Advance care planning for adults with heart failure
Information
- DOI:
- https://doi.org/10.1002/14651858.CD013022.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 27 February 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Heart Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
YN: conceived the concept, developed, and finalised the protocol and the review.
NH: developed and finalised the protocol and the review.
HF: conceived the concept, developed, and finalised the protocol and the review.
EO: developed and finalised the protocol and the review.
AM: developed and finalised the protocol and the review.
MM: developed and finalised the protocol and the review.
DY: developed and finalised the protocol and the review.
JK: developed and finalised the protocol and the review.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health, UK.
Declarations of interest
YN: none.
NH: none.
HF: none.
EO: none.
AM: this research is supported by the 'Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus' from the Japan Agency for Medical Research and Development, AMED.
MM: none.
DY: none.
JK: none.
Acknowledgements
We are grateful to contact editor Mariann Gyongyosi; to peer reviewers Shyh Poh Teo, Maral A'arab‐Amini, Noemi Pavo, and Kit Byatt; and to consumer reviewer Richard Fitzgerald for providing helpful comments on earlier version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Feb 27 | Advance care planning for adults with heart failure | Review | Yuri Nishikawa, Natsuko Hiroyama, Hiroki Fukahori, Erika Ota, Atsushi Mizuno, Mitsunori Miyashita, Daisuke Yoneoka, Joey SW Kwong | |
| 2018 May 04 | Advance care planning for heart failure | Protocol | Yuri Nishikawa, Hiroki Fukahori, Erika Ota, Atsushi Mizuno, Natsuko Hiroyama, Mitsunori Miyashita, Daisuke Yoneoka, Joey SW Kwong | |
Differences between protocol and review
We changed the title of the review from 'Advance care planning for heart failure' to Advance care planning for adults with heart failure.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

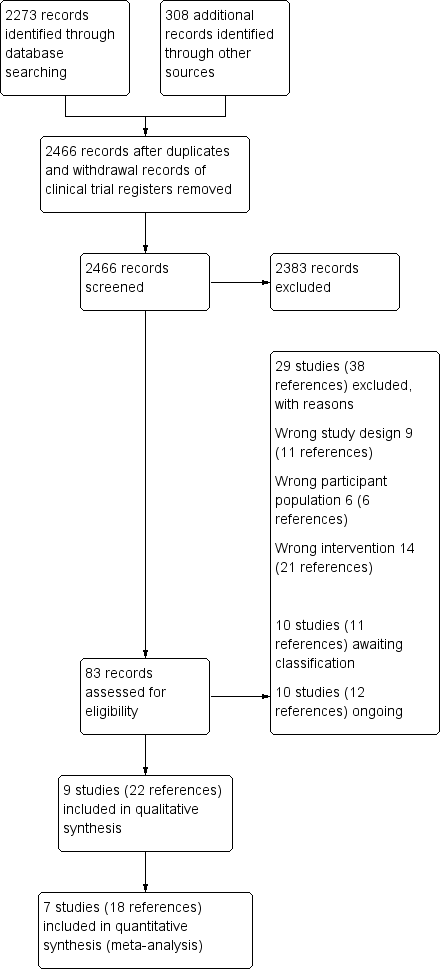
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
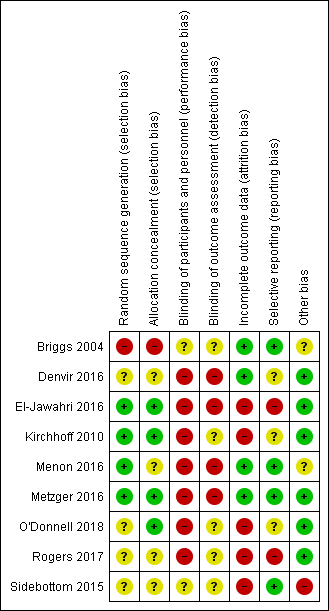
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 4 Participants' depression (overall analysis).

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 5 Participants' depression (subgroup analysis by follow‐up periods).

