Corticosteroides para la parálisis de Bell (parálisis facial idiopática)
Resumen
Antecedentes
La inflamación y el edema del nervio facial están relacionados con la parálisis de Bell. Los corticosteroides tienen una potente acción antiinflamatoria que pueden disminuir el daño nervioso. Ésta es una actualización de una revisión publicada por primera vez en 2002 y actualizada por última vez en 2010.
Objetivos
Determinar la efectividad y la seguridad del tratamiento con corticosteroides en pacientes con parálisis de Bell.
Métodos de búsqueda
El 4 de marzo de 2016 se realizaron búsquedas en el Registro especializado del Grupo Cochrane Neuromuscular (Cochrane Neuromuscular Group), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE, EMBASE y LILACS. Se revisaron las bibliografías de los ensayos aleatorizados y se estableció contacto con expertos conocidos en el área para identificar ensayos adicionales publicados o no publicados. También se realizaron búsquedas de ensayos en curso en registros de ensayos clínicos.
Criterios de selección
Ensayos aleatorizados y cuasialeatorizados que compararon diferentes vías de administración y esquemas de dosis del tratamiento con corticosteroides u hormona adrenocorticotrópica versus un grupo control que no recibió un tratamiento considerado efectivo para esta afección, a menos que el mismo tratamiento se haya administrado de manera similar al grupo experimental.
Obtención y análisis de los datos
Se utilizó la metodología Cochrane estándar. El principal resultado de interés fue la recuperación incompleta de la función motora facial (es decir, la debilidad facial residual). Los resultados secundarios fueron secuelas cosméticamente incapacitantes persistentes, desarrollo de sinquinesia motora o disfunción autonómica (es decir, espasmo hemifacial, lágrimas de cocodrilo) y efectos adversos del tratamiento con corticosteroides manifestados durante el seguimiento.
Resultados principales
Se identificaron siete ensayos con 895 participantes evaluables para esta revisión. Todos proporcionaron datos adecuados para el metanálisis de los resultados primarios. Uno de los ensayos fue nuevo desde la última versión de esta revisión sistemática Cochrane. El riesgo de sesgo en los estudios pequeños más antiguos incluyó algunas evaluaciones poco claras o de alto riesgo, mientras que se consideró que los estudios más grandes tuvieron bajo riesgo de sesgo. En general, 79/452 (17%) de los participantes asignados a los corticosteroides tuvieron una recuperación incompleta de la función motora facial a los seis meses o más después de la asignación al azar; significativamente menos que 125/447 (28%) del grupo control (riesgo relativo [RR] 0,63; intervalo de confianza [IC] del 95%: 0,50 a 0,80). El número de pacientes que necesitan ser tratados con corticosteroides para evitar una recuperación incompleta fue 10 (IC del 95%: 6 a 20). La reducción de la proporción de participantes con secuelas cosméticamente incapacitantes a los seis meses después de la asignación al azar fue muy similar en los grupos de corticosteroides y placebo (RR 0,96; IC del 95%: 0,40 a 2,29; dos ensayos, n = 75, evidencia de calidad baja). Sin embargo, hubo una reducción significativa de la sinquinesis motora durante el seguimiento en los participantes que recibieron corticosteroides (RR 0,64; IC del 95%: 0,45 a 0,91, tres ensayos, n = 485, evidencia de calidad moderada). Tres estudios registraron explícitamente la ausencia de efectos adversos atribuibles a los corticosteroides. Un ensayo informó que tres participantes que recibieron prednisolona presentaron trastornos temporales del sueño y dos ensayos proporcionaron de forma detallada los efectos adversos que ocurrieron en 93 participantes, todos sin gravedad; el análisis combinado de los datos de estos tres ensayos no encontró diferencias significativas en las tasas de efectos adversos entre los pacientes que recibieron corticosteroides y los pacientes que recibieron placebo (RR 1,04; IC del 95%: 0,71 a 1,51; n = 715).
Conclusiones de los autores
La evidencia disponible, de moderada a alta, de ensayos controlados aleatorizados mostró un efecto beneficioso significativo del tratamiento de la parálisis de Bell con corticosteroides.
PICOs
Resumen en términos sencillos
Corticosteroides para la parálisis de Bell
Pregunta de la revisión
¿Cuáles son los efectos de los corticosteroides en la parálisis de Bell?
Antecedentes
La parálisis de Bell es una parálisis o debilidad de los músculos de la cara, habitualmente de un lado, sin causa conocida. Los síntomas se suelen revertir, aunque no siempre. Se cree que la reducción de la inflamación del nervio facial mediante el uso de fármacos corticosteroides (esteroides) limita el daño nervioso. Ésta es una actualización de una revisión publicada por primera vez en 2002 y actualizada por última vez en 2010.
Características de los estudios
Se identificaron siete ensayos clínicos en los que participaron 895 pacientes con parálisis de Bell unilateral leve, moderada o grave de causa desconocida. Todos estos ensayos informaron sobre las tasas de recuperación incompleta (la proporción de pacientes que permanecieron con debilidad facial) y fue posible combinar los resultados. Los pacientes de los estudios tenían entre dos y 84 años. Fueron tratados con corticosteroides o placebo (tratamiento inactivo), ya sea solo o en combinación con otras terapias. Un ensayo sólo involucró a niños, de 24 a 74 meses de edad. La duración de los estudios incluidos de adultos y niños varió entre 157 días y 12 meses.
