Physical therapies for postural abnormalities in people with cystic fibrosis
Abstract
Background
Cystic fibrosis (CF) is the most common life‐threatening, inherited disease in white populations which causes several dysfunctions, including postural abnormalities. Physical therapy may help in some consequences of these postural abnormalities, such as pain, trunk deformity and quality of life.
Objectives
To determine the effects of a range of physical therapies for managing postural abnormalities in people with cystic fibrosis, specifically on quality of life, pain and trunk deformity.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches, hand‐searched journals and conference abstract books. We also searched the reference lists of relevant articles and reviews. Additional searches were conducted on ClinicalTrials.gov and on the WHO International Clinical Trials Registry Platform for any planned, ongoing and unpublished studies.
Date of the last search: 19 March 2020.
Selection criteria
Randomised controlled trials examining any modality of physical therapy considered relevant for treating postural disorders compared with each other, no physical therapy, sham treatment or usual care in people with CF (of any age or disease severity).
Data collection and analysis
Two review authors independently selected eligible trials, assessed the risk of bias in each trial and extracted the data. We contacted trial authors to obtain missing or additional information. We assessed the quality of the evidence using the GRADE criteria.
Main results
Two trials, involving a total of 50 participants with CF and postural abnormalities, were included in this review. One was in people with stable disease (lasting three months) and one in hospital inpatients experiencing an exacerbation (20 days). Both trials compared manual therapy comprising mobilizations to the rib cage and thoracic spine, treatment of specific muscle dysfunction or tight muscle groups; and postural awareness and education versus medical usual care. The age of participants ranged from 17 years to 58 years. Both trials were conducted in the UK.
The following outcomes were measured: change in quality of life, change in pain, change in trunk deformity and change in pulmonary function. Manual therapy may make little or no difference to the change in trunk deformity compared to usual care (low‐quality evidence). No results could be analysed for quality of life (very low‐quality evidence) and pain outcomes (very low‐quality evidence) because of the high heterogeneity between trials. It is uncertain whether the intervention improves lung function: forced vital capacity (very low‐quality evidence); forced expiratory volume in one second (very low‐quality evidence); or Tiffeneau’s index (ratio of forced expiratory volume at one second (FEV1) and forced vital capacity (FVC)). Only one trial (15 participants) measured functional capacity, and the change in walked distance seemed to favour intervention over usual care, but with the possibility of no effect due to wide confidence intervals. The same trial also reported that six participants in the intervention group had positive comments about the intervention and no adverse events were mentioned.
Authors' conclusions
Due to methodological limitations in the included trials, and in addition to the very low to low quality of the current evidence, there is limited evidence about the benefits of physical therapies on postural abnormalities in people with CF. Therefore, further well‐conducted trials with robust methodologies are required considering a prior inclusion criterion to identify the participants who have postural abnormalities.
PICOs
Plain language summary
Physical therapies for postural abnormalities in people with cystic fibrosis
Review question
We reviewed the evidence about the effects of physical therapies that can help the postural abnormalities in people with cystic fibrosis.
Background
Cystic fibrosis is a genetic disease that causes problems in some organs of the body, especially in the lungs. Recently, it has been noticed that postural abnormalities may happen because of the disease progression. Some physical therapies that promote the stretching and strengthening of the muscles connected to the spine may help improving the pain and quality of life in people with cystic fibrosis.
Search date
The evidence is current to: 19 March 2020.
Study characteristics
We included two trials in this review with 50 participants aged between 17 and 58 years and diagnosed with cystic fibrosis and postural abnormalities. The trials compared manual therapy (all physical therapies that promote relaxation of the muscles, as well as mobilization of the spine, such as massage, spinal mobilization) with usual care (the normal treatment they used to receive). Participants were placed in each treatment group at random. One trial was in hospital inpatients and lasted 20 days and the second was in outpatients and lasted three months. Most of the outcomes were reported in both trials.
Key results
The trials did not find any difference between manual therapy and usual care for changes in trunk deformity. We could not combine results for quality of life or pain scores because they were too different. We were not able to find a difference between manual therapy and usual care for lung function. Only one trial (15 participants) measured the change in walked distance which seemed to favour manual therapy over usual care, but this was not clear because there were a large range of results. One trial reported that participants from the manual therapy group enjoyed the type of the treatment they received and that adherence was also high. None of the trials reported any side effects.
Quality of the evidence
Overall, there was only low‐ to very low‐quality of evidence in all outcomes considered. Low‐quality evidence means that our confidence in the effect of manual therapy is limited and the true effect may be very different. We think the fact that people knew which treatment they were receiving can affect the results for the change in quality of life, change in pain and change in lung function outcomes, but this may not affect the other outcomes. Therefore, we are still not confident that manual therapies improve outcomes that we consider important for the treatment of postural abnormalities.
Authors' conclusions
Summary of findings
| Physical therapies compared to usual care for postural abnormalities in people with cystic fibrosis | ||||||
| Patient or population: postural abnormalities in people with cystic fibrosis Settings: outpatients and inpatients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with physical therapies | |||||
| Change in quality of life Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 86%) results were not pooled. Both included trials showed different effects, i.e. one favoured intervention (Sandsund 2010) and the other showed no difference (Sandsund 2011). | |
| Change in pain (mm) Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 83%) results were not pooled. Both included trials showed different effects, i.e. showing no difference in one trial (Sandsund 2010) and favouring usual care in the other (Sandsund 2011). | |
| Change in trunk deformity (cm) Follow‐up: short term | The mean change in trunk deformity in the control group was ‐2.33 cm. | The mean change in trunk deformity in the intervention group was1.01 cm lower (3.11 lower to 1.08 higher). | NA | 50 | ⊕⊝⊝⊝ | |
| Change in FEV1 (L) Follow‐up: short term | The mean change in FEV1 in the control group was 0.04 L. | The mean change in FEV1 in the intervention group was 0.09 L higher (0.04 lower to 0.21 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FEV1 (% predicted) based on the 2010 trial also suggest no statistically significant difference between the treatment groups (Sandsund 2010). |
| Change in FVC (L) Follow‐up: short term | The mean change in FVC in the control group was 0.03 L. | The mean change in FVC in the intervention group was 0.17 L higher (0.02 lower to 0.37 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FVC (% predicted) based on the 2010 trial also suggest a statistically significant difference in favour of the physical therapies group (Sandsund 2010). |
| Change in ERV (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| Change in TLC (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded once due to imprecision; small numbers of participants included in the comparison (small sample size which did not achieve the targeted sample size generated by the power calculation) and the CI overlap showed no effect. | ||||||
Background
Description of the condition
Cystic fibrosis (CF) is an autosomal‐recessive genetic disease which affects the function of various organs in a person. It is the most common life‐threatening, inherited disease in white populations and is caused by mutations in the gene encoding for the CF transmembrane conductance regulator (CFTR) (Lima 2013). Ongoing advances in treatment are leading to an ever‐increasing life expectancy. The current average survival age is approximately 40 years, and it has been predicted that the mean survival age of those born in 2000 may be over 50 years (Dodge 2007). This is possibly due to early diagnosis, multi‐professional management in specialized centers and access to adequate therapy (Yankaskas 2004).
