Rapid initiation of antiretroviral therapy for people living with HIV
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012962Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 19 February 2018see what's new
- Type:
-
- Intervention
- Stage:
-
- Protocol
- Cochrane Editorial Group:
-
Cochrane Infectious Diseases Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
AMU, IEW, and SJ contributed to the protocol design, writing, approved the final version, and responded to the referee comments.
JN helped design the protocol and the methods, approved the final version, and approved the response to the referee comments.
Sources of support
Internal sources
-
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
AMU has no known conflicts of interest.
SJ has no known conflicts of interest.
JBN has no known conflicts of interest.
IEW has no known conflicts of interest.
Acknowledgements
We thank Marcel Kitenge, Marty Richardson, and Paul Garner, who contributed to the planning of this protocol.
IEW, SJ, and the editorial base of the Cochrane Infectious Diseases Group are supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Jun 17 | Rapid initiation of antiretroviral therapy for people living with HIV | Review | Alberto Mateo‐Urdiales, Samuel Johnson, Rhodine Smith, Jean B Nachega, Ingrid Eshun‐Wilson | |
| 2018 Feb 19 | Rapid initiation of antiretroviral therapy for people living with HIV | Protocol | Alberto Mateo‐Urdiales, Samuel Johnson, Jean B Nachega, Ingrid Eshun‐Wilson | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anti-Retroviral Agents [adverse effects, *therapeutic use];
- Developing Countries;
- HIV Infections [*drug therapy, mortality];
- Medication Adherence [statistics & numerical data];
- Pregnancy Complications, Infectious [drug therapy];
- Randomized Controlled Trials as Topic [statistics & numerical data];
- Time Factors;
- *Time-to-Treatment;
Medical Subject Headings Check Words
Adolescent; Adult; Child; Female; Humans; Male; Pregnancy;
PICOs
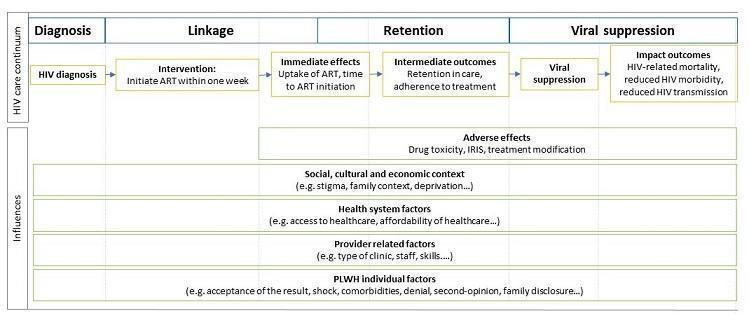
Conceptual model of factors influencing the HIV care continuum. Abbreviations: PLWH: people living with HIV, ART: antiretroviral therapy, IRIS: immune reconstitution inflammatory syndrome
| Options | Treatment for pregnant women with CD4 count < 350 cells/mm³ | Prophylaxis for pregnant women with CD4 count > 350 cells/mm³ |
| Option A | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation and continued until 7 days post‐partum |
| Option B | ART started as soon as HIV is diagnosed, continued for life | Antivirals started as soon as 14 weeks of gestation until childbirth if not breastfeeding or until one week after cessation of breastfeeding |
| Option B+ | ART initiated as soon as HIV diagnosis and continued for life | |
| Source: WHO 2012. | ||