Resultados clave y calidad de la evidencia
Recuperación incompleta
Según evidencia de calidad moderada a alta, los corticoides redujeron el número de pacientes que permanecieron con debilidad facial después de la parálisis de Bell, en comparación con placebo (un fármaco falso). Este hallazgo se basó en los datos de siete estudios que involucraron a 895 participantes con parálisis de Bell de diversos grados de gravedad. Se calculó que para evitar que un paciente quede con debilidad facial, hay que tratar a diez pacientes.
Cinco estudios proporcionaron datos sobre las secuelas a largo plazo de la parálisis de Bell después del tratamiento. Dos de los estudios (75 participantes) examinaron los efectos persistentes sobre la apariencia facial después de seis meses o más. El efecto fue casi el mismo para los corticosteroides y placebo, lo que muestra que los participantes que recibieron corticosteroides se beneficiaron ligeramente, aunque esta evidencia fue de calidad baja. Los datos de tres estudios (485 participantes) mostraron claramente que los pacientes que recibieron corticosteroides desarrollaron menos sinquinesia motora (movimientos faciales no deseados) y lágrimas de cocodrilo (ojos llorosos al comer o masticar), en comparación con los pacientes que recibieron sólo placebo. Este hallazgo se basó en evidencia de calidad moderada.
Efectos secundarios
Tres estudios informaron de que no se pudieron atribuir efectos secundarios al tratamiento con corticosteroides. Según evidencia de calidad moderada de tres estudios (715 participantes), el número de pacientes que presentaron efectos secundarios fue similar al de los que recibieron corticosteroides y placebo.
La evidencia está actualizada hasta marzo de 2016.
Authors' conclusions
Summary of findings
| Corticosteroids compared to placebo or no treatment for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Corticosteroids | |||||
| Incomplete recovery ≥ 6 months after randomisation | 281 per 1000 | 177 per 1000 | RR 0.63 | 895 | ⊕⊕⊕⊕ | NNTB 10, 95% CI 6 to 20 |
| Cosmetically disabling persistent sequelae ≥ 6 months after randomisation | 216 per 1000 | 208 per 1000 | RR 0.96 | 75 | ⊕⊕⊝⊝ | ‐ |
| Motor synkinesis and crocodile tears | 260 per 1000 | 167 per 1000 | RR 0.64 | 485 | ⊕⊕⊕⊝ | ‐ |
| Adverse effects | 127 per 1000 | 133 per 1000 (91 to 192) | RR 1.04 | 715 | ⊕⊕⊕⊝ | 3 other studies recorded that no adverse effects occurred with corticosteroid treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two trials excluded participants who were found to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). In addition, two studies did not use a scoring system such as the House‐Brackmann or Sunnybrook scale to assess facial motor function, relying upon clinical examination, electromyographic tests or photographs (May 1976; Taverner 1954). Taverner 1954 reported outcomes at five months rather than at six months or more. However, we felt that these limitations did not compromise the generalisability of the findings. 2 We downgraded twice: first for imprecision ‐ there was a low number of events and pooled RR allowed the possibility of both no effect and the chance of harm; second for publication bias ‐ of the seven included studies, five did not provide data on the presence of cosmetically disabling sequelae six months or more after randomisation. 3 We downgraded once for imprecision. There was a low number of events. 4 We downgraded the quality of the evidence to moderate because of publication bias. 5 We downgraded for publication bias ‐ only three of seven studies provided data on adverse effects. | ||||||
Background
Description of the condition
Bell's palsy is an acute, generally unilateral, paralysis or weakness of facial musculature consistent with peripheral facial nerve dysfunction, of no detectable cause (Niparko 1993). Additional symptoms frequently include pain around or behind the ear sometimes extending into the occipital or cervical region. Impaired tolerance to ordinary levels of noise and disturbed sense of taste on the same side may also be present (Burgess 1984).
Epidemiological studies have reported an annual incidence of 23 to 25 per 100,000 per year (Martyn 1997; Victor 1994), but one study using a general practitioner (GP) database has suggested it may be even higher at 37 per 100,000 per year (Morales 2013). Bell's palsy affects men and women more or less equally. Until recently was thought to be most common in the 30‐ to 45‐year age group (Bateman 1992; Brandenberg 1993; Katusic 1986; Peitersen 1982; Peitersen 2002; Yanagihara 1988), although the one more recent primary care database study suggested a peak in people over 70 years of age (Morales 2013). The condition presents disproportionately among pregnant women and people who have diabetes, influenza, a cold, or some other upper respiratory ailment. On average, every year a British GP will see one or two people who have developed the condition. Both sides of the face are affected equally often (Prescott 1988).
The aetiology of Bell's palsy is still debated. A viral infection, vascular ischaemia, autoimmune inflammatory disorders and heredity have been proposed as underlying causes (Adour 1982; Burgess 1984; Lackner 2010). A viral aetiology has gained popularity since the isolation of herpes simplex virus‐1 (HSV‐1) genome from the facial nerve endoneurial fluid of people with Bell's palsy (Lackner 2010; Murakami 1996). The prognosis is on the whole favourable. One of the largest series of people with Bell's palsy including those who were not receiving specific therapy, showed that 85% of people with Bell's palsy began to recover within three weeks of onset (Peitersen 1982). For the remaining 15%, partial recovery occurred three to six months later. The same series showed that normal facial expression reappeared in 71% of cases, 13% had insignificant sequelae, and the remaining 16% had permanently diminished function with aberrant innervation (expressed as motor synkinesis or autonomic dysfunction) and post‐paralytic spasms.
Description of the intervention
The treatment of Bell's palsy used to be highly controversial. Corticosteroids were the treatment of choice, based mainly on non‐randomised comparisons (Adour 1972). The authors of numerous clinical series both espoused and condemned corticosteroid therapy with, what appeared to them, equally convincing arguments (Burgess 1984). Four systematic reviews found a significant trend favouring corticosteroid treatment in improving the recovery of facial motor function (de Almeida 2009; Grogan 2001; Ramsey 2000; Williamson 1996). However, their conclusions were affected by including trials of poor quality in the pooled estimate. de Almeida 2009, a state‐of‐the‐art, high‐quality systematic review, differed from our methodology in the way they collected data, in the inclusion of a non‐randomised trial that we excluded (Martinez 1990), and in the exclusion of a trial that we included in our analysis (Taverner 1954).
How the intervention might work
Corticosteroids are known to have an anti‐inflammatory mode of action, which reduces oedema and inflammation of the facial nerve in the acute presentation of Bell's palsy.
Why it is important to do this review
The purpose of this systematic review was to collect and analyse the available evidence from randomised controlled trials concerning the use of corticosteroids for improving the outcome of people with Bell's palsy. This is an update of a review first published in 2002 and last updated in 2010. We updated the review to assess the quality of the evidence regarding the benefit of corticosteroids in Bell's palsy using current methodology, integrate any new evidence and to determine whether further research would be likely to change the estimate of effect or its certainty.
Objectives
To determine the effectiveness and safety of corticosteroid therapy in people with Bell's palsy.
Methods
Criteria for considering studies for this review
Types of studies
Trials of the use of corticosteroid therapy for the treatment of recent‐onset Bell's palsy in which an attempt had apparently been made to conduct a randomised or quasi‐randomised comparison between corticosteroid therapy and a placebo, or open control group. As in the previous version of the review, we used study quality as an exclusion criteria excluding trials with a high risk of bias in several domains.
Types of participants
Participants of any age with clinically diagnosed Bell's palsy, irrespective of the time of evolution of symptoms. We included all participants considered to have Bell's palsy or acute idiopathic facial nerve paralysis by the study authors, irrespective of the ancillary studies they performed to rule out other causes of facial paralysis. We included in the analysis all participants considered eligible by the authors of the studies, irrespective of associated conditions.
Types of interventions
We included trials using any corticosteroid or adrenocorticotrophic hormone therapy, irrespective of the route of administration (oral or parenteral) and length of therapy. We excluded trials in which a drug considered 'effective' for this condition was given to the control group, unless it was given in a similar way to the experimental group. The exception was studies in which control and treatment groups received concomitant antiviral therapy; these are not included in this review, but in the updated Cochrane review 'Antiviral treatment for Bell's palsy (idiopathic facial paralysis)' (Gagyor 2015).
We excluded trials comparing different types of corticosteroids or different dosage schemes, unless the trial included a placebo group.
Types of outcome measures
Primary outcomes
-
Incomplete recovery of facial motor function six months or more after randomisation.
Secondary outcomes
-
Cosmetically disabling persistent sequelae of facial paralysis (poor recovery of facial motor function as defined by the authors of the studies) six months or more after randomisation.
-
Motor synkinesis or autonomic dysfunction during follow‐up (including facial spasm, motor synkinesis and crocodile tears).
-
Adverse effects attributable to the use of corticosteroids.
Search methods for identification of studies
Electronic searches
On 4 March 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL in the Cochrane Register of Studies Online), MEDLINE (January 1966 to February 2016), EMBASE (January 1980 to February 2016) and LILACS (January 1982 to February 2016). The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register (Appendix 1), CENTRAL (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4) and LILACS (Appendix 5).
On 21 June 2016, we searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (who.int/trialsearch/) for ongoing studies. We searched both using the term "Bell's Palsy".
Searching other resources
We reviewed the bibliographies of the randomised trials and contacted the study authors and known experts in the field to identify additional published or unpublished data.
Data collection and analysis
Selection of studies
Six review authors scrutinised published and unpublished papers retrieved by the literature searches to select papers for inclusion. At least two review authors independently assessed each paper for relevance, eligibility and quality. There were no disagreements about inclusion. We applied no language limitations.
Data extraction and management
Review authors collected and analysed data in pairs (FG and VM, DS and MS, IG and FS). We collected data on the following.
-
Methods: study design, study duration, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: number (n), mean age, age range, gender, severity of condition, diagnostic criteria, baseline characteristics, inclusion criteria and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported; definition of recovery status.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
All six review authors had a selection of papers to read, review for quality and extract data from. At least two review authors assessed each trial. All review authors agreed data extraction.
Two review authors (IG and VM) inputted data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
IG completed the 'Risk of bias' table. FS and FD individually reviewed the 'Risk of bias' assessments. We assessed bias by scoring studies as at high, low or unclear risk of bias using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
When comparing studies using different symptom scores to assess outcomes, we used the House‐Brackmann scale when available as this was the most widely used or had comparisons to other scales available. When assessing adverse effects, we reported the number of participants affected, as opposed to the number of events, to facilitate data comparison.
We used dichotomous outcomes and we analysed data as risk ratios (RR) with a corresponding 95% confidence interval (CI). We calculated a treatment effect using the Mantel‐Haenszel method (Egger 2007).
Unit of analysis issues
Where a single trial included multiple trial arms, we included only arms relevant to this review. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) had been combined in the same meta‐analysis, our preference would be to combine groups to create a single comparison if appropriate, or otherwise use one of the other approaches described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
One of the trial authors, who is also a review author (FS), provided unpublished trial data. We also contacted a previous study team member (P Lockhart) for additional information on other studies and received a response. We either extracted the number of participants who completed a treatment originally allocated and the number with the outcome, and considered the potential effect of missing data when presenting results, or reported intention‐to‐treat analyses as presented in trial reports. Where data were unavailable we contacted the original authors, when there was no response, we calculated the numbers from the rates and percentages presented in the papers. We undertook no sensitivity analyses for missing values. For some trials, we calculated either number in the analysis or the number experiencing an event using the percentage experiencing an event.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis, using the thresholds in the Cochrane Handbook for Systematic Reviews of Interventions as guidance (Higgins 2011):
-
0% to 40%: might not be important;
-
30% to 60%: may represent moderate heterogeneity;
-
50% to 90%: may represent substantial heterogeneity;
-
75% to 100%: considerable heterogeneity.
We interpreted the I2 statistic in the context of its CI, the Chi2 test, P value and the size of the effect.
Assessment of reporting biases
Since we were unable to pool more than 10 trials, we could not create a funnel plot to explore possible small‐study biases.
Data synthesis
We used a fixed‐effect model for our analysis and performed a sensitivity analysis with a random‐effects model.
If the review had included more than one comparison that could not be included in the same analysis, we would have reported the results for each comparison separately.
Throughout we have utilised the following notations:
OS: corticosteroid treatment alone
OO: placebo or no treatment only
Using these notations, we conducted the following comparison: OS versus OO.
'Summary of findings' table
At this update, we added a 'Summary of findings' table. We used the following outcomes: incomplete recovery, cosmetically disabling persistent sequelae six months or more after randomisation, motor synkinesis and crocodile tears, and adverse effects.
Under the GRADE approach, the levels of quality of evidence are high, moderate, low or very low. Assessors start at high quality and may downgrade the evidence for the pre‐specified outcomes depending on five GRADE considerations, which are: study limitations, consistency of effect, imprecision, indirectness and publication bias. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used GRADEpro software (GRADEpro 2008). We have justified all decisions to downgrade or upgrade the quality of studies using footnotes and have made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We did not undertake any subgroup analyses for this review. The protocol detailed possible subgroup analyses, although none were appropriate for the included studies.
Sensitivity analysis
We carried out the following sensitivity analyses.
-
Repeated the analyses excluding smaller and underpowered studies.
-
Repeated the analyses excluding studies with short‐term follow‐up (less than nine months).
Results
Description of studies
Results of the search
In the updated searches for this review, we found 59 references in the Cochrane Neuromuscular Specialised Register, 81 in CENTRAL, 818 in MEDLINE, 117 in EMBASE and 15 in LILACS.
There were seven completed randomised controlled trials comparing corticosteroids with no active control when this review was first written. In subsequent updates, review authors identified five other potentially relevant trials (Austin 1993; Bento 1991; Engström 2008; Lagalla 2002; Sullivan 2007).
In all, seven trials including 895 evaluable participants met our inclusion criteria (Austin 1993; Engström 2008; Lagalla 2002; May 1976; Sullivan 2007; Taverner 1954; Unuvar 1999).
See Figure 1 for a flow chart illustrating the study selection process for this update.

Study flow diagram illustrating the study selection process for this update.
1. The previous version of this review listed comparisons involving participants treated with antiviral therapy from Sullivan 2007 and Engström 2008 as two additional separate studies. These two comparisons are not included in this update.
The previous update of this review, Salinas 2010, listed seven excluded studies (Akpinar 1979; Austin 1993; Bento 1991; Bento 1994; Brown 1982; Martinez 1990; Wolf 1978). We have linked two references previously listed as separate trials (Bento 1991 and Bento 1994) under one trial identifier in this update (Bento 1991). We included Austin 1993, previously excluded because of potential attrition bias, as current practice is to include all trials eligible according to the selection criteria.
Authors of the last version of this review included groups receiving antiviral therapy and considered the different comparisons from Engström 2008 and Sullivan 2007 as separate studies (Engström 2008b and Sullivan 2007b). We did not consider the two antiviral‐treated participant groups from those trials in this updated review. These comparisons are included in the companion Cochrane review, 'Antiviral treatment for Bell's palsy (idiopathic facial paralysis)' (Gagyor 2015). In this update, we included only the comparisons of corticosteroid versus placebo or corticosteroids versus no treatment.
Included studies
Summary details of the included trials are given in Characteristics of included studies.
Austin 1993 recruited 107 participants whose symptoms had occurred within five days of presentation to the study centre. Of these, 31 participants did not return for initial follow‐up. The 76 remaining participants subsequently entered a double‐blinded randomised controlled study allocating them to a corticosteroid treatment group (prednisone) or a placebo group. The trial authors gave no details about binding or randomisation. Assessment of participants occurred within five days of onset and at "regular intervals until they experienced recovery from their acute paralysis". Final follow‐up was at six months after recovery. The trial authors stated, "to be included in the analysis, patients must have completed follow up until their acute recovery occurred." Investigators measured disease status using the House‐Brackmann grading system. At first evaluation, participants underwent various tests including blood tests, an audiogram, a nerve excitability test using the maximal stimulation technique and, if indicated, electroneurography. Treatment with prednisone was started at 30 mg twice daily for the first five days followed by a reducing dose stated by the trial authors.
The study reported final outcomes on 53 participants at six months after recovery. The primary outcome was time to recovery, for which trial authors found no statistically significant difference between the two groups. Secondary outcomes included facial paralysis grade at onset versus grade at recovery. There was a statistically significant difference in incomplete recovery rates favouring the treatment group: 5/23 participants receiving prednisone had incomplete recovery at six months versus 10/30 participants receiving placebo (RR 0.65, 95% CI 0.26 to 1.65). Investigators found no significant difference in persistence of pain during recovery, with 3/23 participants in the prednisone group still experiencing pain at six months, compared with 4/30 participants in the placebo group (RR 0.98, 95% CI 0.24 to 3.95).
Engström 2008 had a factorial design that randomised 829 participants into four treatment groups using a two‐stage computerised process. The four treatment groups were prednisolone with placebo, prednisolone with valaciclovir, valaciclovir with placebo, and double placebo. Treatment started within 72 hours of symptom onset. The trial was double‐blind (administrator and participant) for assessment of recovery status until the end of follow‐up. Participants were assessed at onset, after two weeks (11 to 17 days) and after 1, 2, 3, 6 and 12 months. Trialists measured disease status using the House‐Brackmann grading system and the Sunnybrook scale, defining complete recovery status as a Sunnybrook score of 100 and a House‐Brackmann grade of I. Data analysis included an assessment of treatment interaction.
The study reported a positive effect on recovery time due to prednisolone (comparing recovery rates at 12 months in the prednisolone group with the placebo group). For this review, we analysed the corticosteroid‐placebo combination (OS) versus the double placebo combination (OO) 12 months after the onset of facial palsy. Using the Sunnybrook definition, 50/210 participants had an incomplete recovery with prednisolone compared with a greater proportion (73/206) in the placebo group (RR 0.67, 95% CI 0.50 to 0.91).
Lagalla 2002 randomised 62 participants within three days of onset of Bell's palsy to high‐dose prednisone, 1 g daily for three days, then 0.5 g daily for three days, administered intravenously, using saline solution as a placebo. The age range was 15 to 84 years. Exclusion criteria included peptic ulcer disease, pregnancy, severe hypertension, other neurological conditions, diseases of the ear and previous treatment. As in Taverner 1954, investigators excluded participants with herpes zoster oticus from the analyses (four of the participants initially randomised in Lagalla 2002). The investigators monitored participants for 12 months. They assessed recovery of facial motor function using the House‐Brackmann scale. The trial authors reported the development of disabling synkinesis at 12‐month follow‐up.
Lagalla 2002 reported final outcomes on 58 participants at 12 months. In the prednisolone group, 5/30 participants had incomplete recovery, compared with 8/28 participants in the placebo group (RR 0.58, 95% CI 0.22 to 1.57).
May 1976 included 51 participants within two days of Bell's palsy onset and reported outcomes using clinical assessment and photographic documentation without a validated scoring system. The trial report did not specify the age range of participants in this double‐blinded study. In the intervention group, participants received prednisone 410 mg in descending doses over 10 days. The placebo intervention was vitamins. Trialists excluded people with chronic otitis media, trauma, loss of lacrimation, or recurrent or bilateral palsy.
May 1976 was the only study that stated that there was no benefit from corticosteroids for the recovery of Bell's palsy (RR 1.16, 95% CI 0.57 to 2.36).
Sullivan 2007 recruited 551 participants to be treated within 72 hours of onset. Participants were randomised by a dedicated remote telephone‐computerised mechanism in a two‐stage process into four treatment groups in a factorial design: prednisolone with placebo, prednisolone with aciclovir, aciclovir with placebo or double placebo. Participants received prednisolone 25 mg twice daily for 10 days. The trial was blinded for administrator, participant and assessment of recovery status until the end of follow‐up. Assessments took place at onset, after three months and, if participants were still unwell, again after nine months. The investigators measured recovery status on the House‐Brackmann scale, with complete recovery defined as House‐Brackmann grade I. Data analysis included an assessment of treatment interaction.
Sullivan 2007 reported final outcomes on 496 completed participants at three and nine months. In the corticosteroid (OS) group, 5/127 participants had incomplete recovery, compared with 18/122 participants in the placebo (OO) group (RR 0.27, 95% CI 0.10 to 0.70).
Taverner 1954 was a double‐blind trial that included 26 participants with a range of ages from 12 to 76 years within 10 days of onset of Bell's palsy. Participants received either oral cortisone acetate 1 g in descending doses over eight days or placebo tablets. Participants were monitored for up to 157 days. The trial excluded participants with other neurological conditions or diseases of the ear. Taverner 1954 included participants with herpes zoster oticus. These participants were allocated in equal numbers to each treatment arm, and trial authors excluded them from the analyses. The trialists did not used a validated scoring system to measure outcomes.
Taverner 1954 used the recovery of facial motor function as the main outcome measure and reported a small, non‐significant benefit of corticosteroids compared with placebo; CIs allowed for effects in either direction (RR 0.86, 95% CI 0.27 to 2.71).
Unuvar 1999 conducted a non‐blinded, randomised controlled trial on children with severe to complete presentation of Bell's palsy (House‐Brackmann grades IV to V). They randomised 42 children with an age range of 24 to 74 months using a computer‐generated random sequence into two groups. All participants entered the trial within three days of onset of Bell's palsy and received either methylprednisolone 1 mg/kg daily for 10 days then gradually withdrawn over three to five days or no specific treatment. They excluded participants with other neurological conditions, diseases of the ear or systemic diseases. The primary outcome was the recovery of facial motor function measured on the House‐Brackmann facial grading system.
Unuvar 1999 reported a benefit from the treatment with corticosteroids although wide CIs allowed for the possibility of both no effect and a large effect (RR 0.14, 95% CI 0.01 to 2.61).
Ongoing studies
We identified one trial report on the World Health Organization International Clinical Trials Registry Platform search portal (Babl 2015). From the website, it appeared that the study has now started recruitment; we will assess this trial fully in future review updates. See Characteristics of ongoing studies.
Excluded studies
Akpinar 1979 compared three groups of 10 participants each; two groups received different dosage regimens of corticosteroids, and the other received placebo. It was unclear who diagnosed the participants with Bell's palsy and there were varying dosage regimens of corticosteroids used. Two studies were not randomised or quasi‐randomised (Brown 1982; Martinez 1990). It was not possible to extract complete information on the specified outcomes for one study (Wolf 1978). Finally, we excluded Bento 1991 as number of participants and outcome events by treatment groups was not provided; in addition, 50% of the participants were lost to follow‐up.
Risk of bias in included studies
Figure 2 summarises the 'Risk of bias' assessment for each included study.
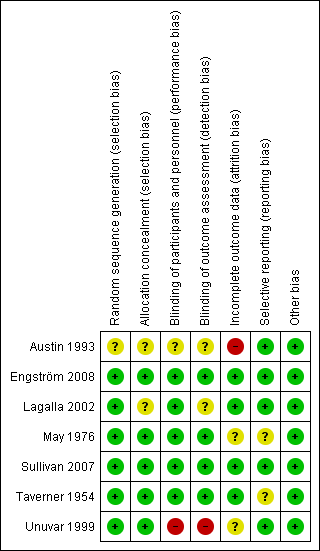
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.
Allocation
All included trials reported the method of randomisation. Four used randomisation centralised at a pharmacy, in accordance with a master sheet of random numbers (May 1976; Taverner 1954), or an automated permuted block technique (Engström 2008; Sullivan 2007). One study used a random number list (Lagalla 2002), and another used a computer‐based program (Unuvar 1999). It was not clear from the reports if there was adequate allocation concealment in Lagalla 2002 or Unuvar 1999. We considered none of the trials to be at high risk of bias for random sequence generation or allocation concealment.
Blinding
Five trials were double‐blind, using placebo in the control group (Engström 2008; Lagalla 2002; May 1976; Sullivan 2007; Taverner 1954). Two of these trials used lactose as the placebo (Sullivan 2007; Taverner 1954), one used vitamins (May 1976), and one used a saline solution (Lagalla 2002). Two trials did not specify what was used as placebo, but they stated that this was formulated to have the same size, smell and colour (Engström 2008; Unuvar 1999). In the trial using vitamins as placebo, the experimental group received similar looking capsules containing prednisone plus vitamins. The trial using saline solution as placebo gave intramuscular vitamins to all participants for 15 days (Lagalla 2002). In one trial, it was not clear from the report if the physicians administering the drug were blinded (Lagalla 2002). There were no serious imbalances in baseline prognostic factors between groups.
Incomplete outcome data
Engström 2008, Sullivan 2007; and Taverner 1954 had drop‐out rates of less than 10%. No participants dropped out in May 1976. Lagalla 2002 included drop‐outs in analyses on an intention‐to‐treat basis, by assuming that drop‐outs had a poor outcome. Austin 1993 had a drop‐out rate of 50% for outcomes six months after recovery. Unuvar 1999 did not report on attrition.
Selective reporting
All studies reported their intended primary outcomes. Engström 2008 reported all primary outcomes, with secondary outcomes reported in subsequent publications (Axelsson 2012; Berg 2012).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
As all trials reported different intervals and lengths of follow‐up, we performed analyses on data reported at the end of the study periods. These were 157 days (Taverner 1954), six months (May 1976; Austin 1993), nine months (Sullivan 2007), and 12 months (Engström 2008; Lagalla 2002; Unuvar 1999).
Primary outcome
Incomplete recovery of facial motor function six months or more after randomisation
All seven trials, with 895 participants, provided data for the outcome complete recovery of facial motor function at six months' follow‐up or more (we included data from Taverner 1954, which reported at five months). The number of people with incomplete recovery at six months' follow‐up was lower in the corticosteroid group compared to the control group (RR 0.63, 95% CI 0.50 to 0.80) (Analysis 1.1; Figure 3).

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.1 Incomplete recovery ≥ 6 months after randomisation.
The tests for statistical heterogeneity were marginally significant (Chi2 = 7.32, P value = 0.29, I2 = 18%). This small degree of heterogeneity was due to the differences in the findings between the large studies (Engström 2008; Sullivan 2007), and small, underpowered studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999). There were better outcomes in two of the studies (Engström 2008; Sullivan 2007).
The number of people who need to be treated with steroids to avoid one person with incomplete recovery was 10 (95% CI 6 to 20).
Sensitivity analyses
We removed smaller underpowered studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999) from the main analysis leaving the two largest studies (Engström 2008; Sullivan 2007). This analysis confirmed the size and direction of effect, but with higher statistical heterogeneity (RR 0.59, 95% CI 0.44 to 0.79, Chi2 = 3.32, I2 = 70%).
We analysed the main outcome for the studies that had the longest follow‐up (i.e. between nine and 12 months) (Engström 2008; Lagalla 2002; Sullivan 2007). This did not significantly change the result.
Secondary outcomes
Cosmetically disabling persistent sequelae six months or more after randomisation
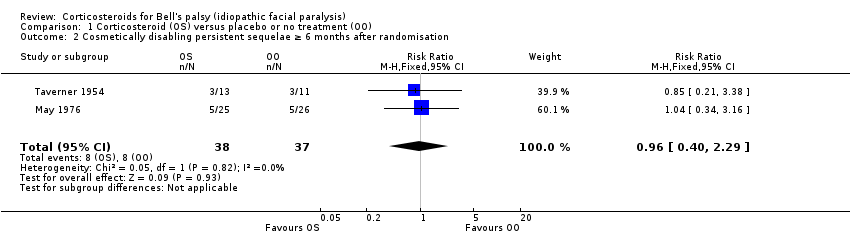
Two trials with 75 participants provided data on the number of participants with severe paralysis, or what may be judged as cosmetically disabling sequelae, at six months' follow‐up or more (May 1976; Taverner 1954). When we combined the data, the number of participants with cosmetically disabling sequelae was similar in the corticosteroid group and the control group (RR 0.96, 95% CI 0.40 to 2.29) (Analysis 1.2; Figure 4)

1.2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.
Motor synkinesis or crocodile tears during follow‐up
Three trials, with 485 evaluable participants, reported separate data on motor synkinesis or crocodile tears during follow‐up (Austin 1993; Engström 2008; Lagalla 2002). Two trials reported the occurrence of disabling synkinesis at 12 months' follow‐up (Engström 2008; Lagalla 2002); the other trial reported at six months' follow‐up (Austin 1993). After pooling these data, we found a significant reduction in the number of people with motor synkinesis in the corticosteroid group (40/241) compared to the placebo group (64/248) (RR 0.64, 95% CI 0.45 to 0.91) (Analysis 1.3; Figure 5). We found no statistical heterogeneity (Chi2 = 0.42, df = 2 (P = 0.81), I2 = 0%).

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.3 Motor synkinesis and crocodile tears.
Adverse effects attributable to the use of corticosteroids
Three studies explicitly recorded the absence of adverse effects attributable to the experimental treatment (May 1976; Taverner 1954; Unuvar 1999). One trial reported that three participants receiving prednisone had temporary sleep disturbances (Lagalla 2002). Another reported "no severe side effects of steroid therapy", although there were single reported cases of mood swings, dyspepsia and minor conjunctivitis by individual participants (Austin 1993). Two trials gave a detailed account of 93 adverse effects (Engström 2008; Sullivan 2007), all of them non‐serious, with no significant difference between people receiving corticosteroids and people receiving placebo (RR 1.04, 95% CI 0.71 to 1.51). Three deaths occurred; all were deemed to be unrelated to treatment, all were in the groups not receiving prednisolone. One man with a history of recurrent atrial fibrillation had a transient relapse while taking prednisolone and valaciclovir (Figure 6).

Forest plot of comparison: 1 Incomplete recovery of facial motor function, outcome: 1.4 Adverse effects.
Subgroup analyses
In this updated version of the review, we did not perform any subgroup analyses.
Discussion
Summary of main results
High‐quality evidence from randomised controlled trials indicated benefit from the use of corticosteroids in Bell's palsy.
Two trials reached a statistically significant difference favouring the use of prednisolone (Engström 2008; Sullivan 2007). Meta‐analysis of the complete high‐quality randomised controlled trial literature convincingly supported a beneficial effect of prednisolone for reducing the numbers of participants with incomplete recovery.
Moderate‐quality evidence indicated that participants who received corticosteroid treatment had less motor synkinesis and crocodile tears than the placebo group.
Based on these two studies, low‐quality evidence revealed no important differences between corticosteroids and placebo in cosmetically disabling persistent sequelae, but wide CIs allowed for the possibility of an effect in either direction.
The included studies did not report differences in the occurrence of adverse effects between corticosteroids and placebo and additionally no serious adverse effects were reported. However, corticosteroids have well‐known adverse effects, of which the incidence rises markedly with prolonged dosages above 10 mg of prednisolone or its equivalent per day (Dollery 1999). Usually corticosteroid courses in Bell's palsy are short and the dose quickly reduces, making the likelihood of adverse effects in practical use less than in longer‐term indications.
Overall completeness and applicability of evidence
No new studies have been published since the previous Cochrane review (Salinas 2010). The two most recent, well‐powered studies (Engström 2008; Sullivan 2007), with five smaller studies (Austin 1993; Lagalla 2002; May 1976; Taverner 1954; Unuvar 1999), provided a significant result based on the evidence provided. These studies sufficiently address the objectives of this review in that they investigate all relevant participants, interventions and outcomes. The findings are in accordance with suggested current clinical practice (Madhok 2009).
Quality of the evidence
For the main outcome of this review (incomplete recovery six months or more after randomisation), all seven studies combined provided high‐quality evidence. In addition, for motor synkinesis and crocodile tears, data from three studies provided moderate‐quality evidence. Cosmetically disabling persistent sequelae six months or more after randomisation was the only analysis performed where the quality of evidence was low. For adverse effects, the evidence was moderate quality. Reasons for downgrading the quality of evidence were that in one study participants and assessors were not blinded to the treatment that they received (Unuvar 1999), and in another study, loss to follow‐up was 50%, representing incomplete outcome data (Austin 1993). Two studies excluded participants who were found after randomisation to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). Two studies did not use a standardised scale for assessment of facial motor function (May 1976; Taverner 1954). We considered the effects of these factors on the quality of the evidence insufficient for further downgrading for indirectness of evidence. The results of the majority of studies in this review were consistent with each other, apart from those of one older, smaller study (May 1976).
Potential biases in the review process
To help ensure that decisions about which studies to include in this review were reproducible, two review authors repeated the process (we divided the studies into three groups). There was no distinction made on the experience and expertise of each author in the reviewing pairs. On applying the eligibility criteria and assessing the relevance of studies, review authors were aware of the names of the study authors, institutions, journal of publication and results. FS and FD did not assess their own trial (Sullivan 2007). There were no final disagreements about which studies should be included. According to previous practice in this review, we excluded several studies and a published abstract that provided insufficient information. Therefore, there might be some risk of publication and selective reporting bias due to data from some studies being unavailable.
Agreements and disagreements with other studies or reviews
Our results are coincident with three previous systematic reviews, which found that corticosteroids significantly improved the prognosis of people with Bell's palsy (de Almeida 2009; Ramsey 2000; Williamson 1996). All three reviews, even though of good quality, included a study that lost 50% of participants to follow‐up (Bento 1991). Only one of them, de Almeida 2009, was carried out after the publication of the largest trials included in our review (Engström 2008; Sullivan 2007). Two of them (de Almeida 2009; Ramsey 2000) also included a non‐randomised study in the analyses (Martinez 1990; Shafshak 1994). A Practice Parameter published by the American Academy of Neurology concluded that corticosteroids were safe and probably effective in improving facial functional outcomes in people with Bell's palsy (Grogan 2001). This Practice Parameter was published, also, before the publication of Sullivan 2007 and Engström 2008.

Study flow diagram illustrating the study selection process for this update.
1. The previous version of this review listed comparisons involving participants treated with antiviral therapy from Sullivan 2007 and Engström 2008 as two additional separate studies. These two comparisons are not included in this update.

'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.1 Incomplete recovery ≥ 6 months after randomisation.

1.2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.3 Motor synkinesis and crocodile tears.

Forest plot of comparison: 1 Incomplete recovery of facial motor function, outcome: 1.4 Adverse effects.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 1 Incomplete recovery ≥ 6 months after randomisation.
Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 3 Motor synkinesis and crocodile tears.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 4 Adverse effects.
| Corticosteroids compared to placebo or no treatment for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Corticosteroids | |||||
| Incomplete recovery ≥ 6 months after randomisation | 281 per 1000 | 177 per 1000 | RR 0.63 | 895 | ⊕⊕⊕⊕ | NNTB 10, 95% CI 6 to 20 |
| Cosmetically disabling persistent sequelae ≥ 6 months after randomisation | 216 per 1000 | 208 per 1000 | RR 0.96 | 75 | ⊕⊕⊝⊝ | ‐ |
| Motor synkinesis and crocodile tears | 260 per 1000 | 167 per 1000 | RR 0.64 | 485 | ⊕⊕⊕⊝ | ‐ |
| Adverse effects | 127 per 1000 | 133 per 1000 (91 to 192) | RR 1.04 | 715 | ⊕⊕⊕⊝ | 3 other studies recorded that no adverse effects occurred with corticosteroid treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two trials excluded participants who were found to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). In addition, two studies did not use a scoring system such as the House‐Brackmann or Sunnybrook scale to assess facial motor function, relying upon clinical examination, electromyographic tests or photographs (May 1976; Taverner 1954). Taverner 1954 reported outcomes at five months rather than at six months or more. However, we felt that these limitations did not compromise the generalisability of the findings. 2 We downgraded twice: first for imprecision ‐ there was a low number of events and pooled RR allowed the possibility of both no effect and the chance of harm; second for publication bias ‐ of the seven included studies, five did not provide data on the presence of cosmetically disabling sequelae six months or more after randomisation. 3 We downgraded once for imprecision. There was a low number of events. 4 We downgraded the quality of the evidence to moderate because of publication bias. 5 We downgraded for publication bias ‐ only three of seven studies provided data on adverse effects. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incomplete recovery ≥ 6 months after randomisation Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.50, 0.80] |
| 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation Show forest plot | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.29] |
| 3 Motor synkinesis and crocodile tears Show forest plot | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.91] |
| 4 Adverse effects Show forest plot | 3 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.51] |

