizadores de las guías. En respuesta a la inquietud continua acerca del uso inconsistente de las GPC, algunos realizadores de guías nacionales han creado e implementado herramientas para apoyar la adopción de sus GPC. La misma es una inversión adicional para el productor de guías y se desconoce la efectividad de este enfoque. El foco de esta revisión es evaluar la efectividad de las herramientas de ejecución, creadas y distribuidas por los realizadores de guías, sobre la adopción de las mismas. Estas herramientas pueden requerir cambios en la presentación de la GPC (p.ej. adaptación de una GPC), o que se publiquen junto con las GPC (p.ej. módulos de aprendizaje en línea).
Objetivos
Evaluar la efectividad de las herramientas de implementación creadas y distribuidas por los realizadores de guías, que acompañan o siguen a la publicación de una GPC, para promover la adopción.
Un objetivo secundario es determinar qué enfoques a la implementación de las guías son más efectivos.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se intentó incluir ensayos controlados aleatorios (ECA), ensayos con asignación al azar por grupos (ECA‐G), estudios controlados de antes y después (CAD) y estudios de series de tiempo interrumpido (STI) que evaluaban los efectos de las herramientas de implementación de guías desarrolladas por realizadores reconocidos de las mismas para mejorar la adopción de sus propias guías. Los estudios CAD reunieron los requisitos para la inclusión si incluían al menos dos sitios de intervención y dos de control, y los estudios STI reunieron los requisitos si tenían al menos tres puntos de datos antes y tres puntos de datos después de la intervención.
Tipos de participantes
Se incluyó a todos los profesionales de la asistencia sanitaria capacitados, los coordinadores de sistemas de salud y los elaboradores de políticas.
Se excluyeron los estudios que incluían a pasantes o a estudiantes de medicina.
Tipos de intervenciones
Se incluyó cualquier intervención desarrollada por los realizadores de GPC para mejorar la implementación de las guías. Los realizadores de guías incluyen, p.ej. la Organización Mundial de la Salud (OMS), NICE y SIGN. Debido a que las guías pueden producirse para una jurisdicción, un sistema de salud o un grupo de profesionales sanitarios específico, las intervenciones para mejorar la implementación de estas GPC pueden distribuirse a las organizaciones pero dirigidas a los individuos dentro de la organización, o pueden dirigirse a las organizaciones completas. Se utilizó la definición de GPC desarrollada por el Institute of Medicine de los EE.UU.: "las guías clínicas son declaraciones desarrolladas sistemáticamente para ayudar en las decisiones del profesional de la asistencia sanitaria y el paciente acerca de la asistencia sanitaria apropiada para circunstancias clínicas específicas" (Field 1990).
Mediante el uso de la taxonomía de EPOC (EPOC taxonomy 2002) como una guía, se desarrolló la siguiente clasificación para organizar y definir las intervenciones a continuación:
1. Herramientas dirigidas al profesional de la asistencia sanitaria
i) Adaptación
-
La adaptación de las GPC para diferentes usuarios con objeto de mejorar la facilidad de uso y la aplicabilidad: algunos ejemplos incluyen el uso de una redacción diferente, variación del contenido, incorporación de estudios de casos de las experiencias de los pacientes en forma de viñetas o descripciones que contextualizan las recomendaciones.
-
Diferentes formatos de GPC adaptados a diferentes usuarios/finalidades, p.ej. electrónico (para el uso en un Personal Digital Assistant), documento, versiones multimedia, resúmenes, inclusión de algoritmos.
ii) Educación
-
Módulos de aprendizaje (para incluir módulos de aprendizaje interactivos) que pueden acreditarse con puntos en la Continuing Medical Education (CME), o para apoyar el uso de auditoría por parte de los médicos auxiliares.
-
Instrucciones/plantillas, p.ej. instrucciones, herramientas o plantillas para adaptar las guías/recomendaciones para el contexto local (también puede usarse a nivel institucional); plantillas/formularios en el punto de atención (evaluación clínica, órdenes estándar).
-
Sistemas de apoyo a las decisiones, p.ej. guías electrónicas con sistemas incorporados de apoyo a las decisiones.
2. Herramientas dirigidas al paciente
-
Producción de versiones de las recomendaciones de las GPC para el público con objeto de mejorar la comunicación profesional‐paciente acerca de las recomendaciones de las guías.
3. Herramientas dirigidas a la organización de la atención
-
Herramientas de evaluación comparativa, p.ej. medidas de las brechas en el rendimiento que van a ser usadas por los que controlan la implementación de las GPC (también pueden ser usadas por profesionales de la asistencia sanitaria individuales).
-
Plantillas de cálculo de costos como una ayuda presupuestaria (también puede ser usado por profesionales de la asistencia sanitaria individuales) para evaluar los recursos requeridos para implementar la GPC.
-
Evaluación de programas, herramientas de auditoría, medidas de rendimiento e indicadores de calidad para evaluar la implementación de la GPC.
4. Intervenciones de medios masivos de comunicación
-
Comunicados de prensa después de la publicación de una GPC.
Las comparaciones consideradas son las siguientes:
-
Herramientas creadas por un productor de guías versus herramienta creada por otra organización o un usuario de guías (es decir herramientas desarrolladas de forma independiente del productor de la GPC).
-
Herramientas creadas por un productor de guías versus ninguna herramienta (es decir GPC solas).
Se excluyeron los siguientes tipos de estudios/intervenciones:
-
Herramientas desarrolladas por grupos de investigadores, grupos de guías a comisión (ya no existente).
-
Estudios que describen las herramientas desarrolladas por los realizadores de guías para mejorar la adopción de las guías sin proporcionar mediciones objetivas del efecto de estas intervenciones sobre la práctica profesional o los resultados del paciente.
-
Encuestas de las barreras/facilitadores a la adopción de las guías.
Tipos de medida de resultado
Se incluyeron estudios que informan las siguientes medidas de resultado:
Resultados primarios
Medidas objetivas del rendimiento de los profesionales de la asistencia sanitaria, uso de recursos de asistencia sanitaria o resultados de los pacientes.
Resultados secundarios
Medidas informadas por el participante del rendimiento de los profesionales de la asistencia sanitaria y el rendimiento del gestor de asistencia sanitaria, incluido el conocimiento o el uso de GPC y los costos.
Se excluyeron los estudios que sólo incluían resultados informados por el participante.
Results
Description of studies
Results of the search
See Characteristics of included studies; Characteristics of ongoing studies and Characteristics of studies awaiting classification tables.
The electronic searches yielded 47,181 citations, down to 26,384 after removal of duplicates. Additional sources searched (including websites and reference lists) yielded 473 citations. Of the these 26,857 citations, we excluded 25,801 irrelevant studies and retrieved and scrutinised 1,056 studies. Of these 1,056 double‐screened studies we excluded 1030 studies and added 14 to the excluded studies table. We listed one study protocol and one conference abstract under 'Ongoing studies' (Salbach 2014; Te Boveldt 2011), and two studies under 'Studies awaiting classification' (Maximov 2012; Van Driel 2007). We judged four studies to be eligible for inclusion in the review. See study flowchart Figure 1.
