Physical therapies for postural abnormalities in people with cystic fibrosis
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: RCT Total duration of trial: 20 days Country: UK Setting: inpatients Method of randomisation: computer‐generated stratified randomisation Method of allocation concealment: not described Outcome assessor blinding: measurements were taken by independent observer blinded to the group allocation | |
| Participants | Inclusion criteria: diagnosis of CF (genotype or sweat sodium > 70 mmol/L or sweat chloride of > 60 mmol/L); 16 years of age or over; inpatient admission for respiratory exacerbation as defined by the UK CF Trust (CF Trust Antibiotic Group 2002); inpatients able to stand for the measurement period without cardiovascular or respiratory compromise Exclusion criteria: current severe haemoptysis; low bone density (z score < ‐3); rib fractures; pregnancy; Inability to give consent for treatment/measurement; planned initiation or continuation of treatment in the home environment; current participation in another trial Total sample: 53 Total number of withdrawals/dropouts: 4 (2 in each group) Mean (SD) age, years: 29.4 (11.8) in intervention group; 25.8 (8.4) in control group Age range, years: 17 to 40 Gender: 14 male and 12 female in intervention group; 16 male and 11 female in control group Diagnosis criteria: genotype or sweat sodium > 70 mmol/L or sweat chloride of > 60 mmol/L After identifying the participants who had postural abnormality we only included these participants in our analysis: 35 participants (20 males and 15 females). | |
| Interventions | Intervention group: physiotherapy (musculoskeletal treatment‐specific, gentle oscillatory mobilisations to the rib cage and thoracic spine of the participants) to improve joint alignment and mobility, and to reduce pain; treatment of specific muscle dysfunction or tight muscle groups to further optimise muscle length and biomechanical relationships in the area, leading to improved efficiency of recruitment and improved power output; postural education and awareness discussions to improve the participant's own joint alignment and ability in a functional manner.; a short programme to reinforce the progress during the treatment sessions may be given Frequency: alternate days Time: 45 minutes Control group: usual care including intravenous antibiotics and airway clearance techniques but no placebo intervention | |
| Outcomes | Thoracic index measured by the Flexicurve® Pulmonary function (FEV1 % predicted; and FVC % predicted) Pain measured by using a 10‐cm VAS Quality of life measured by the Brompton Cystic Fibrosis Questionnaire Hospital Anxiety and Depression Scale Sputum weight Ease of sputum clearance The measures were reported at baseline, and days 5, 10, and pre‐discharge; Hospital Anxiety and Depression Scale was reported at baseline and pre‐discharge; sputum weight was reported at baseline, 5 and 10 days | |
| Notes | Funding: this work was supported by the Trevor Clay Grant from the British Lung Foundation, grant ref: TC08‐7 and sponsorship from the Royal Brompton Hospital Department of Cystic Fibrosis Charitable Fund; the British Lung Foundation had no involvement in the trial design, the data collection, the analysis or interpretation of the data; neither did they have involvement in the writing of the manuscript or the decision to submit for publication The results of this trial were not published, but the author sent all the information and data to the review authors on request Trials Register number: NCT00806884 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation undertaken using a computer‐generated stratified randomisation programme |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "low risk" or "high risk" |
| Blinding of participants and personnel (performance bias) | High risk | Participants knew the group to which they were allocated |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement of "low risk" or "high risk" |
| Selective reporting (reporting bias) | High risk | The trial protocol is available, but not all of the outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
| Methods | Design: RCT Total duration of trial: 12 weeks Country: UK Setting: outpatients Method of randomisation: computer‐generated stratified randomisation schedule Method of allocation concealment: not described Outcome assessor blinding: the independent observers performing the data collection were blinded to the group allocation and participants were requested not to disclose the group they were in to the observers | |