Posture refers to the position in which a person holds their body when standing or sitting. Individuals with CF may present with postural disorders; the most common of these are thoracic hyperkyphosis and scoliosis.
Thoracic hyperkyphosis is characterized by an exaggerated curvature of the spine, with the Cobb angle measuring more than 40° (Fon 1980; Kado 2007). This condition is sometimes known as 'round back' or 'hunchback' and its prevalence in people with CF is higher than in the non‐CF population (Garcia 2011; McIlwaine 2014; Okuro 2012). It has been reported in a systematic review that 62% of people with CF have thoracic hyperkyphosis (Parasa 1999), and the altered posture has an association with significant morbidity, back pain, poor body image, and low mood; all of which impact on quality of life (QoL) (Tattersall 2003). However, it has been observed that this condition has become less common in children and adolescents with CF due to frequent and supervised CF care (Barker 2014).
Scoliosis is an appreciable lateral deviation in the normally straight vertical line of the spine. It is defined as a Cobb angle of more than 10° (Fainardi 2013). It has been shown in a previous study run in the UK that, among 316 children with CF (aged four to 16 years) the prevalence of scoliosis was 15.6%, which is 20 times higher than the prevalence in healthy children of a similar age in the same geographical area (Kumar 2004).
One major cause of altered posture is lung obstruction leading to air trapping and hyperinflation. When ventilation is compromised, changes in posture may occur to allow the chest and trunk muscles to assist with breathing and causing changes in respiratory biomechanics (Kraemer 2006). These changes within the thoracic region of the body lead to decreased trunk mobility which can continue to affect respiratory function as well as gross motor movement and general activity (Botton 2003).
Description of the intervention
As the incidence of musculoskeletal problems in people with CF has increased, physical therapy techniques have been used preventatively to minimize problems resulting from reduced chest wall flexibility, poor posture and musculoskeletal pain (Sandsund 2011). These interventions especially focus on manual therapy (massage, spinal manipulation or mobilisation); educational programs; physical exercises (individualised or group‐based; home‐based; or land‐ or water‐based); Pilates; inspiratory muscle training (IMT); global postural re‐education (GPR); yoga and stretching.
Manual, manipulative or manipulation therapy is an intervention directed towards the spine to restore joint motion. It can use mobilisation (techniques made by the physical therapist using low‐velocity, small or large amplitude within the individual's range of motion) or manipulation (high‐velocity impulses applied to a synovial joint over a short amplitude at or near the end of the passive or physiologic range of motion), or both (Rubinstein 2011; Rubinstein 2012). Massage therapy is the manual manipulation of soft tissue to promote health and well‐being. There are many types of this technique, such classical massage, myofascial release, muscular energy, positional release technique and trigger points therapy.
Educational programs are interventions involving self‐management with minimal contact with healthcare professionals (European Commission 2004).
According to the WHO, physical exercise is "a sub‐category of physical activity that is planned, structured, repetitive, and aims to improve or maintain one or more components of physical fitness" (WHO 2015). There are two types of physical exercise training: aerobic training, involving periods of continuous training with a target intensity below the anaerobic threshold; and anaerobic training, at a high intensity (above the anaerobic threshold) for a short duration (Radkte 2015).
Pilates was developed by Joseph Pilates for the purpose of encouraging better body awareness and improved posture through the optimization of core muscles with stretching and strengthening exercises. These exercises are based on six principles: centering; concentration; control; precision; flow; and co‐ordinated breathing with the exercises (Geneen 2017; Queiroz 2010; Yamato 2015).
IMT involves the training of muscles responsible for inspiration and expiration. The loads applied to the muscles during training are flow, threshold or resistive in nature (Houston 2013).
GPR is a method based on the idea that the muscular system is integrated into muscle chains, which are functional muscle groups responsible for posture and its alterations. In this way, a shortened muscle creates compensations in nearby or distant muscles. To correct these alterations, GPR uses the simultaneous stretching of several muscles belonging to the same muscular chain. This technique provides the correct positioning of the joints and strengthening of the muscles, which corrects dysfunctions of the spine and other joints (Castagnoli 2015; Rossi 2011).
Yoga is based on five principles that include 'proper relaxation', 'proper exercise', 'proper breathing', 'proper diet', as well as positive thinking and meditation (Chanavirut 2006). The yoga exercises systematically train the muscles of the whole body, alternating stretches with holding postures, relaxation and movements (Purenovic‐Ivanovic 2017).
There are two types of stretching: static stretching, which usually involves moving a limb to the end of its range of motion and holding the stretched position between 15 and 60 seconds (Young 2002); and dynamic stretching, which involves controlled movement through the active range of motion for each joint (Behm 2011; Fletcher 2010).
How the intervention might work
There are many hypotheses about the mechanism of action for manual therapy, two of which are mechanical and neurophysiologic. The mechanical approach suggests that manual therapy acts on a manipulable lesion (such as subluxation) that forces a reduction in internal mechanical stresses, lessening symptoms and allowing a return to normal activity (Triano 2001). The neurophysiologic approach suggests that manual therapy impacts the primary afferent neurons from paraspinal tissues, the motor control system and pain processing (Pickar 2002). The biologic and psychological effects of massage and their relationship with the relief of symptoms are unclear and mechanistic studies are needed (Furlan 2015).
Some risk factors for back pain and postural abnormalities can be modified with early education, which is the most inexpensive, realistic and feasible approach (Cardon 2004). In addition, educational programs increase the level of an individual's involvement in their own treatment, resulting in a self‐reliance in physical well‐being, the understanding and management of pain, and the trust in and adherence to treatment recommendations. These aspects improve postural characteristics related to spinal loading (Geldhof 2006; Staal 2002).