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 6 Participants' decisional conflict.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 7 All‐cause mortality.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010).
| Advance care planning compared with usual care for patients with heart failure | ||||||
| Patient or population: people with heart failure with or without their surrogate decision‐makers/carers Settings: inpatient and outpatient hospitals and clinics Intervention: ACP Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | ACP | |||||
| Concordance between participants' preferences and end‐of‐life care (yes/no) Post‐death data: mean days to death, ACP group (388.8 ± 255.7); | 625 per 1000 | 744 per 1000 | RR 1.19 (0.91 to 1.55) | 110 | ⊕⊝⊝⊝ | — |
| Participants' quality of life Measured by EQ‐5D and KCCQ. Higher scores indicate high‐quality of life. Follow‐up: 2 weeks to 6 months | The quality of life score in the ACP groups was on average 0.06 SDs higher (0.26 lower to 0.38 higher) than in the usual care groups. | — | 156 | ⊕⊕⊝⊝ | 1 additional study reported quality of life using the MLHF Questionnaire. The study showed that the quality of life score was improved by 14.86 points in the intervention group compared with 11.80 points in the usual care group at 3 months. Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. | |
| Patients' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
| Completion of documentation by medical staff regarding discussions with participants about ACP processes (yes/no) Follow‐up: 3–6 months | 489 per 1000 | 822 per 1000 | RR 1.68 (1.23 to 2.29) | 92 | ⊕⊕⊝⊝ | 1 additional study reported completion of documentation with HR (HR 2.87, 95% CI 1.09 to 7.59; P = 0.033). |
| Participants' depression Measured on PHQ‐8, PHQ‐9, and HADS. Higher scores indicate high depression Follow‐up: 2 weeks to 6 months | The depression score in the ACP groups was on average 0.58 SDs (0.82 to 0.34) lower than in the usual care groups. | — | 278 | ⊕⊕⊝⊝ Lowc,e | Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. | |
| Caregivers' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
| Quality of communication Measured on Quality of Patient‐Clinician Communication About End‐of‐Life Care. Higher score indicates high satisfaction with the quality of communication Assessed after intervention | 11.2 ± 0.8 (mean ± SD) | MD 0.4 lower (1.61 lower to 0.81 higher) | — | 9 | ⊕⊝⊝⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACP: advanced care planning; CI: confidence interval; EQ‐5D: EuroQol‐5D; HADS: Hospital Anxiety and Depression Survey; HR: hazard ratio; KCCQ: Kansas City Cardiomyopathy Questionnaire; MD: mean difference; MLHF: Minnesota Living with Heart Failure; PHQ: Patient Health Questionnaire; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for indirectness because the study included participants other than people with heart failure. bSince the outcome included only one study, the sample size was too small, and had wide confidence intervals. Therefore, we downgraded two levels for imprecision. cDowngraded one level for risk of bias because most included studies showed unclear selection bias and high attrition bias. dDowngraded one level for imprecision due to small sample size and wide confidence intervals. eDowngraded one level for imprecision due to small sample size. fDowngraded one level for risk of bias due to high risk of selection bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) Show forest plot | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) Show forest plot | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2.1 Follow‐up periods ≤ 3 months | 1 | 44 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.65, 0.54] |
| 2.2 Follow‐up periods > 3 months | 2 | 112 | Std. Mean Difference (Fixed, 95% CI) | 0.11 [‐0.27, 0.48] |
| 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes Show forest plot | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.23, 2.29] |
| 4 Participants' depression (overall analysis) Show forest plot | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5 Participants' depression (subgroup analysis by follow‐up periods) Show forest plot | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5.1 Follow‐up periods ≤ 3 months | 1 | 167 | Std. Mean Difference (Fixed, 95% CI) | ‐0.69 [‐1.01, ‐0.38] |
| 5.2 Follow‐up periods > 3 months | 2 | 111 | Std. Mean Difference (Fixed, 95% CI) | ‐0.41 [‐0.79, ‐0.03] |
| 6 Participants' decisional conflict Show forest plot | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.55, 0.02] |
| 7 All‐cause mortality Show forest plot | 5 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.04, 1.67] |
| 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010) Show forest plot | 4 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.09] |