Long‐term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma
Information
- DOI:
- https://doi.org/10.1002/14651858.CD013047.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 05 August 2019see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Theresa Lawrie and Therese Dowswell wrote the first draft of the review. All authors contributed to study screening and data extraction. All authors advised on and approved the final version of the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
NIHR 16/144 Cochrane Programme Grant Scheme, UK.
Declarations of interest
Theresa Lawrie: none known
Therese Dowswell: none known
David Gillespie: none known
Jonathan Evans: none known
Sara Erridge: none known
Luke Vale: Member of NIHR Health Technology Assessment Clinical Evaluation and Trials Panel until March 2018
Ashleigh Kernohan: none known
Robin Grant: none known
Acknowledgements
We would like to thank the Information Specialist, Jo Platt, and the Gynaecological, Neuro‐oncology and Orphan Cancer Group (GNOC) Managing Editors, Gail Quinn and Clare Jess, for their advice and support in the preparation of this review. In addition, we would like to thank the library staff at the Royal United Hospital, Bath, UK for sourcing many of the articles cited.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Programme Grant funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers, are grateful to the following peer reviewers for their time and comments: Helen Bulbeck ‒ brainstrust, UK; Julia Day ‒ Edinburgh Centre for Neuro‐Oncology (ECNO), UK; Michael Hart ‒ Addenbrooke's Hospital, UK; Riccardo Soffietti ‒ Italy.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Aug 05 | Long‐term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma | Review | Theresa A Lawrie, David Gillespie, Therese Dowswell, Jonathan Evans, Sara Erridge, Luke Vale, Ashleigh Kernohan, Robin Grant | |
| 2018 Jun 12 | Long‐term side effects of radiotherapy, with or without chemotherapy, for glioma | Protocol | Theresa A Lawrie, Jonathan Evans, David Gillespie, Sara Erridge, Luke Vale, Ashleigh Kernohan, Robin Grant | |
Differences between protocol and review
We moved the Health‐Related Quality of Life (HRQoL) outcome from a secondary outcome in the protocol to a primary outcome in the review. This facilitated the inclusion of Taphoorn 2007, which reported HRQoL but not neurocognitive outcomes.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

Study flow diagram (date of search 16/02/18).
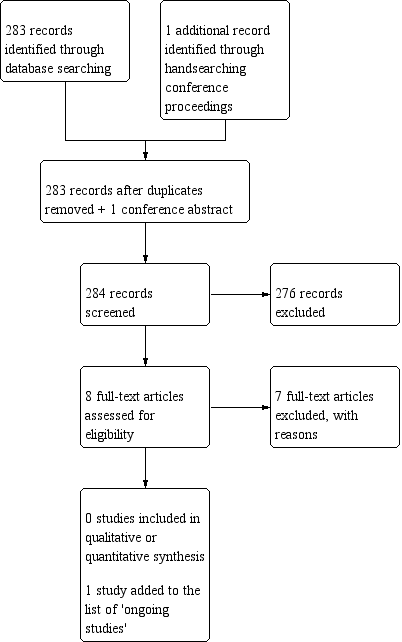
Study flow diagram (date of search 9/10/18).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
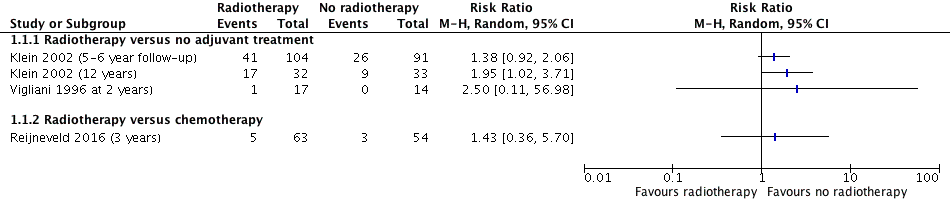
Forest plot of comparison A. Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment. (dichotomous data)
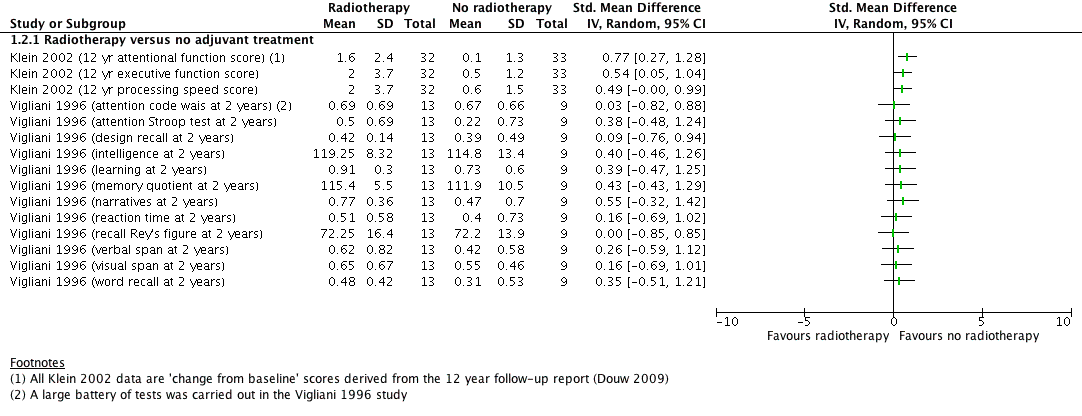
comparison A. Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment. (continuous data)

Forest plot of comparison B: High dose versus low dose radiotherapy, outcome: 2.1 Neurocognitive impairment at 2 years or more after treatment.