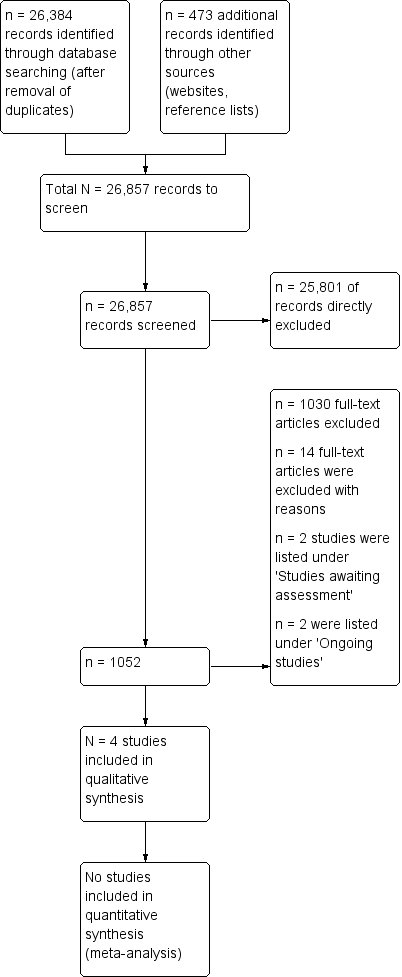
Study flow diagram.
Included studies
We identified four eligible studies of cluster‐RCTs (Bekkering 2005; Daucourt 2003; Fine 2003; Shah 2014) for inclusion in this review, of which one (Shah 2014) consisted of two separate cluster‐RCTs: one a population‐based C‐RCT including all family practices in Ontario, Canada, and the other an embedded C‐RCT including a subsample of the family practices from the larger study.
Populations
Healthcare professionals
In Bekkering 2005 the participants were physiotherapists (n = 113); and in two studies (Daucourt 2003; Fine 2003) the participants were physicians other than general practitioners (GPs) (n = 1913), or family physicians (number not reported), and in one study the intervention was targeted at family physicians (Shah 2014). None of the studies targeted patients, health system managers or policy makers.
Patients
The number and clinical condition of participants in the included studies were as follows: participants (n = 500) with non‐specific low back pain (Bekkering 2005); participants (n = 608) with hospital‐acquired pneumonia (Fine 2003); an unknown number of patients who required a thyroid‐function test (Daucourt 2003); and people with diabetes > 40 years old (n = 933,769) in Ontario (administrative study) and a subgroup of people with diabetes (n = 1592) at high risk of cardiovascular disease (clinical study) (Shah 2014).
Settings
Bekkering 2005 was set in private physiotherapy practices (n = 68); two studies (Daucourt 2003; Fine 2003) were set in hospitals (n = 13), and Shah 2014 was set in family practices (n = 4007 and n = 80 respectively). The studies were conducted in the Netherlands (Bekkering 2005), France (Daucourt 2003), the USA (Fine 2003) and in Canada (Shah 2014) .
Targeted behaviour
The clinical conditions/behaviours targeted by the CPG were as follows: care for people with non‐specific low back pain (Bekkering 2005); appropriate thyroid‐test ordering (Daucourt 2003); timely conversion (and discharge) from intravenous antibiotic therapy to oral antibiotics for people with pneumonia (Fine 2003); and improved cardiovascular risk screening and risk reduction in people with diabetes (Shah 2014).
The guideline recommendations (n = 4) that were implemented were described in one of the studies (Bekkering 2005).
Guideline producers
See Table 1 for details on the guideline development process.
| Author Year Targeted behaviour | Guideline developers | Literature review | Critical appraisal | Consensus processes | Key stakeholder involvement | Barriers/facilitator assessment |
| Targeted behaviour: management of non‐specific low back pain Number of recommendations: 4 main recommendations | The Royal Dutch Physiotherapy Association. | CPGs2 were constructed on the basis of the phases of the physiotherapy process, using the Dutch method of developing physiotherapy guidelines, and evidence from systematic reviews were identified through searching electronic databases | Not mentioned but probably included in the Dutch method of developing CPGs | Based on scientific evidence. If no evidence was available, consensus between experts was obtained | The CPGs were pilot‐tested among 100 physiotherapists and reviewed by an external multidisciplinary panel | Barriers to change were assessed through a survey as part of the CPG development process |
| Targeted behaviour: appropriate thyroid function testing Number of recommendations: not reported | The Committee for Co‐ordinating Clinical Evaluation and Quality in | The CPG developers conducted a comprehensive review of the literature | ‐ | CPG3 development involved an expert consensus process. | ‐ | ‐ |
| Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia Number of recommendations: a 2‐step recommendation | Researchers who were part of the Pneumonia Patient Outcomes Research Team (PORT) | The CPG was based on a review of the medical literature, and empiric evidence on time to reach clinical stability | ‐ | The CPG development process involved the consensus of an 8‐member national guideline panel | The guideline was reviewed by clinical opinion leaders at each study site, and was approved for local use by the relevant utilisation management department. | ‐ |
| Targeted behaviour: management of cardiovascular risk Number of recommendations: no information | Canadian Diabetes Association (and Expert Committee members) | Expert Committee members evaluated the relevant literature, and guidelines were developed and initially reviewed | After formulating new recommendations or modifying existing | Based on scientific evidence/review of the literature | A draft document was circulated nationally and | ‐ |
1Khunti 1998. Development of evidence‐based review criteria for the management of patients with depression in general practice. No published version of the guideline found.
2Bekkering 2003. Dutch physiotherapy guidelines for low back pain.
3Saillour Glénisson 2001. Guidelines for thyroid function tests in adults.
4Shah 2014. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee: Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada.
In Bekkering 2005 the Royal Dutch Physiotherapy Association developed the guidelines; in Daucourt 2003 the Committee for Co‐ordinating Clinical Evaluation and Quality in Aquitaine (CCECQA) developed the guidelines, together with regional groups and national guideline developers; in Fine 2003 members of the Pneumonia Patient Outcomes Research Team (PORT) project developed the guidelines; and in Shah 2014 the Canadian Diabetes Association (CDA) developed the guidelines.
Description of the intervention
| Author Year | Delivery of the intervention | Theoretical models/ frameworks used | Evidence base | Targeted to barriers | Key stakeholder involvement |
| Type of intervention: simple, but multicomponent; active only Intervention target: the healthcare professional Targeted behaviour: management of nonspecific low back pain | Mode: face‐to‐face Provider: The primary investigator and 1 of 2 additional trainers with adequate clinical experience in the management of low back pain supervised the training sessions | ‐ | "The sessions were based on interventions that have all been shown to be effective, such as interactive education and discussion, feedback, and reminders".1,2,3,4,5 | The content of the strategy was determined on the basis of information about the expected barriers for implementation gathered during the development of the CPGs | 2 experts gave advice on the content of the strategy |
| Type of intervention: multicomponent; passive only Intervention target: the healthcare professional Targeted behaviour: appropriate thyroid function testing | Mode: Paper‐based materials Provider: none | ‐ | "Among the clinical guideline diffusion strategies, the most effective are feedback, reminders, academic detailing and financial incentives1,2,4,5 Administrative procedures such as the implementation of test request forms have also proved effective." | ‐ | ‐ |
| Type of intervention: single; active + passive Intervention target: the healthcare professional Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia | Mode: Paper‐based material (detail sheets/treatment recommendations in patient records) and telephone reminder Provider: nurse delivered telephone reminder | ‐ | "The multifaceted guideline dissemination strategy consisted of interventions of proven benefit, including real‐time physician reminders" 1,6,7,8 | ‐ | ‐ |
| Type of intervention: passive Intervention target: family physicians (and diabetes patients at high risk of cardiovascular disease) Targeted behaviour: management of cardiovascular risk factors and outcomes of cardiovascular disease in people with diabetes | Mode: printed educational materials Targeting the family physician: The cardiovascular disease toolkit was a collection of printed educational materials, packaged in a brightly‐coloured box with CDA branding, sent to Canadian family physicians. The contents included an introductory letter from the Chair of the practice Provider: NA13 | The implicit theory behind its | "The literature has demonstrated that the benefits of printed educational interventions are, at best, modest. A systematic review of methods to improve practice guideline adherence demonstrated an absolute improvement of 8% for educational materials. A more recent Cochrane review found that printed educational materials led to a median absolute improvement in performance of only 2% (25). Studies of printed materials specifically tied to clinical practice guidelines also showed modest benefits. A small English study randomised 42 family physicians to receive an algorithm for monitoring and treatment of hypertension of diabetic patients based on practice guidelines, but found no difference in blood pressure control between the intervention and control groups. However, some processes of care were slightly improved: patients in the intervention group were prescribed higher doses of antihypertensive medications, and had more physician visits to monitor blood pressure. In a larger Canadian study, family physicians were randomised to receive a 1‐page summary of a 3‐year‐old practice guideline on anti‐anginal therapy from the local medical governing body. No differences were noted in prescription of β‐blockers, antiplatelet agents, or lipid‐lowering drugs between groups in the 7000 patients reviewed" 9,10,11,12 | ‐ | The toolkit was |
1Bero 1998 Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings.
2Davis 1995 Changing physician performance. A systematic review of the effect of continuing medical education strategies.
3Wensing 1998 Implementing guidelines and innovations in general practice: which interventions are effective?
4Grimshaw 1995 Developing and implementing clinical practice guidelines.
5Davis 1997. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines.
6Murrey 1992 Implementing clinical guidelines: a quality management approach to reminder systems.
7Grimshaw 1993. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations.
8Weingarten 2000. Translating practice guidelines into patient care: guidelines at the bedside.
9Grimshaw 2006. Towards evidence‐based quality improvement: evidence (and its limitation) of the effectiveness of guideline dissemination and implementation strategies 1966–1998.
10Giguère 2012. Printed educational materials: effects on professional practice and healthcare outcomes.
11Bebb 2007. A cluster randomised controlled trial of the effect of a treatment algorithm for hypertension in patients with type 2 diabetes.
12Beaulieu 2004. Drug treatment of stable angina pectoris and mass dissemination of therapeutic guidelines: a randomized controlled trial.
13Not applicable
| Author Year | Tailoring | Feedback | Educational outreach/ Academic detailing/ Small group discussions | Reminders (paper, electronic, telephone) | Decision support tools | Other (test order forms, supportive materials etc.) |
| The content of the strategy was determined on the basis of information about the expected barriers for implementation gathered during the development of the clinical guidelines. | ‐ | 2 interactive training sessions, each lasting 2½ hours, for groups of 8 – 12 physiotherapists (including feedback on current management and reminders). For each session a preparation time of 2 hours was recommended. | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | Pocket memorandum card. | ‐ | Test request form. | |
| ‐ | ‐ | ‐ | Paper‐based detail sheet/ treatment recommendations put into the patient's record + real‐time nurse telephone reminder | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | Printed educational materials |
i) Interventions targeting the healthcare professional
All four studies evaluated guideline implementation tools targeting the healthcare professional.
Tailored interventions
Bekkering 2005 assessed the effectiveness of two (2½ hours) educational training sessions for groups of 8 to 12 physiotherapists on adherence to CPGs for management of non‐specific low back pain. The sessions were based on interventions reported as being effective in the literature (e.g. interactive education and discussion, feedback, and reminders) and were tailored to barriers found in a survey.
Printed materials
Three studies evaluated the effectiveness of paper‐based educational materials or reminders, or both (Daucourt 2003; Fine 2003; Shah 2014).
Daucourt 2003 evaluated the combined effect of two tools: a memorandum pocket card and a test request form to implement guidelines for appropriate thyroid‐test ordering. Orders were made by checking a box, with boxes corresponding to inappropriate test ordering shaded and therefore making ordering impossible.The physician could overrule this by writing the order at the bottom of the sheet. The pocket card summarised the recommendations according to the various clinical or therapeutic situations requiring a thyroid test.
In Fine 2003 physicians received a multifaceted guideline intervention which included placement of a detail sheet in the patient’s medical record once a patient met guideline criteria for stability when receiving intravenous antibiotic therapy for pneumonia, a follow‐up recommendation to the attending physician, and an offer to arrange follow‐up home nursing care. The three site‐specific detail sheets promoted any of three recommended action(s), i.e. conversion from intravenous to oral antibiotic therapy only, conversion and hospital discharge, or hospital discharge only.
Shah 2014 used a cardiovascular disease toolkit which was a collection of printed educational materials, packaged in a brightly‐coloured box with CDA branding, sent to Canadian family physicians. The contents included an introductory letter from the Chair of the practice guidelines’ Dissemination and Implementation Committee; an eight‐page summary of selected sections of the practice guidelines targeted towards family physicians; a four‐page synopsis of the key guideline elements pertaining to cardiovascular disease risk; a small double‐sided laminated card with a simplified algorithm for cardiovascular risk assessment, vascular protection strategies, and screening for cardiovascular disease; and a pad of tear‐off sheets for patients with a cardiovascular risk self‐assessment tool and a list of recommended risk reduction strategies.
The median duration that an intervention was delivered was 22 weeks (range 4 weeks to 12 months).
ii) Interventions targeting the patient
None of the included studies evaluated interventions that targeted the patient.
iii) Interventions targeting the organisation of care
None of the included studies evaluated interventions that exclusively targeted the organisation of care.
iv) Interventions targeting the healthcare professionals and the patients
None of the included studies evaluated targeted both healthcare professionals and patients.
Assessment of barriers
In one of the three included studies (Bekkering 2005), barriers to guideline implementation were assessed through the means of a survey to inform the shape and content (i.e. tailoring) of the guideline implementation strategies. Another aim of the survey was to retrieve information on the most important discrepancies between current practice and recommendations of the guidelines. A model for changing professionals' behaviour and systematic reviews on the effectiveness of implementation interventions was also used to determine the content of the implementation strategy.
Theory base of interventions
None of the interventions used in the included studies was theory‐based.
Evidence base of interventions
The implementation strategies used in the included studies were all supported by some evidence of their effectiveness and cited high‐quality Cochrane Reviews, systematic reviews or overviews to justify their choice of strategies.
Fidelity
None of the included studies provided information on intervention fidelity.
Delivery of the intervention
Mode of delivery:
In Bekkering 2005 the intervention was delivered face‐to‐face. In two studies (Daucourt 2003; Shah 2014) the paper‐based interventions were provided passively. In Fine 2003 one part of the intervention was delivered over the phone, and the rest passively in the form of paper‐based materials.
Provider delivering the intervention (if not electronic, paper‐based, etc):
In Bekkering 2005 the principal investigator and two additional trainers delivered the intervention. In Fine 2003 a nurse delivered part of the intervention.
Comparison interventions
The comparison intervention in all included studies was passive guideline dissemination. Additional material that was delivered together with the guideline was as follows: in Bekkering 2005 four forms: a self‐evaluation form to assess whether their current management was consistent with the recommendations contained in the clinical guidelines, two forms facilitating discussion with other physiotherapists and general practitioners respectively, a copy of the Quebec Back Pain Disability Scale, and a summary of the CPG. In Fine 2003 a cover letter was sent signed by the hospital’s utilisation management director describing the rationale for the guideline. In Daucourt 2003 all physicians were invited to a local information meeting. In Shah 2014 control participants received the CDA newsletter, which included the revised guideline.
Outcomes
Healthcare professional outcomes
Two of the four included studies reported a measure of healthcare professional adherence to guidelines (Bekkering 2005; Daucourt 2003) at four weeks; these were included in the calculations of the median absolute risk difference (ARD).
Healthcare resource use and costs
Fine 2003 reported length of initial hospital stay and re‐admissions at 30 days after index hospitalisation. Shah 2014 reported (primary outcome in clinical study) the proportion of patients with diabetes at high risk of a cardiovascular event who were prescribed a statin (see Table 4 for details on secondary outcomes reported).
| Author Year | Clinical outcomes; Medical complications | Quality of life Satisfaction with care | Mortality | Healthcare resource use (including medications prescribed) | Costs |
| ‐ | Quality of Life (assessed with the EQ‐5D1), mean (SD): BL: Intervention: 0.6730 (0.2042); Control: 0.6134 (0.2661), P = 0.006. At 6 weeks: Intervention: 0.7778 (0.1978); Control: 0.7497 (0.2316) At 12 weeks: Intervention: 0.8141 (0.1988); Control: 0.7873 (0.2210) Note: results for 26 and 52 weeks reported graphically | ‐ | ‐ | Mean annual cost (Euros) per patient (SD): Direct cost2: Intervention: 374 (437). Control: 449 (572). The costs (Euro) of releasing a new guideline for low back pain to 18,000 physiotherapists: Intervention (active strategy): 87,416 Control (passive strategy): 63,101 | |
| ‐ | ‐ | ‐ | ‐ | Cost paper awaits translation | |
| In‐hospital medical complications, number (%): Intervention:157 (55); Control: 206 (63), P = 0.04 Functional status3 SF‐12 physical health composite score: Intervention:45 ± 7, n = 181; SF‐12 mental health composite score: Intervention: 45 ± 6; Control: 45 ± 7, P = 0.71 | Patient satisfaction with care4 , number (%): Not satisfied with overall care: Intervention: 12 (5.3), n = 228; Control: 11 (4.0), n = 273, P = 0.67 Believed length of stay was too short: Intervention: 59 (26.1); Control: 54 (20.2), P = 0.16 Return to usual activities5,Hazard ratio (95% CI): Nonworkers: 1.09 (0.83 to 1.43), P = 0.55 Workers: 0.85 (0.54 to 1.35); P = 0.49 Return to work (workers) 0.99 (0.63 to 1.58), P = 0.98 | Mortality6 all‐cause, number (%): Intervention: 22 (8), n = 283; Control: 29 (9), n = 325, P = 0.70 Pneumonia‐related mortality, number (%): Intervention: 15 (5); Control: 23 (7), P = 0.44 | Length of index hospital (days) stay, median (IQR): Intervention: 5.0 (3.0 to 7.0); Control: 5.0 (3.0 to 8.0); Hazard ratio (95% CI): 1.16 (0.97 to 1.38), P = 0.11 Rehospitalisation7 number (%): Intervention:37 (14); Control:33 (11), P = 0.42 Duration (days) of intravenous antibiotic therapy, median (IQR): Intervention: 3.0 (2.0 to 5.0),n = 283; Control: 4.0 (2.6 to 6.0), n = 325; Hazard ratio (95% CI): 1.23 (1.00 to 1.52), P = 0.06 | ‐ | |
| Clinical data study: Intervention: n = 40 practices/795 patients; Control: n = 40 practices/797 patients Cardiovascular risk reduction (secondary outcomes): Proportion of participants reaching glycaemic control targets (HbA1c < 7.0%): Intervention: 58.5%; Control: 58.8%; OR 0.93 (0.71 to 1.21), P = 0.58 Proportion of participants reaching blood pressure control targets (< 130/80): Intervention: 52.8%; Control: 63.5%, OR 0.72 (0.53 to 0.98), P = 0.04 Proportion of participants reaching LDL‐cholesterol control targets (< 2.0 mmol/L): Intervention: 59.2%; Control: 61.7% , OR 0.90 (0.68 to 1.18), P = 0.43 Proportion of participants reaching Total to HDL‐cholesterol ratio (< 4.0): Intervention: 74.2%; Control: 76.8%, OR 0.85 (0.63 to1.14), P = 0.27 Clinical (secondary outcomes): When HbA1c > 8.0%: Intervention: 11.8%; Control: 13.0%, OR 0.98 (0.48 to 1.98), P = 0.95 When blood pressure > 140/90: Intervention: 5.6%; Control: 7.2%, OR 0.67 (0.25 to 1.82), P = 0.43 When LDL cholesterol > 3.0 mmol/L: Intervention: 43.5%; Control: 45.2%, OR 0.94 (0.53 to 1.67), P = 0.83 Administrative data study : Intervention: 2008 practices (467,713 participants); Control: 1999 practices (466, 076 participants) Secondary outcomes: | ‐ | Administrative data study : Intervention: 2008 practices (467,713 participants); Control: 1999 practices (466,076 participants) Primary outcome: Death or non‐fatal myocardial infarction: Intervention: 2.5%; Control: 2.5%; OR 1.00 (0.96 to 1.03), P = 0.77 Secondary outcomes: Medication initiation (ACEI/ARB > 1 antihypertensive class, or > 2, or > 3, statin, glucose‐lowering drugs, insulin, nitrate): OR, range: from 0.96 to 1.02, P values from 0.03 to 0.94 | Clinical data study: Primary outcome: Proportion of participants prescribed statins (new or renewed prescription): Intervention: 700 (88.1%); Control: 725 (90.1%); OR 0.73, 95% CI 0.42 to 1.26, P = 0.26 Proportion of participants prescribed an ACEI/ARB: Intervention: Control: Secondary outcome. Administrative data study : Secondary outcomes: CAD assessment (electrocardiogram, cardiac stress test, nuclear imaging, coronary angiography, coronary revascularisation, cardiology or internal medicine visit): OR, range: from 0.96 to 1.00, P values from 0.02 to 0.83 | ‐ |
1EQ‐5D: a standardised instrument for use as a measure of health outcome.The EQ‐5D has five dimensions: mobility, self‐care, usual activity,pain/discomfort and anxiety/depression. Each dimension has three levels, no problems, some problems and serious problems. Hence, EQ‐5D has 243 possible health states. Utility values of the general public for these health states as measured with the time tradeoff technique on a random sample of the adult population of the United Kingdom, the MVH‐A1 tariff, were applied in this study. The scores range from −0.594 (worst situation) to 1.0 (perfect health).
2The direct costs consisted of costs of the dissemination of the guideline and the costs of the healthcare utilisation of the patients. Prices for the year 2002.
3SF‐12 health scores were assessed in all patients able to provide reliable self‐report data during the 30‐day interview, excluding 6 intervention‐arm and 6 control‐arm patients with missing data.
4Patient satisfaction with care was assessed for all patients with a 30‐day interview that was not completed by a paid caregiver, excluding four intervention‐arm and two control‐arm patients with missing data. An additional two patients in the intervention arm and six patients in the control arm were hospitalised for the full 30 days and were not asked about length of hospital stay. SF‐12, 12‐Item Short Form was used.
5Return to usual activities among non‐workers was assessed for 183 intervention arm and 219 control arm patients not employed at baseline who completed a 30‐day interview. Return to usual activities among workers was assessed in 59 intervention‐arm and 59 control‐arm patients employed at baseline. Return to work was assessed among 54 intervention‐arm and 53 control‐arm patients employed at baseline.
6Mortality, medical complications, and return to work and usual activities were adjusted for pneumonia severity risk class.
7Rehospitalisation within 30 days of the index admission was assessed for all patients who were discharged alive from either the index hospitalisation or another acute‐care facility (if transferred to an acute‐care facility from the index hospitalisation).
8Fluid fasting times assessed by local investigator asking the patient about the fasting time, and checking this information against medical notes.
9Cost for designing, editing, reproducing, and posting need when applied to 170 acute trusts.
10Cost of providing 170 acute trusts with implementation support through a web‐based resource championed through opinion leadership. This includes development costs for the tool (which for this project were in‐house costs, in other cases external agencies may have to be used which are likely to be three times higher), publicity materials, training materials and opinion leader time and activity.
Two studies reported on costs (Bekkering 2005; Saillour‐Glénisson 2005 (belonging to Daucourt 2003)). One of the studies reported mean annual cost per patient, total cost for releasing the guideline and cost of active implementation intervention (Bekkering 2005). The other article awaits translation (Saillour‐Glénisson 2005).
Patient outcomes
Bekkering 2005 reported quality‐of‐life measures at four weeks. Fine 2003 reported all‐cause and pneumonia‐related mortality, medical complications, functional status and patient satisfaction with care at 30 days after the initial hospitalisation. Shah 2014 reported (primary outcome in administrative data study) death or non‐fatal myocardial infarction. Daucourt 2003 reported the number of requests for a thyroid function test that complied with the guidelines (Guideline Conformity Rate (GCR)) at 4 weeks. (See Table 4 for details of the secondary outcomes reported).
Excluded studies
After scrutinising the full text we excluded 1030 studies and added 14 to the excluded studies table. See Characteristics of excluded studies table.
Risk of bias in included studies
See 'Risk of bias' tables within the Characteristics of included studies,Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. White space indicate studies not reporting non‐objective outcomes and for which risk of bias could not be assessed.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. White spcace indicate studies not reporting non‐objective outcomes and for which risk of bias could not be assessed.
The randomisation sequence and the allocation concealment were adequate in three studies (Bekkering 2005; Daucourt 2003; Shah 2014), and unclear in Fine 2003. In Shah 2014 blinding was adequate (clinical data study assessed), and in Daucourt 2003 it was unclear whether or not the healthcare professionals were blinded, while in two studies (Bekkering 2005; Fine 2003) it was clear that they were not. In one study the healthcare professional selected a maximum of 10 consecutive patients for the study, and we therefore judged the risk of performance bias to be high (Bekkering 2005). Performance bias was also judged high in Fine 2003 as treatment assignment was not concealed. Blinding of outcome assessment was adequate in two studies (Bekkering 2005; Shah 2014), and unclear in the other two. Baseline characteristics were reported to be similar in one study (Fine 2003), not similar in one study (Shah 2014), and unclear in the other two studies. The outcome data were complete in two studies (Daucourt 2003; Shah 2014), and unclear in the other two (with losses to follow‐up of more than 20%). In Shah 2014 some of the outcomes that were listed in the trial protocol were not in the study report, while in the other three studies the risk of selective reporting was low. Shah 2014 had unclear risk of other bias (contamination), while the other three were at low risk.
Effects of interventions
See: Summary of findings for the main comparison
i) Interventions targeting the health care professionals
Healthcare professional outcomes
See summary of findings Table for the main comparison; Table 4 and Table 5.
| Author Year | Adherence Outcomes | Participants (Settings) | Control Adherence | Intervention Adherence | Median ARD |
| (Hoijenboos 2005) Targeted behaviour: management of non specific low back pain GL tool used: interactive training workshop X2 | Adherence to 4 guideline recommendations: i) Limit number of sessions in normal course back pain ii) Set functional treatment goals iii) Use mainly active interventions iv) Give adequate information Note: an increase was desirable for all outcomes | 113 physiotherapists (68 private physiotherapy practices) | i) Post: 14 (13), n = 253 ii) Post: 180 (71) iii) Post: 154 (60) iv) Post: 221 (87) | i) Post: 32 (27), n = 247 ii) Post: 188 (79) iii) Post: 183 (77) iv) Post: 229 (96) | +0.115 11.5% higher adherence in the intervention group i) 0.14% ii) 0.08% iii) 0.17% iv) 0.09% |
| Targeted behaviour: appropriate thyroid function testing Guideline tool used: | Global Guideline Conformity Rate | 1412 physicians (6 general hospitals) | Pre: 62.0% (95% CI 47.7 to 76.4) | Dual intervention group: Post: 77.9% (95% CI 68.9 to 87.0) Note: only results for the dual intervention presented here | +0.159% |
| Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia GL tool used: detail sheet/ treatment recommendations+ telephone reminder | No adherence outcomes reported, only proxies | 545 physicians (7 not‐for‐profit hospitals) | ‐ | ‐ | ‐ |
| Targeted behaviour: improved cardiovascular risk factor management in people with diabetes Guideline tool used: printed educational material | No adherence outcomes reported, only proxies | 2 separate studies: Administrative data study: n = 4007 practices: Intervention: 2008; Control: 1999 Clinical data study. n = 80 practices (1592 patients); Intervention: 40 practices (8795 patients); Control: 40 practices (8797 patients) | ‐ | ‐ | ‐ |
Two of the four included studies (Bekkering 2005; Daucourt 2003) reported one or more measures of healthcare professionals' adherence to guidelines. The overall median absolute risk difference (range) (five comparisons) was (range: 0.115 to 0.159), i.e. a median difference in adherence of 13.5%, with the effects ranging from 11.5% to 15.9% increase in adherence.
Fine 2003, in which physicians received an educational mailing, a daily assessment of (pneumonia) patient stability and an additional sheet to the medical notes with a follow‐up recommendation for converting from intravenous to oral antibiotic and hospital discharge, compared with education mailing alone, reported that those in the intervention group had a more rapid rate of conversion to oral antibiotics (hazard ratio (HR) 1.23, 95% confidence interval (CI) 1.00 to 1.52, P = 0.06). Shah 2014 did not report health professional outcomes.
Healthcare resource use and costs
Fine 2003 reported similar percentages in each group of patients being readmitted (intervention group 14% versus 11% in the control group), and a similar length of initial hospital stay (median of five days in each group) at 30 days after index visit.
Shah 2014 reported similar or slightly lower (= undesired effect) use of different types of coronary artery disease (CAD) assessment tools in practices that received the guideline tool compared to those who received the updated guideline only (administrative data study), as was the case for the medication initiation outcomes (both were secondary outcomes).
Bekkering 2005 reported mean annual direct medical costs for the intervention group of EUR 374 versus EUR 449 in the control group. Direct costs included costs of the dissemination of the guideline and healthcare resource use by the patient. Daucourt 2003 reported prescribing cost data in a paper in French (Saillour‐Glénisson 2001) which awaits translation.
Patient outcomes
SeeTable 4 for details
Bekkering 2005 reported similar quality‐of‐life scores for patients with non‐specific low back pain at 12 months.
Fine 2003 reported similar scores on the SF‐12 physical component score (intervention group 45 (standard deviation (SD) 7) versus control group 45 (SD 7)) and the mental component score (intervention group 45 (SD 6) versus control group 45 (SD 7)) at 30 days after index stay, and little or no difference for mortality (intervention group 8% versus control group 9%), and return to work (HR 0.99, 95% CI 0.63 to 1.58). The same authors reported fewer hospital complications in the intervention group compared with control (157 (55%) and 206 (63%) respectively, P = 0.04).
Shah 2014 reported little or no difference between groups (Intervention 2.5%; Control 2.5%; odds ratio (OR) 1.00, 95% CI 0.96 to 1.03, P = 0.77) for death and non‐fatal myocardial infarction (primary outcome in the administrative data study), and also little or no difference for any of the other (secondary) clinical events reported (see Table 4 for details).
ii) Interventions targeting the organisation of care
No studies reported results for this comparison.
iii) Interventions targeting the patient
No studies reported results for this comparison.
iv) Interventions targeting the healthcare professional, the organisation of care and/or the patient
No study reported results for this comparison.
Effectiveness of different approaches of guideline dissemination
We include four studies in this review, of which one evaluated the effectiveness of two short tailored educational workshops, and the other three studied the effects of using paper‐based tools, including order forms or reminders, or both. As the types of multifaceted interventions, the clinical condition and behaviour targeted varied across studies it was not possible to determine which of the different approaches used to improve implementation of guidelines was most effective.
Discusión
Resumen de los resultados principales
Se identificaron cuatro ECA con asignación al azar por grupos aptos para la inclusión en esta revisión, que evaluaban los efectos de las herramientas creadas por realizadores de guías existentes para mejorar la implementación de las guías.
Todos los estudios incluidos evaluaron herramientas dirigidas al profesional de la asistencia sanitaria. Sin embargo, el metanálisis no fue factible, debido a que las afecciones clínicas y el comportamiento estudiados, así como las herramientas usadas para la implementación de las guías, variaron entre los estudios. La variación en la duración de las intervenciones y el seguimiento también dificultó las comparaciones. Las herramientas creadas por los realizadores de las guías, y proporcionadas a los profesionales de la asistencia sanitaria como una ayuda para mejorar el cumplimiento, probablemente dan lugar a un mayor cumplimiento de las guías (diferencia de riesgo absoluto [DRA] mediana 13,5%) al momento del seguimiento promedio de cuatro de semanas (pruebas de calidad moderada). El efecto varió de un 11,5% en un estudio (dos talleres educacionales cortos adaptados para mejorar el tratamiento del lumbago no específico) a un 15,9% en el otro (una tarjeta recordatoria de bolsillo y un formulario de prueba‐solicitud para mejorar el pedido de pruebas de tiroides). Ningún estudio informó el cumplimiento inicial, y pareció que previamente no había ninguna guía para los comportamientos y afecciones específicas estudiados. Hubo pruebas de baja certidumbre provenientes de un ensayo de poca o ninguna diferencia en los costos entre los grupos.
Debido a los pocos estudios aptos identificados y a la variedad de intervenciones implementadas, no fue posible determinar qué enfoques son más efectivos, que fue el objetivo secundario de esta revisión. Dos de los estudios incluidos informó los datos del costo, y uno de los mismos está en espera de traducción. Aunque no es posible considerar directamente la inversión hecha por los realizadores de guías al crear las herramientas de implementación, es probable que el costo no sea significativamente diferente al de otras organizaciones que crean herramientas para apoyar la implementación de las guías. Debe señalarse que incluso los efectos pequeños a moderados de la intervención pueden ser sumamente eficaces en función de los costos si la afección clínica estudiada es sumamente prevalente y la creación y distribución de las herramientas de implementación usadas son de bajo costo.
No hay pruebas disponibles sobre la efectividad de las intervenciones dirigidas a la organización de la atención o al paciente.
Compleción y aplicabilidad general de las pruebas
En todos los estudios incluidos las intervenciones se dirigieron al profesional de la asistencia sanitaria. Ninguno de los estudios incluidos usó herramientas dirigidas a los coordinadores de sistemas de salud o a los elaboradores de políticas, al paciente (p.ej. versiones de la guía desarrolladas para el paciente) o a la organización de la atención (p.ej. herramientas de evaluación comparativa, plantillas de cálculo de costos o evaluación de los programas, herramientas de auditoría, medidas del rendimiento e indicadores de calidad para evaluar la implementación de la GPC), y ningún estudio evaluó los efectos de las intervenciones relacionadas con los medios masivos. Las herramientas de implementación utilizadas se proporcionaron junto con la GPC, y ninguna estaba integrada a la GPC (p.ej. adaptación de la GPC para una audiencia específica). Además, sólo se han evaluado herramientas para implementar guías que procuran promover el uso de GPC para unas pocas afecciones clínicas y comportamientos.
Calidad de la evidencia
Las pruebas provinieron de ECA con asignación al azar por grupos que habían tenido en cuenta la agrupación en el análisis. Se disminuyó la certidumbre de las pruebas de todos los estudios incluidos de alta a moderada para el resultado principal (cumplimiento de las guías), debido al alto riesgo de sesgo. Debido a que sólo un único estudio aportó pruebas de la efectividad de una determinada implementación en los costos, la confianza en las pruebas se disminuyó de forma adicional a baja debido a la imprecisión.
Sesgos potenciales en el proceso de revisión
Se efectuaron búsquedas en un gran número de bases de datos mediante una estrategia que fue diseñada por un especialista senior en información, y luego se adaptó para diferentes bases de datos. También se realizaron búsquedas en un gran número de sitios web de los realizadores de guías relevantes. Cuatro revisores examinaron varias referencias identificadas por las búsquedas electrónicas, con la exclusión de los artículos que eran irrelevantes y claramente no aptos, lo cual produjo una larga lista que repasó un segundo revisor. Dos revisores evaluaron de forma independiente todos los títulos y resúmenes potencialmente elegibles teniendo en cuenta los criterios de elegibilidad para asegurar que no se omitiera ninguna referencia importante. También se realizó la extracción de datos y la evaluación del riesgo de sesgo por duplicado.
Acuerdos y desacuerdos con otros estudios o revisiones
No se conocen otras revisiones que hayan evaluado la efectividad de las herramientas desarrolladas por los realizadores de guías reconocidos para mejorar la implementación de sus propias GPC. Sin embargo, los resultados de un cumplimiento mediano de 13,5% mayor de las guías en el grupo de intervención (dos estudios: uno que evaluó una intervención basada en documentos y uno una intervención que constaba de dos talleres educacionales cortos) son mayores que la mejoría absoluta mediana informada en el rendimiento para los recordatorios por computadora en el punto de atención de alrededor del 4% (Shojania 2009), 2% para el material didáctico impreso (Giguère 2012) y 6% para las reuniones educacionales (Forsetlund 2009). Estas revisiones, sin embargo, incluyeron a un número mucho mayor de estudios y participantes, lo cual puede explicar las diferencias en el efecto.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. White space indicate studies not reporting non‐objective outcomes and for which risk of bias could not be assessed.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. White spcace indicate studies not reporting non‐objective outcomes and for which risk of bias could not be assessed.
