Natriuretic peptide‐guided treatment for the prevention of cardiovascular events in patients without heart failure
Information
- DOI:
- https://doi.org/10.1002/14651858.CD013015.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 15 October 2019see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Heart Group
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
CS: publication screening; assessment of relevance and quality of papers; data extraction; correspondence with study authors; organisation, analysis, and interpretation of data; and writing the manuscript.
-
FR: responsibility for design of the protocol; assessment of all trials for inclusion or exclusion; and checking data entry.
-
ML: contributions to analysis, review, and interpretation of data; discussion of the protocol and final manuscript; statistical advice provided; and approval of the final version.
-
CR: contributions to the review and discussion of the protocol; statistical advice provided; acting as third party to resolve disagreements; assessment of quality of papers and risk of bias.
-
KMD: contributions to the review and discussion of the protocol and final manuscript; approval of the final version.
-
CW: contributions to the review and discussion of the protocol.
-
RBP: data extraction and risk of bias assessment.
-
JG: contributions to the review and discussion of the protocol and final manuscript; approval of the final version.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health, UK.
Declarations of interest
-
CS: none known.
-
FR: none known.
-
ML: author of an eligible study. Prof Ledwidge reports board membership and shares in Solvotrin Therapeutics and H&L Pharma and is a named inventor on several patents related to isosorbide prodrugs of salicylates and iron‐protein formulations. Co‐Principal Investigator on the prospective, personalised comparison of ARni with ArB in patients with natriuretic peptide elevation (PARABLE) study, sponsored by Heartbeat Trust with unrestricted grant support from Novartis. Funded by an EU FP7 grant investigating biomarkers of cardiovascular disease and a Health Research Board, Ireland, project grant.
-
CR: none known.
-
KMD: author of an eligible study. Prof McDonald is co‐Principal Investigator on the prospective, personalised comparison of ARni with ArB in patients with natriuretic peptide elevation (PARABLE) study, sponsored by Heartbeat Trust with unrestricted grant support from Novartis.
-
CW: author of an eligible study.
-
RBP: none known.
-
JG: author of an eligible study. Dr Gallagher has received consultancy fees from Novartis and payment for preparation of educational presentations by PCM Scientific Servier Laboratories and Novartis. Received payment for talk preparation by Roche Diagnostics and has received conference and travel fees from Servier, Novartis, and Menarini.
Acknowledgements
We would like to thank Charlene Bridges, Cochrane Heart Information Specialist, for assistance provided with the search strategy; Andrea Takeda, Systematic Review Specialist, for assistance with methodological issues; and Nicole Martin, Managing Editor, for considerable help and input throughout the review process. We are grateful to Marco Antonio A Peña Duqu, Contact Editor, for feedback provided. We also gratefully acknowledge peer review by J.C. Kelder, Department of Cardiology, St Antonius Hospital, Nieuwegein, The Netherlands; and Lenny Vasanthan, Physical Medicine and Rehabilitation Department, Christian Medical College, Vellore, India. Finally, we are thankful to the consumer reviewer Carlotta Raby, United Kingdom, for valuable feedback provided.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Oct 15 | Natriuretic peptide‐guided treatment for the prevention of cardiovascular events in patients without heart failure | Review | Claire Sweeney, Fiona Ryan, Mark Ledwidge, Cristin Ryan, Ken McDonald, Chris Watson, Rebabonye B Pharithi, Joe Gallagher | |
| 2018 Apr 27 | Natriuretic peptide‐guided treatment for the prevention of cardiovascular events in patients without heart failure | Protocol | Fiona Ryan, Cristin Ryan, Mark Ledwidge, Ken McDonald, Chris Watson, Ciara Keane, Joe Gallagher | |
Differences between protocol and review
In the review protocol, we planned to perform subgroup analysis according to those with (1) BNP 35 pg/mL to 100 pg/mL or NT‐proBNP 125 pg/mL to 300 pg/mL and (2) BNP ≥ 100 pg/mL and NT‐proBNP ≥ 300 pg/mL. We derived the lower limit for these subgroups from the European Society of Cardiology Guidelines (Ponikowski 2016); the upper limit for these subgroups represents the cut‐off points for implementation of strategies to prevent heart failure, as specified in the 2014 Canadian Cardiovascular Society Heart Failure Management Guidelines (Moe 2015).
However the more recent 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure recommends a value of BNP > 50 pg/mL or NT‐proBNP > 125 pg/mL for the implementation of strategies to prevent heart failure (Ezekowitz 2017). We therefore believe it was appropriate to change the cut‐off values for these subgroups in a post hoc amendment to (1) BNP < 50 pg/mL and NT‐proBNP > 125 pg/mL and (2) BNP ≥ 50 pg/mL and NT‐proBNP ≥ 125 pg/mL.
PICOs
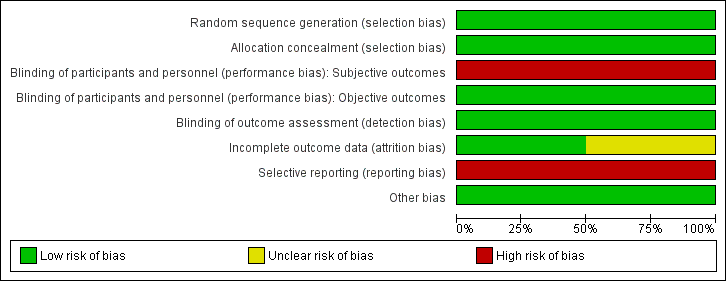
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 1 Cardiovascular mortality (as defined by trialists).