Forest plot of comparison C: Conventional versus stereotactic conformal radiotherapy, outcome: Neurocognitive impairment at 2 years or more after treatment.
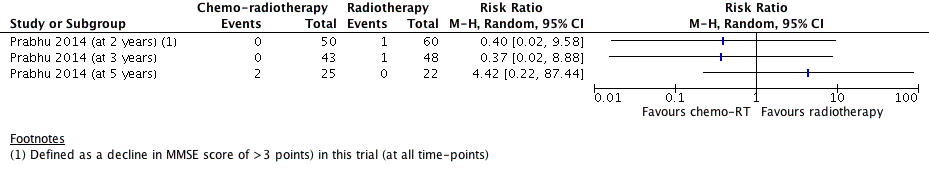
Forest plot of comparison D: Chemoradiotherapy versus radiotherapy, outcome: Neurocognitive impairment at 2 years or more after treatment.

Forest plot of comparison A (exploratory with totals): Radiotherapy versus no radiotherapy, outcome: Neurocognitive impairment at 2 or more years after treatment.
| Long‐term neurocognitive and other side effects of radiotherapy, with or without chemotherapy, for glioma | ||||
| Patient or population: people with glioma surviving at least two years Settings: tertiary care | ||||
| Comparison and Outcomes | Relative effect | No of participants andstudies | Quality of the evidence | Comments |
| Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 5‐ to 6‐year follow‐up | RR 1.38 (0.92 to 2.06) | 1 study with data for 195 participants | ⊕⊝⊝⊝ | Outcome defined as cognitive disability deficits in at least 5 of 18 neuropsychological tests |
| Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 12 year follow‐up | RR 1.95 (1.02 to 3.71) | 1 study with data for 65 participants | ⊕⊝⊝⊝ | Outcome defined as cognitive disability deficits in at least 5 of 18 neuropsychological tests |
| Intervention: radiotherapy Comparison: no adjuvant treatment Outcome: neurocognitive impairment at 2 year follow‐up | RR 2.50 (0.11 to 56.98) | 1 study with data for 31 participants | ⊕⊝⊝⊝ | There was a single event for this outcome in this observational study. The outcome was defined as a significant deterioration (≥ 1 SD) in 8 out of 12 neuropsychological tests |
| Intervention: radiotherapy Comparison: chemotherapy Outcome: neurocognitive impairment at 3 year follow‐up | RR 1.43 (0.36 to 5.70) | 1 study with data for 117 participants | ⊕⊕⊝⊝ | Outcome defined as a MMSE score of 26 or less |
| Intervention: high‐dose radiotherapy Comparison: low‐dose radiotherapy Outcome: neurocognitive impairment at 2 years after treatment | RR 0.53 (0.06, 4.85) | 1 study with data for 65 participants | ⊕⊝⊝⊝ | Outcome defined as decrease in MMSE score from baseline (more than 3 points).There was serious and uneven attrition between groups in this study. |
| Intervention: high‐dose radiotherapy Comparison: low‐dose radiotherapy Outcome: neurocognitive impairment at 5 years after treatment | RR 0.16 (0.01 to3.20) | 1 study with data for 38 participants | ⊕⊝⊝⊝ | Outcome defined as decrease in MMSE score from baseline (more than 3 points). There was serious and uneven attrition between groups in this study. |
| Intervention: chemoradiotherapy Comparison: radiotherapy Outcome: neurocognitive impairment at 3 years after treatment | RR 0.37 (0.02 to 8.88) | 1 study with data for 91 participants | ⊕⊕⊝⊝ | Outcome defined as a decline (of more than 3 points in MMSE score) in cognitive state compared with baseline |
| Intervention: stereotactic conformal radiotherapy Comparison: radiotherapy Outcome: neurocognitive impairment at 5 years after treatment | RR 4.62 (95% CI 0.25 to 86.72) | 1 study with data for 23 participants | ⊕⊕⊝⊝ | Outcome defined as a decline (of more than 3 points in MMSE score) in cognitive state compared with baseline. There was serious sample attrition at 5 years. |
| GRADE Working Group grades of evidence | ||||
| Abbreviations: SD = standard deviation; MMSE = Mini Mental State Exam 1. Single study contributing data had very serious study design limitations (−2) | ||||