| Clinical practice guideline (CPG) + implementation tool compared with CPG only for adherence to guidelines | ||||
| Patient or population: Healthcare professionals (physiotherapists, hospital physicians) providing care for people with different clinical conditions (patients with non specific low back pain, patients with symptoms indicating a need for a thyroid function test) Setting: Private physiotherapy clinics in the Netherlands, general hospitals in France Intervention: CPG + guideline implementation tool (e.g. training workshops, paper‐based materials and order forms, reminders, web‐based tools) | ||||
| Outcomes | Median ARD (Absolute risk difference) | No of Participants | Certainty of the evidence | Comments |
| Adherence to guidelines | Guideline tools provided to healthcare professionals as an aid to improve the use of a CPG probably lead to improved adherence with the CPG, as compared to guidelines only. Median ARD: 0.135 (0.115 to 0.15.9) at mean 4 weeks follow‐up | 68 physio‐ therapy practices; and 6 hospitals (2 C‐RCTs) | ⊕⊕⊕⊝ | 2 of the 4 included studies reported a proxy measure for adherence to guidelines, and results from these studies could therefore not be included in the ARD calculation |
| Costs | Guideline tools aimed at improving the use of guidelines may lead to little or no difference in healthcare costs | 68 physio‐ therapy clinics (1 C‐RCT) | ⊕⊕⊝⊝ low2 | 1 trial reported no difference in mean direct annual cost* per patient between intervention and control groups. 1 French paper belonging to 1 of the included trials (6 hospitals) and reporting on costs awaits translation |
| * Direct costs included costs of the dissemination of the guideline and healthcare resource use by the patient GRADE Working Group grades of evidence | ||||
| 1We downgraded the certainty of the evidence one step due to high risk of bias. 2We downgraded the certainty of the evidence two steps due to imprecision. | ||||
| Author Year Targeted behaviour | Guideline developers | Literature review | Critical appraisal | Consensus processes | Key stakeholder involvement | Barriers/facilitator assessment |
| Targeted behaviour: management of non‐specific low back pain Number of recommendations: 4 main recommendations | The Royal Dutch Physiotherapy Association. | CPGs2 were constructed on the basis of the phases of the physiotherapy process, using the Dutch method of developing physiotherapy guidelines, and evidence from systematic reviews were identified through searching electronic databases | Not mentioned but probably included in the Dutch method of developing CPGs | Based on scientific evidence. If no evidence was available, consensus between experts was obtained | The CPGs were pilot‐tested among 100 physiotherapists and reviewed by an external multidisciplinary panel | Barriers to change were assessed through a survey as part of the CPG development process |
| Targeted behaviour: appropriate thyroid function testing Number of recommendations: not reported | The Committee for Co‐ordinating Clinical Evaluation and Quality in | The CPG developers conducted a comprehensive review of the literature | ‐ | CPG3 development involved an expert consensus process. | ‐ | ‐ |
| Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia Number of recommendations: a 2‐step recommendation | Researchers who were part of the Pneumonia Patient Outcomes Research Team (PORT) | The CPG was based on a review of the medical literature, and empiric evidence on time to reach clinical stability | ‐ | The CPG development process involved the consensus of an 8‐member national guideline panel | The guideline was reviewed by clinical opinion leaders at each study site, and was approved for local use by the relevant utilisation management department. | ‐ |
| Targeted behaviour: management of cardiovascular risk Number of recommendations: no information | Canadian Diabetes Association (and Expert Committee members) | Expert Committee members evaluated the relevant literature, and guidelines were developed and initially reviewed | After formulating new recommendations or modifying existing | Based on scientific evidence/review of the literature | A draft document was circulated nationally and | ‐ |
| 1Khunti 1998. Development of evidence‐based review criteria for the management of patients with depression in general practice. No published version of the guideline found. | ||||||
| Author Year | Delivery of the intervention | Theoretical models/ frameworks used | Evidence base | Targeted to barriers | Key stakeholder involvement |
| Type of intervention: simple, but multicomponent; active only Intervention target: the healthcare professional Targeted behaviour: management of nonspecific low back pain | Mode: face‐to‐face Provider: The primary investigator and 1 of 2 additional trainers with adequate clinical experience in the management of low back pain supervised the training sessions | ‐ | "The sessions were based on interventions that have all been shown to be effective, such as interactive education and discussion, feedback, and reminders".1,2,3,4,5 | The content of the strategy was determined on the basis of information about the expected barriers for implementation gathered during the development of the CPGs | 2 experts gave advice on the content of the strategy |
| Type of intervention: multicomponent; passive only Intervention target: the healthcare professional Targeted behaviour: appropriate thyroid function testing | Mode: Paper‐based materials Provider: none | ‐ | "Among the clinical guideline diffusion strategies, the most effective are feedback, reminders, academic detailing and financial incentives1,2,4,5 Administrative procedures such as the implementation of test request forms have also proved effective." | ‐ | ‐ |
| Type of intervention: single; active + passive Intervention target: the healthcare professional Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia | Mode: Paper‐based material (detail sheets/treatment recommendations in patient records) and telephone reminder Provider: nurse delivered telephone reminder | ‐ | "The multifaceted guideline dissemination strategy consisted of interventions of proven benefit, including real‐time physician reminders" 1,6,7,8 | ‐ | ‐ |
| Type of intervention: passive Intervention target: family physicians (and diabetes patients at high risk of cardiovascular disease) Targeted behaviour: management of cardiovascular risk factors and outcomes of cardiovascular disease in people with diabetes | Mode: printed educational materials Targeting the family physician: The cardiovascular disease toolkit was a collection of printed educational materials, packaged in a brightly‐coloured box with CDA branding, sent to Canadian family physicians. The contents included an introductory letter from the Chair of the practice Provider: NA13 | The implicit theory behind its | "The literature has demonstrated that the benefits of printed educational interventions are, at best, modest. A systematic review of methods to improve practice guideline adherence demonstrated an absolute improvement of 8% for educational materials. A more recent Cochrane review found that printed educational materials led to a median absolute improvement in performance of only 2% (25). Studies of printed materials specifically tied to clinical practice guidelines also showed modest benefits. A small English study randomised 42 family physicians to receive an algorithm for monitoring and treatment of hypertension of diabetic patients based on practice guidelines, but found no difference in blood pressure control between the intervention and control groups. However, some processes of care were slightly improved: patients in the intervention group were prescribed higher doses of antihypertensive medications, and had more physician visits to monitor blood pressure. In a larger Canadian study, family physicians were randomised to receive a 1‐page summary of a 3‐year‐old practice guideline on anti‐anginal therapy from the local medical governing body. No differences were noted in prescription of β‐blockers, antiplatelet agents, or lipid‐lowering drugs between groups in the 7000 patients reviewed" 9,10,11,12 | ‐ | The toolkit was |
| 1Bero 1998 Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. | |||||
| Author Year | Tailoring | Feedback | Educational outreach/ Academic detailing/ Small group discussions | Reminders (paper, electronic, telephone) | Decision support tools | Other (test order forms, supportive materials etc.) |
| The content of the strategy was determined on the basis of information about the expected barriers for implementation gathered during the development of the clinical guidelines. | ‐ | 2 interactive training sessions, each lasting 2½ hours, for groups of 8 – 12 physiotherapists (including feedback on current management and reminders). For each session a preparation time of 2 hours was recommended. | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | Pocket memorandum card. | ‐ | Test request form. | |
| ‐ | ‐ | ‐ | Paper‐based detail sheet/ treatment recommendations put into the patient's record + real‐time nurse telephone reminder | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | Printed educational materials |
| Author Year | Clinical outcomes; Medical complications | Quality of life Satisfaction with care | Mortality | Healthcare resource use (including medications prescribed) | Costs |
| ‐ | Quality of Life (assessed with the EQ‐5D1), mean (SD): BL: Intervention: 0.6730 (0.2042); Control: 0.6134 (0.2661), P = 0.006. At 6 weeks: Intervention: 0.7778 (0.1978); Control: 0.7497 (0.2316) At 12 weeks: Intervention: 0.8141 (0.1988); Control: 0.7873 (0.2210) Note: results for 26 and 52 weeks reported graphically | ‐ | ‐ | Mean annual cost (Euros) per patient (SD): Direct cost2: Intervention: 374 (437). Control: 449 (572). The costs (Euro) of releasing a new guideline for low back pain to 18,000 physiotherapists: Intervention (active strategy): 87,416 Control (passive strategy): 63,101 | |
| ‐ | ‐ | ‐ | ‐ | Cost paper awaits translation | |
| In‐hospital medical complications, number (%): Intervention:157 (55); Control: 206 (63), P = 0.04 Functional status3 SF‐12 physical health composite score: Intervention:45 ± 7, n = 181; SF‐12 mental health composite score: Intervention: 45 ± 6; Control: 45 ± 7, P = 0.71 | Patient satisfaction with care4 , number (%): Not satisfied with overall care: Intervention: 12 (5.3), n = 228; Control: 11 (4.0), n = 273, P = 0.67 Believed length of stay was too short: Intervention: 59 (26.1); Control: 54 (20.2), P = 0.16 Return to usual activities5,Hazard ratio (95% CI): Nonworkers: 1.09 (0.83 to 1.43), P = 0.55 Workers: 0.85 (0.54 to 1.35); P = 0.49 Return to work (workers) 0.99 (0.63 to 1.58), P = 0.98 | Mortality6 all‐cause, number (%): Intervention: 22 (8), n = 283; Control: 29 (9), n = 325, P = 0.70 Pneumonia‐related mortality, number (%): Intervention: 15 (5); Control: 23 (7), P = 0.44 | Length of index hospital (days) stay, median (IQR): Intervention: 5.0 (3.0 to 7.0); Control: 5.0 (3.0 to 8.0); Hazard ratio (95% CI): 1.16 (0.97 to 1.38), P = 0.11 Rehospitalisation7 number (%): Intervention:37 (14); Control:33 (11), P = 0.42 Duration (days) of intravenous antibiotic therapy, median (IQR): Intervention: 3.0 (2.0 to 5.0),n = 283; Control: 4.0 (2.6 to 6.0), n = 325; Hazard ratio (95% CI): 1.23 (1.00 to 1.52), P = 0.06 | ‐ | |
| Clinical data study: Intervention: n = 40 practices/795 patients; Control: n = 40 practices/797 patients Cardiovascular risk reduction (secondary outcomes): Proportion of participants reaching glycaemic control targets (HbA1c < 7.0%): Intervention: 58.5%; Control: 58.8%; OR 0.93 (0.71 to 1.21), P = 0.58 Proportion of participants reaching blood pressure control targets (< 130/80): Intervention: 52.8%; Control: 63.5%, OR 0.72 (0.53 to 0.98), P = 0.04 Proportion of participants reaching LDL‐cholesterol control targets (< 2.0 mmol/L): Intervention: 59.2%; Control: 61.7% , OR 0.90 (0.68 to 1.18), P = 0.43 Proportion of participants reaching Total to HDL‐cholesterol ratio (< 4.0): Intervention: 74.2%; Control: 76.8%, OR 0.85 (0.63 to1.14), P = 0.27 Clinical (secondary outcomes): When HbA1c > 8.0%: Intervention: 11.8%; Control: 13.0%, OR 0.98 (0.48 to 1.98), P = 0.95 When blood pressure > 140/90: Intervention: 5.6%; Control: 7.2%, OR 0.67 (0.25 to 1.82), P = 0.43 When LDL cholesterol > 3.0 mmol/L: Intervention: 43.5%; Control: 45.2%, OR 0.94 (0.53 to 1.67), P = 0.83 Administrative data study : Intervention: 2008 practices (467,713 participants); Control: 1999 practices (466, 076 participants) Secondary outcomes: | ‐ | Administrative data study : Intervention: 2008 practices (467,713 participants); Control: 1999 practices (466,076 participants) Primary outcome: Death or non‐fatal myocardial infarction: Intervention: 2.5%; Control: 2.5%; OR 1.00 (0.96 to 1.03), P = 0.77 Secondary outcomes: Medication initiation (ACEI/ARB > 1 antihypertensive class, or > 2, or > 3, statin, glucose‐lowering drugs, insulin, nitrate): OR, range: from 0.96 to 1.02, P values from 0.03 to 0.94 | Clinical data study: Primary outcome: Proportion of participants prescribed statins (new or renewed prescription): Intervention: 700 (88.1%); Control: 725 (90.1%); OR 0.73, 95% CI 0.42 to 1.26, P = 0.26 Proportion of participants prescribed an ACEI/ARB: Intervention: Control: Secondary outcome. Administrative data study : Secondary outcomes: CAD assessment (electrocardiogram, cardiac stress test, nuclear imaging, coronary angiography, coronary revascularisation, cardiology or internal medicine visit): OR, range: from 0.96 to 1.00, P values from 0.02 to 0.83 | ‐ | |
| 1EQ‐5D: a standardised instrument for use as a measure of health outcome.The EQ‐5D has five dimensions: mobility, self‐care, usual activity,pain/discomfort and anxiety/depression. Each dimension has three levels, no problems, some problems and serious problems. Hence, EQ‐5D has 243 possible health states. Utility values of the general public for these health states as measured with the time tradeoff technique on a random sample of the adult population of the United Kingdom, the MVH‐A1 tariff, were applied in this study. The scores range from −0.594 (worst situation) to 1.0 (perfect health). | |||||
| Author Year | Adherence Outcomes | Participants (Settings) | Control Adherence | Intervention Adherence | Median ARD |
| (Hoijenboos 2005) Targeted behaviour: management of non specific low back pain GL tool used: interactive training workshop X2 | Adherence to 4 guideline recommendations: i) Limit number of sessions in normal course back pain ii) Set functional treatment goals iii) Use mainly active interventions iv) Give adequate information Note: an increase was desirable for all outcomes | 113 physiotherapists (68 private physiotherapy practices) | i) Post: 14 (13), n = 253 ii) Post: 180 (71) iii) Post: 154 (60) iv) Post: 221 (87) | i) Post: 32 (27), n = 247 ii) Post: 188 (79) iii) Post: 183 (77) iv) Post: 229 (96) | +0.115 11.5% higher adherence in the intervention group i) 0.14% ii) 0.08% iii) 0.17% iv) 0.09% |
| Targeted behaviour: appropriate thyroid function testing Guideline tool used: | Global Guideline Conformity Rate | 1412 physicians (6 general hospitals) | Pre: 62.0% (95% CI 47.7 to 76.4) | Dual intervention group: Post: 77.9% (95% CI 68.9 to 87.0) Note: only results for the dual intervention presented here | +0.159% |
| Targeted behaviour: appropriate duration of intravenous antibiotic therapy for treatment of pneumonia GL tool used: detail sheet/ treatment recommendations+ telephone reminder | No adherence outcomes reported, only proxies | 545 physicians (7 not‐for‐profit hospitals) | ‐ | ‐ | ‐ |
| Targeted behaviour: improved cardiovascular risk factor management in people with diabetes Guideline tool used: printed educational material | No adherence outcomes reported, only proxies | 2 separate studies: Administrative data study: n = 4007 practices: Intervention: 2008; Control: 1999 Clinical data study. n = 80 practices (1592 patients); Intervention: 40 practices (8795 patients); Control: 40 practices (8797 patients) | ‐ | ‐ | ‐ |