| Participants | Inclusion criteria: a diagnosis of CF (confirmed by genotype or a sweat sodium concentration of >70 mmol/L or sweat chloride of > 60 mmol/L); reported awareness of postural changes including stiffness, discomfort and/or pain of musculoskeletal origin in the thoracic spine or chest wall; a stable clinical state with lung function at the time of entry within 10% of the mean of the last 2 recordings (separated by at least 1 month); and a FEV1 of ≥ 30% predicted at time of entry Exclusion criteria: medical diagnosis of cor pulmonale, bone density z score < ‐3, history of spontaneous or spinal fractures, active arthropathy or spinal disease process such as hypertrophic pulmonary osteoarthropathy, currently receiving musculoskeletal treatment (e.g. physiotherapy, osteopathic or chiropractic), pregnant, unable to give consent or currently enrolled in another research trial Total sample: 20 participants Total number of withdrawals/drop‐outs: 7 (4 in intervention group and 3 in control group) Mean age, years: 27 in both intervention and control group Age range, years: 25 to 34 Gender: 5 male and 5 female (both groups) Diagnosis criteria: a diagnosis of CF (confirmed by genotype or a sweat sodium concentration of > 70 mmol/L or sweat chloride of > 60 mmol/L) After identifying the participants who had postural abnormality and we only included these participants in our analysis: 15 participants (8 males and 7 females). | |
| Interventions | Intervention group: musculoskeletal assessment at the first of 6 weekly visits, during which musculoskeletal treatments were given; specific mobilisations to the rib cage and thoracic spine; treatment of specific muscle dysfunction or tight muscle groups; and postural awareness, education and advice based on the principles of the Alexander technique Frequency of intervention: once a week for 6 weeks Time of intervention: up to 45 minutes Control group: received no further intervention | |
| Outcomes | Primary outcome measure: FEV1 The measures were taken at week 0, 3, 6, and 12 | |
| Notes | Funding: Royal Brompton Hospital (CF) Charitable Trust Fund provided monies for participants' travel reimbursement Trial Register number: NCT00716664 The author has responded to our enquiries with further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation undertaken using a computer‐generated stratified randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of "low risk" or "high risk" |
| Blinding of participants and personnel (performance bias) | High risk | Participants knew the group in which they were allocated |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | The trial reported dropouts along with reasons, and further claimed to have done intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | The trial protocol is available, but not all of the outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | High risk | There was a large baseline imbalance between groups in MST and thoracic index |
CF: cystic fibrosis
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity
PEFR: peak expiratory flow rate
RCT: randomised controlled trial
SD: standard deviation
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A study of Tai Chi ‐ not relevant for treating postural disorders. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: RCT Total duration of trial: 3 months Country: Brazil Setting: outpatients Method of randomisation: random allocation program Method of allocation concealment: not described Outcome assessor blinding: the researcher who performed all evaluations was blinded to the group allocation |
| Participants | Inclusion criteria: individuals with a diagnosis of CF, aged 7 ‐ 20 years, clinically stable disease, and regular follow‐up at the outpatient clinic Exclusion criteria: children and adolescents with cognitive alterations or osteomuscular changes that would make it impossible to perform the tests Total sample: n = 34 Total number of withdrawals/dropouts: 0 Mean (SD) age, years: 13.2 (3.3) years Age range, years: 7 to 20 Gender: 20 male and 14 female Diagnosis criteria: consensus statement |
| Interventions | Intervention group: given an illustrated handbook of guidelines for practicing aerobic physical exercises (e.g. running, swimming, walking, dancing, playing games, cycling, skipping rope, or other activities of interest to them); the handbook also contained 12 illustrated stretching figures, including stretches for the shoulder girdle, upper limbs, trunk, and lower limbs Frequency: they were instructed to exercise at least 3 times per week Duration of intervention: at least 20 minutes Control group: received the usual recommendation that is part of the routine of the care team, including verbal orientations to perform exercise and stretching, without the delivery of specific written information for the practice of physical exercise and stretching |
| Outcomes | Anthropometrics Lung function (FEV1, FVC, FEF25%‐75%) Postural evaluation: head tilt, scapular girdle tilt, pelvic tilt, A‐P trunk tilt, cervical lordosis, thoracic kyphosis, lumbar lordosis, lateral chest distance, anteroposterior chest distance, abdominal protusion Static baropodometry Dynamic baropodometry |