Physical exercise has multiple beneficial effects. Benefits include decreased muscle fatigue and tension as well as improvements in activity tolerance levels, muscle strength, the range of joint motion, cartilage nutrition, cardiovascular fitness, co‐ordination and the increased muscular stabilization of the lumbar region (Staal 2002). Similarly, Pilates exercises increase the stability and control of spinal deep muscles and reduce the activity of superficial muscles. These factors improve the QoL, posture and body awareness of an individual (Yamato 2015).
IMT can also reduce postural abnormalities. Respirations involve the back and abdominal muscles; furthermore, the diaphragm muscle is connected to the lumbar vertebrae. These muscles contribute to postural control and are strengthened during a program of IMT (Obayashi 2012).
Stretching increases the range of motion of the joint, probably due to changes in the length and stiffness of the affected musculotendinous unit (Behm 2011). Sustained use of the type of static stretching in GPR can modify body alignment and reduce stress on joints and tense muscles (Rossi 2011), improve thoraco‐abdominal mobility (Moreno 2007) and decrease lower‐back pain (Castagnoli 2015). Yoga provides the necessary range of movement to maintain the proper health of muscles, ligaments, cartilage and joint capsules, which is essential for their function (Purenovic‐Ivanovic 2017).
Why it is important to do this review
When CF was first described, airway clearance was the mainstay of respiratory management. However, the role of physiotherapy in this condition has significantly changed due to alterations in the disease patterns from infancy to adulthood. Physiotherapy is, therefore, no longer limited to airway clearance; it also emphasizes the importance of physical exercise and postural care, as well as addressing the unique complications which occur as people with CF live longer. Therefore, health professionals are facing secondary complications that need to be investigated and treated with the intention of maintaining the QoL of people with CF. Postural abnormalities promote changes in ventilatory mechanics, besides causing pain and discomfort. Interventions that cause a change in thoracic range of motion or prevent immobility of the thoracic spine, or both, may help breathing mechanics and reduce pain. To date, there is no systematic review of the currently available evidence describing the possibilities of physical therapies for postural changes observed in people with CF.
Objectives
To determine the effects of a range of physical therapies for managing postural abnormalities in people with CF, specifically on:
-
QoL;
-
pain;
-
trunk deformity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (including cross‐over trials).
Types of participants
People with CF with a diagnosis based on clinical criteria and sweat testing or genotype analysis and who had thoracic hyperkyphosis or scoliosis. We considered participants regardless of age and degree of disease severity. Trials including participants with more than one type of postural disorder were included; however, we evaluated the effects of interventions for each disorder separately.
Types of interventions
We included trials examining any modality of physical therapy considered relevant for treating postural disorders such as manual therapy (e.g. massage, spinal manipulation, and mobilisation), educational programs, exercise training (individualised or group‐based or home‐based), Pilates, stretching, GPR, IMT, and yoga. Each intervention of physical therapy was compared with each other, to no physical therapy, sham treatment or usual care.
Types of outcome measures
The outcomes listed below are not selection criteria for this review, but they are outcomes of interest within the included trials.
Primary outcomes
-
Change in QoL in carrying out activities of daily living over the short, medium and long term*, measured by a validated instrument
-
overall (e.g. Medical Outcomes Study 36 – Item Short – Form Health Survey (SF‐36))
-
health‐related QoL (HRQoL) (e.g. Cystic Fibrosis Questionnaire Revised (CFQ‐R) (Quittner 2009))
-
-
Change in pain in carrying out activities of daily living over the short, medium and long term*, measured by a validated instrument (e.g. visual analogue scale (VAS)), or other available pain scales (Numerical Rating Scale for Pain, Chronic Pain Grade Scale)
-
Change in trunk deformity in carrying out activities of daily living over the short, medium and long term*, measured by a validated instrument (e.g. Cobb method, biophotogrammetry)
Secondary outcomes
-
Treatment success (measured by a participant‐reported global impression of clinical change (no improvement, much or very much improvement) or similar measures)
-
Change in pulmonary function over the short, medium and long term*, measured by a validated instrument (in L and % predicted)
-
forced vital capacity (FVC)
-
forced expiratory volume in one second (FEV1)
-
Tiffenau’s index (FEV1/FVC)
-
expiratory reserve volume (ERV)
-
total lung capacity (TLC)
-
-
Change in functional capacity over the short, medium and long term*, measured by a validated instrument (e.g. 3‐minute step test or 6‐minute walk test)
-
Adverse effects
-
Adherence to treatment
-
Ease of access to intervention
*We considered 'short term' to mean less than three months, 'medium term' to mean longer than three months but less than one year, and 'long term' to mean longer than one year.
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a search of the Group's Cystic Fibrosis Trials Register for relevant trials using the following terms: musculoskeletal and postural abnormalities.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group's website.
Date of the last search of the Group’s Cystic Fibrosis Trials Register: 19 March 2020.
We also searched the following trial registries:
-
the US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 22 May 2018);
-
the WHO International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; searched 22 May 2018).
For details of our search strategies, please see Appendix 2.
Searching other resources
We searched the reference lists of all included trials and any relevant systematic reviews identified for additional trials. We contacted experts and organisations in the field to obtain additional information on relevant trials.
Data collection and analysis
Selection of studies
Two review authors (TS and KM) independently screened the titles and abstracts for inclusion of all the potential trials identified from the search. Where possible, we retrieved the full‐text reports and two review authors (TS and KM) independently screened these and identified trials for inclusion. When trials were only in abstract form, we contacted the trial authors for additional information. We resolved any disagreements through discussion or, if required, we consulted a third review author (PN). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009) and also the tables of characteristics of included and excluded trials. We did not impose any language restrictions.
Data extraction and management
If the reports had required translation, the translator would have extracted the data directly using a data extraction form or extracted data from the translation provided. If this happens in the future, a review author will check numerical data extracted in the original trial report. For the current review version we used a data collection form for trial characteristics and outcome data which was piloted on one trial in the review. We extracted the following information.
-
Methods ‐ trial design duration of the trial, details of any 'run‐in' period, the number of centres and locations, trial settings, withdrawals, and date of trial.
-
Participants ‐ number (total and in each intervention group), mean age, age range, gender, the severity of condition, diagnostic criteria, baseline characteristics, inclusion criteria, and exclusion criteria.
-
Interventions ‐ intervention and comparison.
-
Outcomes ‐ primary and secondary outcomes specified and collected, and time points reported. When reports do not present the change from baseline, we extracted the final values.
-
Notes ‐ funding for the trial, and notable conflicts of interest of trial authors.
Two review authors (VO, KSM) extracted information on characteristics from the included trials. We resolved disagreements by consensus or by involving a third review author (IS). One review author (VO) transferred data into the Review Manager software (RevMan 2014). We double‐checked that data were entered correctly by comparing with the trial reports.
We present the included trials in a single comparison (intervention versus usual care); however, in future updates, if we include more than one intervention in the results, we will present comparisons of each different intervention separately. Similarly, since different postural disorders may not respond similarly to same intervention, if we include such results in the future, we plan to report the results for each disorder separately. For the trials that include a mixture of postural disorders, we will attempt to contact the investigators for separate data.
When extracting data from the included trials, it became clear that neither trial listed the presence of postural abnormality as an inclusion criterion and both trials included individuals with and without postural abnormality. On request, the lead investigator (same person for both trials) sent the raw data from the trials for analysis and we applied the 9.2 value Flexicurve® as a criterion to identify those participants who had postural abnormalities (Hinman 2004). After identifying the participants who had postural abnormality and only including these participants in our analysis, we calculated the means and standard deviations (SDs) for the assessed outcomes.
For those outcomes combining scales with different directions, we used the method recommended by Cochrane of multiplying by ‐1 to ensure results run in the same direction (Sambunjak 2017).
We evaluated the outcomes over the short term, medium term and long term (as defined above) and present the data in the analysis separately for each time point.
Assessment of risk of bias in included studies
The authors (VO, KSM) independently assessed the risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreements by discussion or by involving another review author (IS). We assessed the risk of bias according to the following domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other bias.
We judged each potential source of bias as high, low or unclear and provided quotes from the trial report with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different trials for each of the domains listed.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Oliveira 2018), and reported any deviations from it in the section 'Differences between protocol and review'.
Measures of treatment effect
We entered outcome data for each trial into the data tables in the Review Manager software to calculate treatment effects (RevMan 2014). For continuous outcomes, we reported the mean difference (MD) with 95% confidence intervals (CIs), or the standardised mean difference (SMD) with 95% CIs if the trials report different units of measurement. If the trials had not reported the mean and SD for each group, but had reported a difference between groups, we would have used the generic inverse variance (GIV) to enter data in the analyses. If we had been able to analyse data for any dichotomous outcomes, we would have presented results as a relative risk (RR) with corresponding 95% CIs. We planned to narratively describe any skewed data which the investigators may have reported as medians and interquartile ranges.
Unit of analysis issues
If we identify further studies in the future, we will include cross‐over trials and report data from the first treatment arm only.
If a single trial had reported multiple treatment groups, we would have included all the relevant arms, that is, those in which participants received our pre‐specified interventions and comparators. We planned to compare different physical therapy modalities with each other, so all the relevant arms are be compared to a control arm and to each other (e.g. therapy 1 versus therapy 2, therapy 1 versus usual therapy, therapy 2 versus usual therapy). For the analysis, we planned to divide the control group evenly between the relevant intervention arms, as it ensures that each person in the control group is counted only once.
Cluster RCTs are not eligible for inclusion because the focus of this review is related to individuals with CF.
Dealing with missing data
In cases of incomplete data or a lack of data from included trials (e.g. when a trial was identified as abstract only), we contacted the investigators or trial sponsors. As discussed above, we were able to obtain additional data from the two included trials (Sandsund 2010; Sandsund 2011). If they had not sent the requested information, we would still have included the trial and attempted to clarify the reason why the access to the missing data was not possible. If the trial authors contact us with data in the future we will add the information to the review at the following update. In future updates, we will explore the impact of including such trials in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We evaluated the heterogeneity of trial results by looking at the forest plots in order to detect non‐overlapping CIs, with application of the Chi² test (with a P value of 0.10 to indicate statistical significance) and by applying the I² statistic. According to the Cochrane Handbook for Systematic Reviews of Interventions, values up to 40% indicate that heterogeneity may not be important, while values between 30% and 60% indicate moderate heterogeneity, between 50% and 90% substantial heterogeneity and between 75% and 100% considerable heterogeneity (Higgins 2011b). We considered the I² statistic with a value of 50% as a moderate level of heterogeneity (Higgins 2011b).
Assessment of reporting biases
There were insufficient included trials to construct funnel plots to assess reporting bias among trials. We plan to re‐assess the possibility of constructing funnel plots, if we include sufficient trials in future review updates as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If we then note asymmetry, we will explore possible causes including publication bias, the risk of bias, selective outcome or analysis reporting and true heterogeneity (Sterne 2011).
Data synthesis
We used a fixed‐effect model to determine the actual effects of the intervention and we have planned a sensitivity analysis using a random‐effects model if we could not explain any heterogeneity identified.
We performed individual participant data (IPD) meta‐analyses by two‐stage approach to analysis. In the first stage, we analysed each individual trial in the same way and entered the data in the usual way, as set out in the analysis plan. In the second step, we combined the results of each of these individual trial analyses to provide a pooled estimate of effect in the same way as for a conventional systematic review (Stewart 2011).
Subgroup analysis and investigation of heterogeneity
If we had been able to include sufficient data and had identified substantial heterogeneity, we planned to analyse the possible causes of the heterogeneity using subgroup analysis in terms of the following parameters:
-
age of participants (up to 18 years of age versus 18 years and over);
-
gender.
We planned to use the following outcomes in subgroup analyses:
-
QoL;
-
pain;
-
trunk deformity
-
pulmonary function;
-
treatment success;
-
functional capacity;
-
adverse effects;
-
adherence to treatment;
-
ease of access to intervention;
We planned to use the formal test for subgroup interactions in Review Manager (RevMan 2014).
Sensitivity analysis
If we had been able to include sufficient data, we planned to carry out the following sensitivity analyses.
-
Repeat the analysis excluding trials at a high or unclear risk of bias for allocation concealment.
-
Repeat the analysis excluding quasi‐RCTs.
-
Repeat the analysis excluding trials with missing data.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table for each comparison using the following outcomes:
-
QoL;
-
pain;
-
trunk deformity;
-
FEV1;
-
FVC;
-
ERV;
-
TLC.
In future updates, if insufficient data are available to present the change from baseline, we will report the final values. Since the mean values and SDs for the two types of outcome (change and final values) may differ substantially, we planned to place them in separate subgroups to avoid confusion for the reader, but the results of the subgroups will be pooled together (Higgins 2011b)
We used the five GRADE considerations (trial limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence (studies that contribute data to the pre‐specified outcomes) (Atkins 2004). We employed methods and recommendations described in chapter 11 and chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), using the GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of the evidence using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The database searches identified 21 references, of which five trials were considered as possibly being eligible. After full‐text screening, two trials (three references) were included (Sandsund 2010; Sandsund 2011), one trial (six references) was excluded (Lorenc 2015), one trial (one reference) is currently listed as ongoing (NCT03295201) and one trial (three references) is listed as 'awaiting classification' (until more information is available to allow a judgement regarding eligibility) (Schindel 2015). Full details on the results of the search are presented in a PRISMA diagram (Figure 1).

Flow diagram.
Included studies
Two trials met the inclusion criteria; one was published as a full paper (Sandsund 2011) and the second was only available as an abstract (Sandsund 2010), but additional data for both were provided by the authors. Neither included trial randomised only participants with diagnosed postural abnormalities. The authors provided the IPD from all participants, and we applied the 9.2 value Flexicurve® as a criterion to identify the participants who had postural abnormalities (Hinman 2004). The total number of participants recruited to each trial was 53 (Sandsund 2010) and 20 (Sandsund 2011); after exclusion of participants without postural abnormalities, the sample considered for this review was 50 participants in total, 35 participants in one trial (Sandsund 2010) and 15 in the second (Sandsund 2011).
Full details of the included studies can be found in the table (Characteristics of included studies)
Study design
Both trials were RCTs; the duration of the intervention ranged from 20 days (Sandsund 2010) to 12 weeks (Sandsund 2011) and all outcomes were recorded at the end of the trial period. One trial recruited outpatients (Sandsund 2011) and one recruited inpatients (Sandsund 2010). Both were single‐centre trials conducted in the UK (Sandsund 2010; Sandsund 2011).
Participants
The number of participants in each trial was respectively 53 (Sandsund 2010) and 20 participants (Sandsund 2011). Both trials included participants with and without postural abnormalities and after the exclusion of participants without postural abnormalities, the sample in the included trials was 35 participants (Sandsund 2010) and 15 participants (Sandsund 2011). Therefore, a total of 50 participants provided data for the two included trials. These comprised 20 males and 15 females (Sandsund 2010); eight males and seven females (Sandsund 2011). Both trials included adults, with ages ranging from 17 years to 58 years in one trial (Sandsund 2010) and 23 years to 41 years in the second trial (Sandsund 2011). The diagnosis criteria for CF in both trials was confirmed by genotype analysis or a sweat sodium concentration of over 70 mmol/L or sweat chloride of over 60 mmol/L (Sandsund 2010; Sandsund 2011).
Interventions
The two included trials used the same method of intervention (Sandsund 2010; Sandsund 2011). All participants were submitted to a musculoskeletal assessment followed by a 45‐minute session of the intervention that included specific rib cage and thoracic spine mobilisations, treatment of specific muscle dysfunction or tight muscle groups and postural awareness and education. Both control groups received only usual medical care. However, in one trial the control group also received an airway clearance therapy (Sandsund 2010). In one trial, interventions were conducted in alternate days while the participants were hospital inpatients; however, the number of sessions were unclear (Sandsund 2010). In the second trial, the intervention was conducted once a week, for six weeks (Sandsund 2011).
Outcomes
Both trials reported the change in the QoL measured by health‐related instruments. One trial used the Brompton Cystic Fibrosis Questionnaire (Sandsund 2010), the second trial used the Cystic Fibrosis Quality of Life Questionnaire (CFQoL) (Sandsund 2011). The BCFQ combines a number of symptoms domains, which a score of up to 100. A higher score is indicative of worse QoL. (Sandsund 2010)The CFQoL score ranges from 0 to 100, where higher scores indicates a better QoL, and it is estimated that scores above 50 are considered a good QoL. (Rozov 2006) Change in pain was measured in both trials with a 10‐cm VAS (Sandsund 2010; Sandsund 2011). The change in trunk deformity was measured by the thoracic index by the Flexicurve® (Sandsund 2010; Sandsund 2011). Both trials measured the change in pulmonary function (L) (Sandsund 2010; Sandsund 2011) and one additionally measured this outcome as % of predicted (Sandsund 2010). Only one trial assessed the change in functional capacity and used the modified shuttle test (Sandsund 2011).
The included trials reported the outcomes at baseline, day five, day 10, and pre‐discharge (Sandsund 2010); and at baseline, three weeks, six weeks and 12 weeks (Sandsund 2011).
Excluded studies
One trial (six references) has been excluded from this review (Lorenc 2015).
Studies awaiting classification
There is one trial currently awaiting classification until more information is available to allow a judgement regarding eligibility (Schindel 2015). The trial is described as a RCT and was conducted in Brazil. It recruited 34 participants aged from seven to 20 years. The intervention lasted for 12 weeks and the outcomes were measured at baseline and at the end of the trial. The outcomes assessed were lung function (FVC, FEV1, FEV1/FVC, and FEF25%‐75%), postural evaluation by postural assessment software (PAS/SAPO) and baropodometric measures (both static and dynamic) (Schindel 2015).
Ongoing studies
One paediatric RCT is ongoing (NCT03295201). Participants are aged from six to 14 years and the trial is being conducted in Turkey. The primary outcome measure is the change in exercise tolerance by the modified shuttle test. Secondary outcome measures include the change in QoL using the Cystic Fibrosis Questionnaire‐Revised (CFQR), postural stability using the balance master device ‐ Limits of Stability Test (LOS), spinal deformity by the Cobb Angle and the modified Cobb Angle, and pulmonary function.
Risk of bias in included studies
Summary figures for the risk of bias are presented in the figures (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
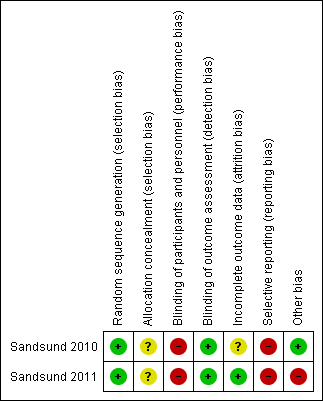
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Both included trials used a computer randomisation program to allocate the participants to treatment groups and therefore these judged as having a low risk of bias (Sandsund 2010; Sandsund 2011).
Allocation concealment
Neither trial reported on allocation concealment, so we judged them as having an unclear risk of bias (Sandsund 2010; Sandsund 2011).
Blinding
Performance bias
Both trials were judged as high risk of bias since all the participants knew the group to which they were allocated (Sandsund 2010; Sandsund 2011).
Detection bias
Both trials blinded the outcome assessors, so we judged them as having a low risk of bias (Sandsund 2010; Sandsund 2011). In one trial, participants were requested not to disclose the group they were in to outcome assessors (Sandsund 2011).
Incomplete outcome data
One trial reported the dropouts with the reasons, but did not report using an intention‐to‐treat analysis. As this trial is not yet published, and we received only one manuscript with information to include in the review, we classified this trial as having an unclear the risk of bias for this domain (Sandsund 2010). The second trial reported dropouts along with reasons, and further stated that an intention‐to‐treat analysis was undertaken; thus, we judged this trial to have a low risk of bias (Sandsund 2011).
Selective reporting
Neither trial reported all the pre‐specified outcomes according to their protocols (accessed via the online trials registry clinicaltrials.gov), therefore we judged them as high risk of bias (Sandsund 2010; Sandsund 2011).
Other potential sources of bias
Both RCTs included in this review seem to be free from other sources of bias (Sandsund 2010; Sandsund 2011).
We judged one RCT to be free from other sources of bias (Sandsund 2010). In the second included RCT, despite stratified randomisation, a large baseline imbalance between groups in MST and thoracic index was observed, therefore, this could suggests an exaggeration of effect estimate (Sandsund 2011).
Effects of interventions
In the summary of findings table, the quality of the evidence has been graded for pre‐defined outcomes (see above) and definitions of these gradings are provided within the table (summary of findings Table for the main comparison).
Intervention versus usual care
Two trials compared a form of intervention for postural abnormalities with usual care (Sandsund 2010; Sandsund 2011). Both trials reported short‐term outcomes (at less than three months) but neither trial provided medium‐term (between three months and one year) or long‐term data (at time points greater than one year).
Primary outcomes
1. Change in QoL
a. overall
Neither trial reported this outcome.
b. HRQoL
Both trials (n = 50) reported a measure of this outcome, one using the Brompton Cystic Fibrosis Questionnaire (Sandsund 2010) and the second using the Cystic Fibrosis Quality of Life Questionnaire – Section One (physical functioning) (Sandsund 2011). As the scales works in different directions, we multiplied the results of one trial (Sandsund 2010) by ‐1 in order to combine them. When we performed a meta‐analysis of these two trials, there was substantial heterogeneity (Chi² = 7.02, df = 1, (P = 0.008); I² = 86%), and the forest plot showed different effects, i.e. showing no difference in one trial, MD ‐0.95 (95% CI ‐2.05 to 0.16) (Sandsund 2011) and favouring intervention in the other, MD 0.82 (95% CI 0.12 to 1.51) (Sandsund 2010); therefore, we decided not to pool data. However, data were entered into a forest plot just for visual interpretation of the results (Analysis 1.1) (very low‐quality evidence).
2. Change in pain
Both trials measured pain using a VAS (Sandsund 2010; Sandsund 2011). When we performed a meta‐analysis of these two trials, there was substantial heterogeneity (Chi² = 5.81, df = 1 (P = 0.02); I² = 83%), and the forest plot showed different effects, i.e. showing no difference in one trial, MD ‐3.44 (95% CI ‐15.64 to 8.76) (Sandsund 2010) and favouring usual care in the other, MD 18.73 (95% CI 5.46 to 32.00) (Sandsund 2011); therefore, we decided not to pool data. However, data were entered into a forest plot just for visual interpretation of the results (Analysis 1.2) (very low‐quality evidence).
3. Change in trunk deformity
The thoracic index was used to measure posture in both trials (n = 50); there was no difference between groups, MD ‐1.01 (95% CI ‐3.11 to 1.08) with no heterogeneity (I² = 0%) (very low‐quality evidence) (Analysis 1.3).
Secondary outcomes
1. Treatment success
In one trial (15 participants), participants in the intervention group were asked if they thought the sessions had been beneficial (Sandsund 2011). Six participants returned comments and they were all positive. However, this outcome could not be meta‐analysed because the control group (usual care) was not assessed.
2. Change in pulmonary function over the short, medium and long term
a. FVC
It is uncertain whether intervention improves FVC because the analysis of data for the change in FVC from baseline (L) from both trials (50 participants) showed no difference and the quality of the evidence is very low, MD 0.17 L (95% CI ‐0.02 to 0.37) (I² = 0%) (Analysis 1.4). One of the trials (35 participants) additionally reported the change from baseline in FVC % predicted (Sandsund 2010) and showed a small difference between groups favouring intervention, MD 5.14% predicted (95% CI 0.37 to 9.91) (Analysis 1.5).
b. FEV1
Both included trials (50 participants) reported the change from baseline in FEV1 (L) (Sandsund 2010; Sandsund 2011). It is uncertain whether intervention improves FEV1 because the data analysis shows no difference between groups and the quality of the evidence is very low, MD 0.09 L (95% CI ‐0.04 to 0.21) (I² = 39%) (Analysis 1.6). One trial (35 participants) also reported the change from baseline in FEV1 % predicted (Sandsund 2010), which also showed no difference between groups, MD 0.43% predicted (95% CI ‐9.50 to 10.36) (Analysis 1.7).
c. Tiffenau’s index (FEV1/FVC)
Both included trials (50 participants) reported this outcome measured in L (Sandsund 2010; Sandsund 2011). There was no difference in change from baseline between groups, MD ‐0.12 L (95% CI ‐4.49 to 4.24) (I² = 0%) (Analysis 1.8).
d. ERV
Neither included trial reported this outcome.
e. TLC
Neither included trial reported this outcome.
3. Change in functional capacity
One trial (15 participants) measured functional capacity using the modified shuttle test (Sandsund 2011). The analysis showed no between‐group difference; the mean distance walked by the intervention group was further than that in the usual care group, but the CIs included the possibility of no effect, MD 72.22 m (95% CI ‐101.79 to 246.23) (Analysis 1.9).
4. Adverse effects
One trial (n = 35) did not provide information regarding adverse events (Sandsund 2010). As for the second trial, only six out of 10 participants of the intervention group returned no negative comments and stated that the suggested intervention was acceptable and of benefit. (Sandsund 2011).
5. Adherence to treatment
Neither included trial reported this outcome.
6. Ease of access to intervention
Neither included trial reported this outcome.
Discussion
Summary of main results
The aim of this review was firstly to determine the effects of physical therapies for managing postural abnormalities in people with CF and, secondly, to determine what would be the best intervention among the possibilities described in the protocol. We identified two trials for inclusion in this review, one of which was published as full paper (Sandsund 2011) and one as an abstract only; the authors of the abstract have kindly provided the full report of the study (Sandsund 2010).
A total of 73 participants were randomised in the two trials, but postural abnormalities were found in only 50 participants. One trial enrolled 15 clinically stable people with CF and postural abnormalities (Sandsund 2011) and one enrolled 35 participants with this condition experiencing an exacerbation of respiratory infection (Sandsund 2010). Despite the wide possibilities for treating postural changes, only the manual therapy approach compared to usual care was studied in the trials and included in the review.
The included trials reported all the review's primary outcome measures. Results for the outcome of QoL and pain have not been combined because of the high heterogeneity. Initially, we planned a sensitivity analysis using a random‐effects model if any heterogeneity identified could not be explained. However, there are too few included trials to undertake this analysis. The high heterogeneity identified may be due to differences between the two trials. The earlier trial randomised inpatients (Sandsund 2010), while the later trial recruited outpatients (Sandsund 2011). We believe that the experience of hospitalisation reduces QoL and increases pain perception. Thus, the intervention would make the participant feel a great improvement in QoL and reduced pain. It is likely that participants in the outpatient trial already had a high QoL and lower baseline pain levels because they were not put into a stressful experience such as hospitalisation, therefore the intervention was not as effective as expected (Sandsund 2011). We also noted that the heterogeneous data came from subjective outcomes, which makes the values less accurate. Combined results from both trials suggest that there is no difference in changes to trunk deformity when manual therapy is compared to usual care.
Regarding secondary outcome measures, based on results from both trials, it is uncertain whether intervention improves changes in FVC, FEV1 and Tiffeneau’s index. Only one trial measured functional capacity, and the change in the distance walked favoured the intervention over usual care, but with the possibility of no effect due to wide CIs. Only one trial (15 participants) reported that six participants of the intervention group had positive comments about the intervention and no adverse events were mentioned (Sandsund 2011). Other secondary outcomes were not measured.
None of these trials provided medium‐term (between three months and one year) or long‐term data (at time points longer than one year) or evaluated overall QoL and the others secondary outcomes. Since, only two trials could be included in this review, the data about the real benefits of manual therapy in the chosen outcomes are inconclusive.
Overall completeness and applicability of evidence
The trials evaluated only adults, either as outpatients (Sandsund 2011) or hospitalised inpatients (Sandsund 2010). The small number of trials did not allow meaningful subgroup analyses. Thus, we did not investigate the effects of physical therapies for postural abnormalities by age of participants (up to 18 years of age versus 18 years and over) or gender. Although postural abnormalities are more evident in adults, trials in young people presenting with the early development of this condition should be conducted, so that professionals know the best technique that should be used in this early phase. The presence of postural abnormalities was not used as an inclusion criteria in the included trials because the aim of these trials was to verify the effects of the manual therapy on posture. Therefore, the trials randomised a mixed sample of people with CF and with or without trunk deformity.
The included trials in our review compared manual therapy with usual care. Neither trial measured or reported on the overall QoL, treatment success, ERV, TLC, adherence to treatment and ease of access to the intervention. Several interventions we considered important to treat postural abnormalities have also not been investigated, such as physical exercise, Pilates, yoga, IMT, GPR, stretching. Consequently, all the evidence should be viewed with caution. We are still not able to discuss all the possibilities of treatment for postural abnormalities in a clinical setting.
The effectiveness of a trial to detect real difference between interventions strongly depends on sample size. Very small samples undermine the internal and external validity of a trial, while very large samples tend to transform small differences into statistically significant differences, even when they are clinically insignificant (Faber 2014). The CONSORT (Consolidated Standards of Reporting Trials) statement recommends that the clinical trials should indicate how the sample size was determined (Moher 2010). This was seen in both trials (Sandsund 2010; Sandsund 2011).
Quality of the evidence
Two trials were included in this review. One trial was published as a full paper (Sandsund 2011) and one in abstract form only (Sandsund 2010). A copy of the unpublished paper was obtained following correspondence with the authors (Sandsund 2010). A total of 73 people with CF were randomised in the two trials, but only 50 participants had postural abnormalities and were included in our analysis, resulting in one trial with 35 participants (Sandsund 2010) and one with 15 participants (Sandsund 2011). The same intervention was used in both trials, but the participants had different settings.
Overall, the quality of the evidence from the trials was judged to be mainly low or very low for the only comparison we were able to make (summary of findings Table for the main comparison). The small number of participants in each group made the results imprecise, and furthermore the participants were not blinded in either trial.
Both included trials were judged to have a high risk of bias in at least one domain. The major methodological limitations were the lack of information about allocation concealment, lack of blinding (particularly with subjective outcomes highly susceptible to biased assessment), and selective reporting of outcomes.
Although the effect sizes for some of the outcomes included in our analysis were statistically significant, the clinical significance of these results remains unclear. Furthermore, the tools used to assess adverse effects and personal preference in the included trials were generally not well‐described or validated; and neither trial incorporated measures of adherence to treatment and ease of access to intervention.
Potential biases in the review process
In attempt to reduce potential biases, we performed a systematic process according to Cochrane's recommendations. Thus, all decisions regarding to trial selection, data extraction and risk of bias were checked independently by two review authors, and when disagreement existed, an independent third author was consulted.
We contacted the trial authors for clarification on some missing information from three trials (Sandsund 2010; Sandsund 2011; Schindel 2015). Only investigators from two of these trials sent the requested information and these were included (Sandsund 2010; Sandsund 2011). Therefore, we were able to access to the 'raw' data and additional information from these two included trials. Since investigators from the earlier trial shared the data (IPD), we could analyse on an intention‐to‐treat basis (Sandsund 2010). However, we are uncertain of what the impact of this has on the results and conclusions of the review.
As the potentially eligible trials did not determine the postural abnormality as an inclusion criteria, we had to apply a criterion to identify the participants who had postural abnormalities based on a Flexicurve® cutoff point of 9.2 (Hinman 2004). Therefore, this criterion could have introduced bias in the review for some reasons. Firstly, Hinman determined the Flexicurve® value in healthy adults in an erect posture, so we are not sure if this value would be the same for individuals with CF (Hinman 2004). Secondly, no trial has sought to determine the exact Flexicurve® cutoff point that determines hyperkyphosis. Thirdly, postural evaluation by Flexicurve® correlates well with evaluation by Cobb angle, but this finding does not suggest a good agreement between the measures (Barrett 2018). However, the Flexicurve® has been largely used in most trials because it is easy to use and avoids unnecessary exposure to radiographs which is necessary to obtain the Cobb angle measure (Bansal 2015; Greendale 2011).
Due to the small number of included trials, we could not undertake a sensitivity analysis, which could be another potential source of bias.
Agreements and disagreements with other studies or reviews
The increased risk for developing kyphosis and scoliosis in people with CF was already being discussed four decades ago, and the retarded bone age seemed to be more likely related to a significant spinal deformity (Erkkila 1978). However, despite the progress in CF treatment, there is a paucity of evidence about interventions for postural abnormalities. As far as we know, there have not been any other systematic reviews or RCTs (other than the trials considered in this review) comparing physical therapies for postural abnormalities in people with CF.
A recent trial, which we have listed as 'awaiting classification' pending further information, will be considered for this review (Schindel 2015). The trial does not evaluate the thoracic index, but showed that in the intervention group regular exercise reduced cervical lordosis, thoracic hyperkyphosis, lumbar lordosis, lateral chest distance, and abdominal protrusion, as well as in the baropodometric mean pressure and contact area.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Usual care versus Intervention, Outcome 1 Change in quality of life.

Comparison 1 Usual care versus Intervention, Outcome 2 Change in pain (mm).

Comparison 1 Usual care versus Intervention, Outcome 3 Change in trunk deformity (cm).

Comparison 1 Usual care versus Intervention, Outcome 4 Change in FVC (L).

Comparison 1 Usual care versus Intervention, Outcome 5 Change in FVC (% predicted).
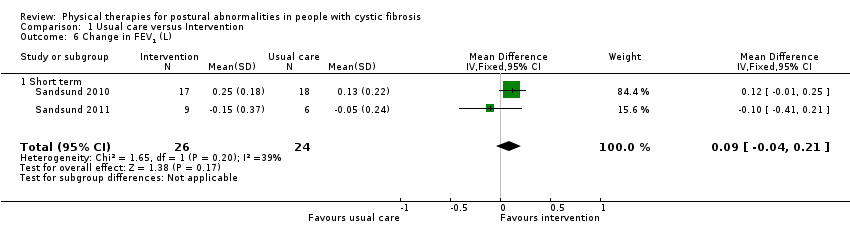
Comparison 1 Usual care versus Intervention, Outcome 6 Change in FEV1 (L).

Comparison 1 Usual care versus Intervention, Outcome 7 Change in FEV1 (% predicted).

Comparison 1 Usual care versus Intervention, Outcome 8 Change in Tiffenau’s index (FEV1/FVC).

Comparison 1 Usual care versus Intervention, Outcome 9 Change in functional capacity.
| Physical therapies compared to usual care for postural abnormalities in people with cystic fibrosis | ||||||
| Patient or population: postural abnormalities in people with cystic fibrosis Settings: outpatients and inpatients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with usual care | Risk with physical therapies | |||||
| Change in quality of life Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 86%) results were not pooled. Both included trials showed different effects, i.e. one favoured intervention (Sandsund 2010) and the other showed no difference (Sandsund 2011). | |
| Change in pain (mm) Follow‐up: short term | See comment. | NA | 50 | ⊕⊝⊝⊝ | Due high heterogeneity (I² = 83%) results were not pooled. Both included trials showed different effects, i.e. showing no difference in one trial (Sandsund 2010) and favouring usual care in the other (Sandsund 2011). | |
| Change in trunk deformity (cm) Follow‐up: short term | The mean change in trunk deformity in the control group was ‐2.33 cm. | The mean change in trunk deformity in the intervention group was1.01 cm lower (3.11 lower to 1.08 higher). | NA | 50 | ⊕⊝⊝⊝ | |
| Change in FEV1 (L) Follow‐up: short term | The mean change in FEV1 in the control group was 0.04 L. | The mean change in FEV1 in the intervention group was 0.09 L higher (0.04 lower to 0.21 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FEV1 (% predicted) based on the 2010 trial also suggest no statistically significant difference between the treatment groups (Sandsund 2010). |
| Change in FVC (L) Follow‐up: short term | The mean change in FVC in the control group was 0.03 L. | The mean change in FVC in the intervention group was 0.17 L higher (0.02 lower to 0.37 higher) | NA | 50 | ⊕⊝⊝⊝ | The results for change in FVC (% predicted) based on the 2010 trial also suggest a statistically significant difference in favour of the physical therapies group (Sandsund 2010). |
| Change in ERV (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| Change in TLC (L) Follow‐up: short term | Outcome not reported. | NA | NA | NA | ||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded once due to imprecision; small numbers of participants included in the comparison (small sample size which did not achieve the targeted sample size generated by the power calculation) and the CI overlap showed no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in quality of life Show forest plot | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| 1.1 Short term | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.27, 0.91] |
| 2 Change in pain (mm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| 2.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 6.72 [‐2.27, 15.70] |
| 3 Change in trunk deformity (cm) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| 3.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐3.11, 1.08] |
| 4 Change in FVC (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| 4.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.02, 0.37] |
| 5 Change in FVC (% predicted) Show forest plot | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| 5.1 Short term | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [0.37, 9.91] |
| 6 Change in FEV1 (L) Show forest plot | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| 6.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.04, 0.21] |
| 7 Change in FEV1 (% predicted) Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐8.52, 4.88] |
| 7.1 Short term | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐9.50, 10.36] |
| 7.2 Cutt off | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐12.77, 5.37] |
| 8 Change in Tiffenau’s index (FEV1/FVC) Show forest plot | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.79, 2.98] |
| 8.1 Short term | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐4.49, 4.24] |
| 8.2 Cutt off | 2 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐3.58, 4.11] |
| 9 Change in functional capacity Show forest plot | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |
| 9.1 Short term | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 72.22 [‐101.79, 246.23] |

