Corticosteroides para la parálisis de Bell (parálisis facial idiopática)
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Double‐blind, randomised, controlled study | |
| Participants | 107 people were initially randomised; 76 completed follow‐up until acute recovery and were included in the study analyses Participants were treated within 5 days of onset Sex: male 39 (51%), female 37 (49%) Age: mean 36.8 years, range 18 to 70 years 37 cases (49%) right side palsy and 39 cases (51%) left side palsy | |
| Interventions |
| |
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up up to 9 months | |
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | 1 October 1989 to 31 December 1990 | |
| Notes | Good recovery defined as grade I or II of the House‐Brackmann scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised at the pharmacy but details not given |
| Allocation concealment (selection bias) | Unclear risk | Allocation of participants not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The study was blinded to both the patient and the clinical investigators". Further details of blinding not given |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The study was blinded to both the patient and the clinical investigators". Further details of blinding not given |
| Incomplete outcome data (attrition bias) | High risk | 107 participants randomised, 31 did not attend for follow‐up assessment. 76 allocated to prednisone or placebo Analysis 6 months after resolution of Bell's palsy included 53 participants (23 prednisolone and 30 placebo), representing over 50% loss to follow‐up at this point. Reasons for additional drop‐outs at 6 months not described |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Low risk | Single‐centre study |
| Methods | Randomised, placebo‐controlled trial with 4 treatment groups | |
| Participants | 829 participants randomised within 72 hours of facial palsy onset No contraindications to corticosteroid or antiviral use Sex: male 341 (41%), female 488 (59%) Age: mean 40 years, range 31‐54 years | |
| Interventions | Participants allocated into 1 of 4 treatment groups:
Dosages: valaciclovir 1000 mg 3 times daily for 7 days; prednisolone 60 mg daily for 5 days | |
| Outcomes | Primary outcome:
Complete recovery was taken as Sunnybrook scale 100 and House‐Brackmann scale grade I Other outcomes:
Follow‐up at 2 weeks, and 1, 2, 3, 6 and 12 months after randomisation, according to recovery Final outcomes reported at 12 months | |
| Funding | Uppsala University; GlaxoSmithKline (Sweden); Pfizer AB (Sweden); Acta Otolaryngologica Foundation; Rosa and Emanuel Nachmanssons Foundation; Stig and Ragna Gorthon Foundation; Torsten Birger Segerfalk Foundation; Margit Arstrups Foundation; County Council of Skåne; Helsinki University Central Hospital Research Funds | |
| Conflicts of interest | One author was paid by GlaxoSmithKline for a lecture on Bell's Palsy | |
| Date conducted | May 2001 to September 2006 | |
| Notes | Multicentre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computer number generator. Sequentially numbered identical containers allocated to participants on entry into the trial, by the recruiting physician |
| Allocation concealment (selection bias) | Low risk | Allocation sequence double‐blind and generated by a computer number generator in random permuted blocks of 8 |
| Blinding of participants and personnel (performance bias) | Low risk | Study drugs issued in identical containers. All participants blinded to treatment group until study completion. All study personnel and data analysts blinded to treatment group until study completion |
| Blinding of outcome assessment (detection bias) | Low risk | All study personnel and data analysts blinded to treatment group until study completion |
| Incomplete outcome data (attrition bias) | Low risk | Numbers lost to follow‐up and reasons given:
'Modified' intention‐to‐treat analyses used. All randomised participants who received at least 1 dose of study medication included, but participants who did not start therapy excluded. Last observation carried forward method used for the modified intention‐to‐treat analysis, and missing data points imputed in the post‐baseline follow‐up visits from the last observation available for each participant |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported. Other outcomes reported in another paper due to space constrictions |
| Other bias | Low risk | No other biases identified |
| Methods | Randomised, placebo‐controlled, double‐blind trial | |
| Participants | 62 participants within 3 days of onset of onset of Bell's palsy; 4 people excluded after randomisation because of acute herpes zoster infection Sex: male 34, female 28 Age: mean (± SD) 47.5 (± 19) years, range 15 to 84 years Participants with contraindications to corticosteroids (peptic ulcer disease, pregnancy, severe hypertension), or previously treated excluded. No losses to follow‐up 34 cases left palsy, 28 cases right palsy | |
| Interventions |
All participants received intramuscular vitamins for 15 days | |
| Outcomes | Primary outcome:
Used House‐Brackmann scale for assessment. "Good recovery" was grades I or II Secondary outcomes:
Follow‐up at 1, 3, 6 and 12 months Final outcomes reported at 6 and 12 months | |
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | Not stated | |
| Notes | Single centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated by authors |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to the treatment. Saline solution was used as a placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated if assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Numbers lost to follow‐up and reasons given: 62 people randomised, 4 excluded after randomisation (2 in each group) because of herpes zoster infection; the remaining 58 completed study Trial authors stated: "Comparative statistics was carried out on an intention‐to‐treat basis, by assuming that drop‐outs had a poor outcome. Data analysis concerning basal features included all patients enrolled. The analysis of clinical grading changes included data from only 58 subjects" |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported |
| Other bias | Low risk | No other bias identified |
| Methods | Double‐blind, placebo‐controlled, randomised trial with 2 treatment groups | |
| Participants | 51 participants within 2 days of onset of Bell's palsy People with chronic otitis, trauma, loss of lacrimation, and bilateral or recurrent palsy, and herpes zoster excluded Sex: male 28, female 23 Age: ranges not clearly stated "30 years or less and 31 years or older" | |
| Interventions |
| |
| Outcomes | Primary outcome:
Assessment made clinically using photographs (examples given in the paper) at 6 months after onset Secondary outcomes:
No adverse effects reported | |
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | 1972 to 1974 | |
| Notes | Single centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random stratified sequence generated by a statistician, administered in a pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation of treatment group unknown to both participants and physicians |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded. Placebo was similar‐looking tablets containing vitamins |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded to treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on loss to follow‐up rates |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported, adverse effects not reported |
| Other bias | Low risk | No other bias identified |
| Methods | Double‐blind, placebo‐controlled, randomised, factorial trial | |
| Participants | 552 participants randomised and 496 included in final outcome assessment Referred for assessment and treatment within 72 hours of paralysis onset. All participants aged ≥ 16 years and no contraindications to corticosteroids or antiviral therapy Sex: male 253 (51%), female 243 (49%) Age: mean (± SD) 44 (± 16.4) years | |
| Interventions | Participants allocated to 1 of 4 treatment groups to receive:
Participants received prednisolone 25 mg twice daily for 10 days or aciclovir 400 mg 5 times daily for 10 days, both treatments or neither treatment, depending on allocation | |
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up at 3 and 9 months Final outcomes reported at 9 months | |
| Funding | Supported by a grant (02/09/04) from the Health Technology Assessment Programme of the National Institute for Health Research (Department of Health, England). The Scottish Executive (Chief Scientist Office and National Health Service Education for Scotland) funded the Scottish School of Primary Care during the study. Practices were reimbursed for their contributions through national Support for Science mechanisms | |
| Conflicts of interest | Drs Sullivan and Donnan reported receiving grant support from GlaxoSmithKline for projects unrelated to the trial. No other potential conflict of interest relevant to this article reported | |
| Date conducted | June 2004 to June 2006 | |
| Notes | Multicentre: 17 hospitals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... patient was randomly assigned to a study group by an independent, secure, automated telephone randomisation service" |
| Allocation concealment (selection bias) | Low risk | All parties blinded to allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Participants not receiving active drug received placebo. All administered medication was identical and in identical containers |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded to treatment group |
| Incomplete outcome data (attrition bias) | Low risk | Frequency and reason for drop‐outs documented: 138 assigned to prednisolone, of whom 127 completed the trial: 3 received an incorrect drug and 11 were lost to follow‐up (4 withdrew consent, 2 sought active treatment, 1 did not provide primary outcome data, 4 could not be contacted after the 1st visit) 141 assigned to placebo of whom 122 completed the trial: 19 were lost to follow‐up (6 withdrew consent, 3 could not be contacted, 3 sought active treatment, 1 did not provide primary outcome data, 3 could not be contacted after the 1st visit, 2 died, 1 withdrawn by investigator) |
| Selective reporting (reporting bias) | Low risk | All planned outcome measures reported |
| Other bias | Low risk | No other potential sources of bias identified |
| Methods | Double‐blind, randomised controlled trial | |
| Participants | 26 participants within 10 days of onset of Bell's palsy Sex: male 13 and female 13 Age: range 12 to 76 years | |
| Interventions |
| |
| Outcomes | Primary outcomes:
Secondary outcome:
Final outcome reported at 157 days | |
| Funding | Medical Research Council supplied cortisone acetate | |
| Conflicts of interest | Not stated | |
| Date conducted | August 1953 to June 1954 | |
| Notes | Single‐centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation centralised in pharmacy, in accordance with a master sheet of random numbers |
| Allocation concealment (selection bias) | Low risk | Central allocation in pharmacy |
| Blinding of participants and personnel (performance bias) | Low risk | Assessors and participants both blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors and participants both blinded (the point to which assessors were blinded was not clear) |
| Incomplete outcome data (attrition bias) | Low risk | 2 people excluded from analysis because of herpes of the external meatus |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported |
| Other bias | Low risk | No other bias identified |
| Methods | Randomised controlled trial | |
| Participants | 42 children within 3 days of onset of Bell's palsy Sex: male 21, female 21 Age: mean (± SD) 56.9 (± 4.7) months, range 24 to 74 months Children with chronic neurological conditions, other reasons for facial palsy and acute otitis media excluded | |
| Interventions |
| |
| Outcomes | Primary outcome:
Secondary outcome:
Follow‐up at 4, 6 and 12 months | |
| Funding | Not stated | |
| Conflicts of interest | Not stated | |
| Date conducted | Not stated | |
| Notes | Single‐centre study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Participants allocated to groups by concealed computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) | High risk | Participants not blinded to the treatment that they were receiving |
| Blinding of outcome assessment (detection bias) | High risk | Assessors not blinded to the treatment that participants were receiving |
| Incomplete outcome data (attrition bias) | Unclear risk | Losses to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Outcomes given by authors |
| Other bias | Low risk | No other potential biases were identified |
n: number of participants; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Allocation to treatment group was according to the day of admission, which is a method that is highly susceptible to bias. It was not clear who diagnosed participants with Bell's palsy and they used varying dosage regimens of corticosteroids. Follow‐up 3 weeks | |
| Did not provide the number of participants and outcome events by treatment groups. 50% of participants lost to follow‐up | |
| Not randomised or quasi‐randomised. There was no placebo group or open control group | |
| Not randomised or quasi‐randomised. 27% of participants lost to follow‐up | |
| Unable to extract complete information on the specified outcomes |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Bell's palsy in children: a multi‐centre, double‐blind, randomised, placebo‐controlled trial to determine whether prednisolone improves recovery at 1 month |
| Methods | Randomised control trial (computer‐generated randomisation schedule), parallel assignment |
| Participants | Participants aged 6 months to 18 years, weight ≥ 5 kg, diagnosed with Bell's palsy by their treating doctor and have an acute onset of symptoms of Bell's palsy for < 72 hours prior to randomisation |
| Interventions |
|
| Outcomes | Primary outcome:
|
| Starting date | 1 July 2015 |
| Contact information | Prof Franz Babl Emergency Research Department, Murdoch Children's Research Institute, Royal Children's Hospital, VIC, Australia +61399366748 |
| Notes | www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12615000563561 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incomplete recovery ≥ 6 months after randomisation Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.50, 0.80] |
| Analysis 1.1  Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 1 Incomplete recovery ≥ 6 months after randomisation. | ||||
| 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation Show forest plot | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.29] |
| Analysis 1.2 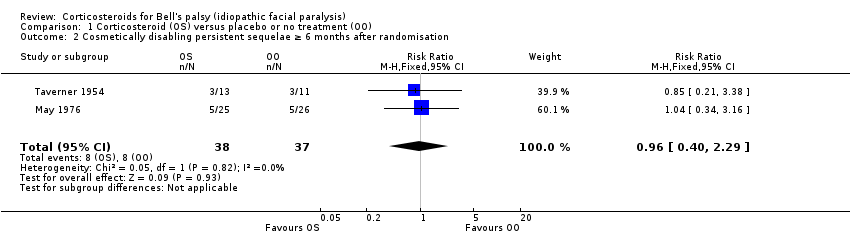 Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation. | ||||
| 3 Motor synkinesis and crocodile tears Show forest plot | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.91] |
| Analysis 1.3  Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 3 Motor synkinesis and crocodile tears. | ||||
| 4 Adverse effects Show forest plot | 3 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.51] |
| Analysis 1.4  Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 4 Adverse effects. | ||||

Study flow diagram illustrating the study selection process for this update.
1. The previous version of this review listed comparisons involving participants treated with antiviral therapy from Sullivan 2007 and Engström 2008 as two additional separate studies. These two comparisons are not included in this update.
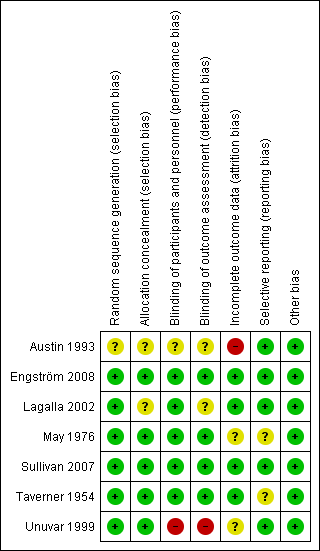
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study. Green (+) = low risk of bias; yellow (?) = unclear risk of bias; red (‐) = high risk of bias.

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.1 Incomplete recovery ≥ 6 months after randomisation.

1.2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.

Forest plot of comparison: 1 Corticosteroid (OS) versus placebo or no treatment (OO), outcome: 1.3 Motor synkinesis and crocodile tears.

Forest plot of comparison: 1 Incomplete recovery of facial motor function, outcome: 1.4 Adverse effects.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 1 Incomplete recovery ≥ 6 months after randomisation.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 3 Motor synkinesis and crocodile tears.

Comparison 1 Corticosteroid (OS) versus placebo or no treatment (OO), Outcome 4 Adverse effects.
| Corticosteroids compared to placebo or no treatment for Bell's palsy | ||||||
| Patient or population: people with Bell's palsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Corticosteroids | |||||
| Incomplete recovery ≥ 6 months after randomisation | 281 per 1000 | 177 per 1000 | RR 0.63 | 895 | ⊕⊕⊕⊕ | NNTB 10, 95% CI 6 to 20 |
| Cosmetically disabling persistent sequelae ≥ 6 months after randomisation | 216 per 1000 | 208 per 1000 | RR 0.96 | 75 | ⊕⊕⊝⊝ | ‐ |
| Motor synkinesis and crocodile tears | 260 per 1000 | 167 per 1000 | RR 0.64 | 485 | ⊕⊕⊕⊝ | ‐ |
| Adverse effects | 127 per 1000 | 133 per 1000 (91 to 192) | RR 1.04 | 715 | ⊕⊕⊕⊝ | 3 other studies recorded that no adverse effects occurred with corticosteroid treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two trials excluded participants who were found to have clinical evidence of herpes zoster infection (Lagalla 2002; Taverner 1954). In addition, two studies did not use a scoring system such as the House‐Brackmann or Sunnybrook scale to assess facial motor function, relying upon clinical examination, electromyographic tests or photographs (May 1976; Taverner 1954). Taverner 1954 reported outcomes at five months rather than at six months or more. However, we felt that these limitations did not compromise the generalisability of the findings. 2 We downgraded twice: first for imprecision ‐ there was a low number of events and pooled RR allowed the possibility of both no effect and the chance of harm; second for publication bias ‐ of the seven included studies, five did not provide data on the presence of cosmetically disabling sequelae six months or more after randomisation. 3 We downgraded once for imprecision. There was a low number of events. 4 We downgraded the quality of the evidence to moderate because of publication bias. 5 We downgraded for publication bias ‐ only three of seven studies provided data on adverse effects. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incomplete recovery ≥ 6 months after randomisation Show forest plot | 7 | 895 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.50, 0.80] |
| 2 Cosmetically disabling persistent sequelae ≥ 6 months after randomisation Show forest plot | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.29] |
| 3 Motor synkinesis and crocodile tears Show forest plot | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.91] |
| 4 Adverse effects Show forest plot | 3 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.51] |