| Notes | Funding: not described Register number: RBR‐3r4h5s We have contacted the trial author to seek further information |
FEF25%‐75%: mid‐expiratory flow
FEV1: forced expiratory volume in 1 second
FVC: forced vital capacity
RCT: randomised controlled trial
SD: standard deviation
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Clinical effects of exercise program added to pulmonary rehabilitation in patients with cystic fibrosis |
| Methods | Design: triple blinded (participant, care provider, outcomes assessor) RCT Parallel assignment Location: Turkey |
| Participants | Inclusion criteria: diagnosed with CF; aged 6 ‐ 14 years; able to understand commands Exclusion criteria: FEV1 < 30%; presence of cor pulmonale; advanced gastroesophageal reflux; current hospital admission due to lung infection; diagnosed with neuromuscular disease |
| Interventions | Intervention group: ACBT and postural exercise program, once per week for 6 weeks Control group: ACBT alone once per week for 6 weeks ACBT involves 3 phases (breathing control, chest expansion exercise, and huff coughing) which are applied in a sequence to remove secretions The postural exercise program includes thoracic vertebra mobilization, pectoral stretching, scapula and thoracic extensors strengthening and core stability exercises |
| Outcomes | Primary outcome: change in exercise tolerance by the modified shuttle test Secondary outcomes: change in quality of life by the CFQR, postural stability by the balance master device ‐ Limits of Stability Test, spinal deformity by the Cobb Angle and the modified Cobb Angle, pulmonary function. |
| Starting date | 05 March 2017 |
| Contact information | Prof Evrim Karadag Saygi, MD ([email protected]) Marmara University School of Medicine, Department of Physical Medicine and Rehabilitation Turkey |
| Notes | NCT03295201 |
ACBT: active cycle of breathing techniques
CF: cystic fibrosis
CFQR: Cystic Fibrosis Questionnaire‐Revised
FEV1: forced expiratory volume in 1 second
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in quality of life Show forest plot | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| Analysis 1.1  Comparison 1 Usual care versus Intervention, Outcome 1 Change in quality of life. | ||||
| 1.1 Short term | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| 2 Change in pain (mm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| Analysis 1.2  Comparison 1 Usual care versus Intervention, Outcome 2 Change in pain (mm). | ||||
| 2.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| 3 Change in trunk deformity (cm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| Analysis 1.3  Comparison 1 Usual care versus Intervention, Outcome 3 Change in trunk deformity (cm). | ||||
| 3.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| 4 Change in FVC (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| Analysis 1.4  Comparison 1 Usual care versus Intervention, Outcome 4 Change in FVC (L). | ||||
| 4.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| 5 Change in FVC (% predicted) Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| Analysis 1.5  Comparison 1 Usual care versus Intervention, Outcome 5 Change in FVC (% predicted). | ||||
| 5.1 Short term | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| 6 Change in FEV1 (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| Analysis 1.6 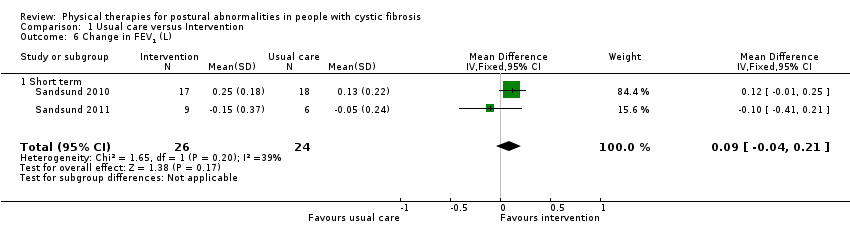 Comparison 1 Usual care versus Intervention, Outcome 6 Change in FEV1 (L). | ||||
| 6.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| 7 Change in FEV1 (% predicted) Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐8.52, 4.88] |
| Analysis 1.7  Comparison 1 Usual care versus Intervention, Outcome 7 Change in FEV1 (% predicted). | ||||
| 7.1 Short term | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐9.50, 10.36] |
| 7.2 Cutt off | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐12.77, 5.37] |
| 8 Change in Tiffenau’s index (FEV1/FVC) Show forest plot | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.79, 2.98] |
| Analysis 1.8  Comparison 1 Usual care versus Intervention, Outcome 8 Change in Tiffenau’s index (FEV1/FVC). | ||||
| 8.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐4.49, 4.24] |
| 8.2 Cutt off | 2 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.58, 4.11] |
| 9 Change in functional capacity Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |
| Analysis 1.9  Comparison 1 Usual care versus Intervention, Outcome 9 Change in functional capacity. | ||||
| 9.1 Short term | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
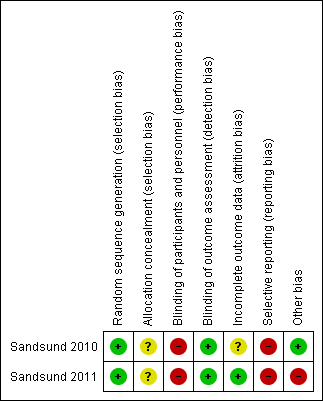
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Usual care versus Intervention, Outcome 1 Change in quality of life.

Comparison 1 Usual care versus Intervention, Outcome 2 Change in pain (mm).

Comparison 1 Usual care versus Intervention, Outcome 3 Change in trunk deformity (cm).

Comparison 1 Usual care versus Intervention, Outcome 4 Change in FVC (L).

Comparison 1 Usual care versus Intervention, Outcome 5 Change in FVC (% predicted).

Comparison 1 Usual care versus Intervention, Outcome 6 Change in FEV1 (L).

Comparison 1 Usual care versus Intervention, Outcome 7 Change in FEV1 (% predicted).

Comparison 1 Usual care versus Intervention, Outcome 8 Change in Tiffenau’s index (FEV1/FVC).

Comparison 1 Usual care versus Intervention, Outcome 9 Change in functional capacity.
| Physical therapies compared to usual care for postural abnormalities in people with cystic fibrosis | ||||||
| Patient or population: postural abnormalities in people with cystic fibrosis Settings: outpatients and inpatients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with physical therapies | |||||
| Change in quality of life Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 86%) results were not pooled. Both included trials showed different effects, i.e. one favoured intervention (Sandsund 2010) and the other showed no difference (Sandsund 2011). | |
| Change in pain (mm) Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 83%) results were not pooled. Both included trials showed different effects, i.e. showing no difference in one trial (Sandsund 2010) and favouring usual care in the other (Sandsund 2011). | |
| Change in trunk deformity (cm) Follow‐up: short term | The mean change in trunk deformity in the control group was ‐2.33 cm. | The mean change in trunk deformity in the intervention group was1.01 cm lower (3.11 lower to 1.08 higher). | NA | 50 | ⊕⊝⊝⊝ | |
| Change in FEV1 (L) Follow‐up: short term | The mean change in FEV1 in the control group was 0.04 L. | The mean change in FEV1 in the intervention group was 0.09 L higher (0.04 lower to 0.21 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FEV1 (% predicted) based on the 2010 trial also suggest no statistically significant difference between the treatment groups (Sandsund 2010). |
| Change in FVC (L) Follow‐up: short term | The mean change in FVC in the control group was 0.03 L. | The mean change in FVC in the intervention group was 0.17 L higher (0.02 lower to 0.37 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FVC (% predicted) based on the 2010 trial also suggest a statistically significant difference in favour of the physical therapies group (Sandsund 2010). |
| Change in ERV (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| Change in TLC (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded once due to imprecision; small numbers of participants included in the comparison (small sample size which did not achieve the targeted sample size generated by the power calculation) and the CI overlap showed no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in quality of life Show forest plot | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| 1.1 Short term | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| 2 Change in pain (mm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| 2.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| 3 Change in trunk deformity (cm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| 3.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| 4 Change in FVC (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| 4.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| 5 Change in FVC (% predicted) Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| 5.1 Short term | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| 6 Change in FEV1 (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| 6.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| 7 Change in FEV1 (% predicted) Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐8.52, 4.88] |
| 7.1 Short term | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐9.50, 10.36] |
| 7.2 Cutt off | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐12.77, 5.37] |
| 8 Change in Tiffenau’s index (FEV1/FVC) Show forest plot | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.79, 2.98] |
| 8.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐4.49, 4.24] |
| 8.2 Cutt off | 2 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.58, 4.11] |
| 9 Change in functional capacity Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |
| 9.1 Short term | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |