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 2 Cardiovascular hospitalisation (as defined by trialists).

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 3 Cardiovascular hospitalisation (as defined by trialists) by natriuretic peptide level.

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 4 All‐cause mortality.

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 5 All‐cause hospitalisation (all occurrences).

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 6 Ventricular dysfunction (as defined by trialists).

Comparison 1 Natriuretic peptide‐guided treatment versus standard care, Outcome 7 Change in NP level at the end of follow‐up.
| Natriuretic peptide‐guided treatment compared to standard care for the prevention of cardiovascular events in patients without heart failure | ||||||
| Patient or population: patients without heart failure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with standard care | Risk with natriuretic peptide‐guided treatment | |||||
| Cardiovascular mortality Follow‐up: 2 years | Study population | RR 0.33 | 300 | ⊕⊕⊝⊝ | Cardiovascular deaths not available, so cardiac deaths used | |
| 20 per 1000 | 7 per 1000 | |||||
| Cardiovascular hospitalisation (as defined by trialists) Follow‐up range: 2 years to mean 4.2 years | Study population | RR 0.52 | 1674 | ⊕⊕⊕⊝ | ||
| 163 per 1000 | 85 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.90 | 1354 | ⊕⊕⊝⊝ | ||
| 60 per 1000 | 54 per 1000 | |||||
| All‐cause hospitalisation (all occurrences) | Study population | RR 0.83 | 1354 | ⊕⊕⊕⊝ | ||
| 601 per 1000 | 499 per 1000 | |||||
| Ventricular dysfunction (as defined by trialists) | Study population | RR 0.61 | 1374 | ⊕⊕⊕⊕ | Ventricular dysfunction was defined by trialists as left ventricular dysfunction and heart failure. Left ventricular dysfunction (LVD) included all patients with asymptomatic left ventricular systolic dysfunction (LVSD) and/or asymptomatic left ventricular diastolic dysfunction (LVDD). Heart failure included all patients with LVD (LVSD and/or LVDD) and with symptoms of heart failure requiring emergency admission to hospital | |
| 87 per 1000 | 53 per 1000 | |||||
| Change in NP level at the end of follow‐up | Mean change in NP level at the end of follow‐up was 9.52 pg/mL | MD 4.06 pg/mL higher | ‐ | 1374 | ⊕⊕⊕⊝ | These data come from Ledwidge 2013; NP level was reported as BNP. Huelsmann 2013 also reported change in NT‐proBNP after 1 year of treatment. However, these data were presented as median and interquartile range; therefore we could not calculate mean difference |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aImprecision: very wide 95% confidence interval, which includes the line of null effect and is consistent with the possibility of both important benefits and harms. The sample size is small. Downgraded by two levels. bRisk of bias: both studies did not blind patients or personnel. Downgraded by one level. cImprecision: wide 95% confidence interval, which includes the line of null effect and is consistent with the possibility of important benefits and harms. Downgraded by one level. dRisk of bias: downgraded by one level due to attrition bias as one study excluded from analysis patients who were lost to follow‐up and those who withdrew, who did not have at least one year of detailed follow‐up cost data. eRisk of bias: echocardiographer and cardiologist reviewing end of study echo were blinded and diagnosis of ventricular dysfunction was based on clearly defined echocardiographic measures, which removed subjectivity in outcome assessment. Not downgraded. fImprecision: wide CIs that cross the line of no effect (zero) and include the possibility of the intervention causing both benefit and harm. Downgraded by one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality (as defined by trialists) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Cardiovascular hospitalisation (as defined by trialists) Show forest plot | 2 | 1674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.40, 0.68] |
| 3 Cardiovascular hospitalisation (as defined by trialists) by natriuretic peptide level Show forest plot | 2 | 1674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.38, 0.65] |
| 3.1 BNP < 50 pg/mL and NT‐proBNP < 125 pg/mL | 1 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.95] |
| 3.2 BNP > 50 pg/mL and NT‐proBNP > 125 pg/mL | 2 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.65] |
| 4 All‐cause mortality Show forest plot | 2 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.35] |
| 5 All‐cause hospitalisation (all occurrences) Show forest plot | 2 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.92] |
| 6 Ventricular dysfunction (as defined by trialists) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Change in NP level at the end of follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |


