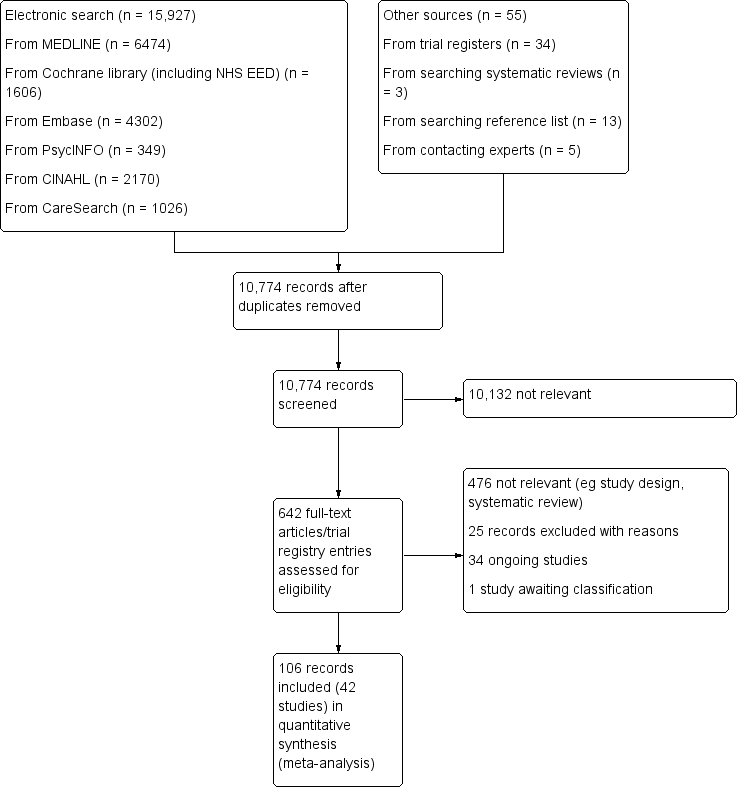
The effectiveness and cost‐effectiveness of hospital‐based specialist palliative care for adults with advanced illness and their caregivers
Information
- DOI:
- https://doi.org/10.1002/14651858.CD012780.pub2Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 30 September 2020see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
SB won funding for the review from National Institute for Health Research Health Services and Delivery Research programme (Project Number 16/02/17) and was Chief Investigator on the grant. SB, DY, CJE, GG, CT, FEM, MC and IJH contributed to the writing of the 2017 protocol. AO carried out the searches. SB and AO screened the studies and extracted the data. All authors reviewed the final studies for inclusion. AO entered the data. AO and DY carried out the analyses. SB, AO, DY and IJH prepared the final text. ll authors reviewed and contributed to the final draft.
Sources of support
Internal sources
-
Department of Palliative Care, Policy and Rehabilitation, Cicely Saunders Institute, King's College London, London, UK
-
Institute of Psychiatry, King's College London, London, UK
External sources
-
School of Nursing, Midwifery and Social Work, University of Manchester, Manchester, UK
-
Amsterdam Institute of Social Science Research, University of Amsterdam, Amsterdam, Netherlands
-
Regional Palliative Care Network, IRCCS AOU San Martino‐IST, Genoa, Italy
-
National Institute for Health Research (NIHR), UK
Earlier drafts of this review were completed by the MORECare project, which was funded by the NIHR and managed by the Medical Research Council as part of the Methodology Research Programme (project number: G0802654/1).
-
The Atlantic Philanthropies and Cicely Saunders International, Other
The review was finalised through support from The Atlantic Philanthropies and Cicely Saunders International
-
NIHR Health Services and Delivery Research (HS&DR), UK
Ongoing work for this project has been funded by NIHR HS&DR Project number: 16/02/17
Declarations of interest
SB: none known; SB is a Consultant in Palliative Medicine and manages patients with advanced life‐threatening illness.
AO: none known.
DY: none known.
WG: none known.
GG: none known.
CT: none known.
MC: none known.
FEM: none known; FEM is a Consultant in Palliative Medicine and manages patients with advanced life‐threatening illness.
CJE: none known; CJE is an Honorary Nurse Consultant and manages patients with advanced life‐threatening illness.
IJH: none known; IJH is a Consultant in Palliative Medicine and manages patients with advanced life‐threatening illness.
Acknowledgements
We acknowledge Barbara Daveson, Melinda Smith, Hamid Benalia, Emily West, Sue Hall, Barbara Gomes and Nancy Preston who contributed to earlier drafts of the protocol.
BuildCARE members: Emma Bennett, Francesca Cooper, Barbara Daveson, Susanne de Wolf‐Linder, Mendwas Dzingina, Clare Ellis‐Smith, Catherine Evans, Taja Ferguson, Lesley Henson, Irene Higginson, Bridget Johnston, Pauline Kane, Peter Lawlor, Paul McCrone, Regina McQuillan, Diane Meier, Sean Morrison, Fliss Murtagh, Charles Normand, Steve Pantilat, Ana Reison, Karen Ryan, Lucy Selman, Melinda Smith, Katy Tobin, Rowena Vohora and Gao Wei.
We are grateful to the following peer reviewers for their time and comments: Brian Duncan, Ollie Minton, Claudia Virdun, and Abhijna Vithal Yergolkar.
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
This research was funded by the National Institute for Health Research Health Services and Delivery Research programme (Project Number 16/02/17). It was also supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South London, now recommissioned as NIHR Applied Research Collaboration South London. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Sep 30 | The effectiveness and cost‐effectiveness of hospital‐based specialist palliative care for adults with advanced illness and their caregivers | Review | Sabrina Bajwah, Adejoke O Oluyase, Deokhee Yi, Wei Gao, Catherine J Evans, Gunn Grande, Chris Todd, Massimo Costantini, Fliss E Murtagh, Irene J Higginson | |
| 2017 Sep 02 | The effectiveness and cost‐effectiveness of inpatient specialist palliative care in acute hospitals for adults with advanced illness and their caregivers | Protocol | Sabrina Bajwah, Deokhee Yi, Gunn Grande, Chris Todd, Massimo Costantini, Fliss E Murtagh, Catherine J Evans, Irene J Higginson | |
Differences between protocol and review
There are a number of differences between the published protocol (Bajwah 2017), and this review.
Study design
In the published protocol, we stated that we will include a number of study designs including randomised trials, non‐randomised trials, controlled before‐and‐after studies, interrupted time series studies and repeated measures studies. Due to the expansion of our review from only inpatient specialist palliative care to include other models of HSPC (HSPC) and given that RCTs are the most rigorous study design, we refrained from analysing studies that were not RCTs in order to reduce heterogeneity and allow meta‐analyses where possible. We initially wanted to minimise cross‐contamination by including only cluster‐randomised studies. However, our project advisory group suggested that both cluster and non‐cluster‐RCTs should be included to capture the breadth of evidence from RCTs that met our eligibility criteria.
Intervention
The published protocol was focussed on assessing the effectiveness and cost‐effectiveness of inpatient specialist palliative care in acute hospitals for adults with advanced illness and their unpaid caregivers. However, we expanded the scope of our review from inpatient specialist palliative care to all models of HSPC, and the title has been amended to reflect this. Given that models of HSPC are evolving, we broadened the review to increase relevance for clinical practice and policy makers with the potential to aid the future development, funding and implementation of evidence‐based HSPC. As a result of expanding the scope of our review to cover models of HSPC, we also expanded the scope of usual care to "inpatient or outpatient hospital care without specialist palliative care input at the point of entry into the study, community care or hospice care provided outside of the hospital setting".
In our protocol, we stated that the intervention should be administered by hospital staff who have completed specialist training in palliative care or who had obtained clinical competencies and professional characteristics required for the delivery of inpatient specialist palliative care through clinical experience. Experts in our project advisory group recommended that we include studies where the training of the palliative care team was unclear, with eligibility informed by activity of delivering specialist palliative care rather than level of specialist training. In order to capture this difference, we included studies where the training/clinical competence of the palliative care team was described as well as studies that simply stated the involvement of a palliative care team.
Outcomes
We changed our primary outcome from pain to two primary outcomes, patient HRQoL (previously, a secondary outcome) and patient symptom burden assessed using a composite measure of two or more symptoms (a new outcome we introduced following expert advice). The clinical experts on our project advisory group suggested that pain may not be an appropriate outcome for those with non‐malignant conditions, where pain may be less prevalent compared to patients with cancer. Furthermore, the aim of palliative care is to improve quality of life, while also ensuring effective symptom management.
We have further provided clarity around the outcomes we presented in our protocol.
-
We included number of home deaths in the review as a proxy for achieving patient preferred place of death, as people’s preference is mostly to die at home (Gomes 2012).
-
In our protocol, one of our secondary outcomes was patient's other symptoms (e.g. physical, psychological, social or spiritual domains). We specifically presented data on patient anxiety and patient depression for this outcome.
-
Another secondary outcome in our protocol was satisfaction with care, which we reported as patient satisfaction with care and unpaid caregiver satisfaction with care in this review.
-
We had unpaid caregiver symptom control (e.g. physical, psychological, social or spiritual domains) as an outcome in our protocol. In this review, we presented unpaid caregiver anxiety and caregiver depression for caregiver symptom control.
-
For the caregiver pre‐ and post‐bereavement outcome we reported in the protocol, we presented caregiver grief and caregiver quality of life.
-
Although we presented achieving preferred place of care or death as one outcome in the protocol, we split it into two outcomes in the review: achieving patient preferred place of death and achieving patient preferred place of care.
-
We added a new secondary outcome (breathlessness) to this review because of the recommendations we received from clinical experts in our project advisory group on its relevance as an appropriate outcome in non‐malignant conditions.
Given the expansion of these outcomes, there has been a change in the order of the outcomes reported in this review compared to the protocol. Compared to our protocol, we now have two economic outcomes: resource use; costs and cost‐effectiveness. Resource use encompasses institutional care services use, outpatient clinic services use, community care services use, unpaid caregiver care and medication and other resources. Where possible, we summarised data on cost and cost‐effectiveness of HSPC.
Data analysis and assessments
We added early versus late palliative care as a subgroup analysis which was recommended for inclusion in our review by clinical experts because of its relevance to practice. Although we had initially specified that pain and other outcomes presented as binary data will be treated as binary outcomes in our published protocol, this was not possible as most studies presented their outcomes as continuous data. The only outcome where we were able to calculate an odds ratio and 95% confidence intervals in addition to standardised mean differences was patient depression.
We expanded our risk of bias (ROB) methods by carrying out separate assessments for all subjective outcomes (e.g. health‐related quality of life) and all objective outcomes (e.g. mortality). Where studies did not include either subjective or objective outcomes, we left the domain that was not included blank. We added the domain 'Other bias (other sources of bias)' in the full review in order to assess whether groups were balanced at baseline and whether differences at baseline were adjusted for. We further expanded on the response options for 'size of study bias'. In particular, we assessed the following as unclear risk of bias under 'size of study bias': studies that had < 50 participants in one treatment arm and 50 to 199 participants in another treatment arm; and studies that had 50 to 199 participants in one treatment arm and > 200 participants in another treatment arm.
We had planned to use either a fixed‐effects or random‐effects model for meta‐analysis. Due to the different models of HSPC in our review, we presented only random‐effects models as we are estimating the average effect across HSPC rather than any single true effect. We had planned to estimate an intra‐class correlation coefficient (ICC) where the authors of cluster‐RCTs did not carry out adjustment or provide an ICC. However, we decided to use an estimate of ICC we obtained from a previous Cochrane Review in adjusting for clustering in McCorkle 2015. We contacted the authors of McCorkle 2015 for their ICC but at the time of publication they have not responded. In our protocol we stated that we would contact the original investigators for missing data and describe any strategy used for imputing missing data. We decided to only contact authors for missing data without carrying out imputations as this is the preferred method for dealing with missing data (Higgins 2011). We initially wanted to explore reasons for heterogeneity in sensitivity analysis. However, Cochrane editors recommended the use of subgroup analysis for assessing heterogeneity. Consequently, we explored heterogeneity using subgroup analysis, while we used sensitivity analysis to test the estimate we used in adjusting for clustering in the cluster‐RCT. As we did not include nonrandomised studies, we did not have to pay particular attention to selection bias and reporting bias in such studies. We did not carry out a subgroup analysis assessing provision of single or few components of HSPC because very few studies provided a single component of HSPC. One of our subgroup analyses in the protocol was models of specialist palliative care. In our protocol, we have clarified this as models of HSPC because we expanded our review to include more models of HSPC.
Given that combining endpoint scores and change scores is not recommended when using standardised mean differences (SMDs) and also that Cochrane does not recommend pooling adjusted and unadjusted estimates together, we pooled studies presenting adjusted endpoint scores as our main meta‐analysis, while we carried out sensitivity analyses with studies reporting unadjusted endpoint scores, adjusted change scores and unadjusted change scores. This is a change from our protocol based on advice from Cochrane editors.
In our protocol, we planned to include three 'Summary of Findings' tables: inpatient hospital specialist palliative care and usual care versus inpatient hospital care without any specialist palliative care input (e.g. oncological care only); inpatient hospital specialist palliative care and usual care versus community care (e.g. primary or specialist care provided in the patient’s place of residence); and inpatient hospital specialist palliative care and usual care versus hospice care provided outside of the hospital setting. We decided to present only one 'Summary of Findings' (SoF) table, rather than three, for the comparison of HSPC (plus or minus usual care) versus usual care as experts in our project advisory group advised us this comparison alone would be the most informative for decision‐makers. We expanded usual care to "inpatient or outpatient hospital care without specialist palliative care input at the point of entry into the study, community care or hospice care provided outside of the hospital setting". We presented results on both cost and cost‐effectiveness in our SoF table as opposed to only cost‐effectiveness in our protocol.
We initially stated that we would rate the strength of the evidence using a tool by Van Tulder 2003. However, we decided to use the GRADE approach in accordance with Cochrane standards.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Ambulatory Care [economics];
- Bias;
- Caregivers [psychology, *statistics & numerical data];
- Cost-Benefit Analysis;
- Family;
- Heart Failure [mortality, therapy];
- Home Care Services, Hospital-Based [*economics];
- Hospitalization [economics];
- Neoplasms [mortality, therapy];
- Pain Management [statistics & numerical data];
- Palliative Care [*economics, *methods];
- Patient Satisfaction;
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Symptom Assessment [statistics & numerical data];
- Terminal Care [*economics, *methods];
Medical Subject Headings Check Words
Humans;
PICOs

A figure describing the power of included studies at recruitment and follow‐up
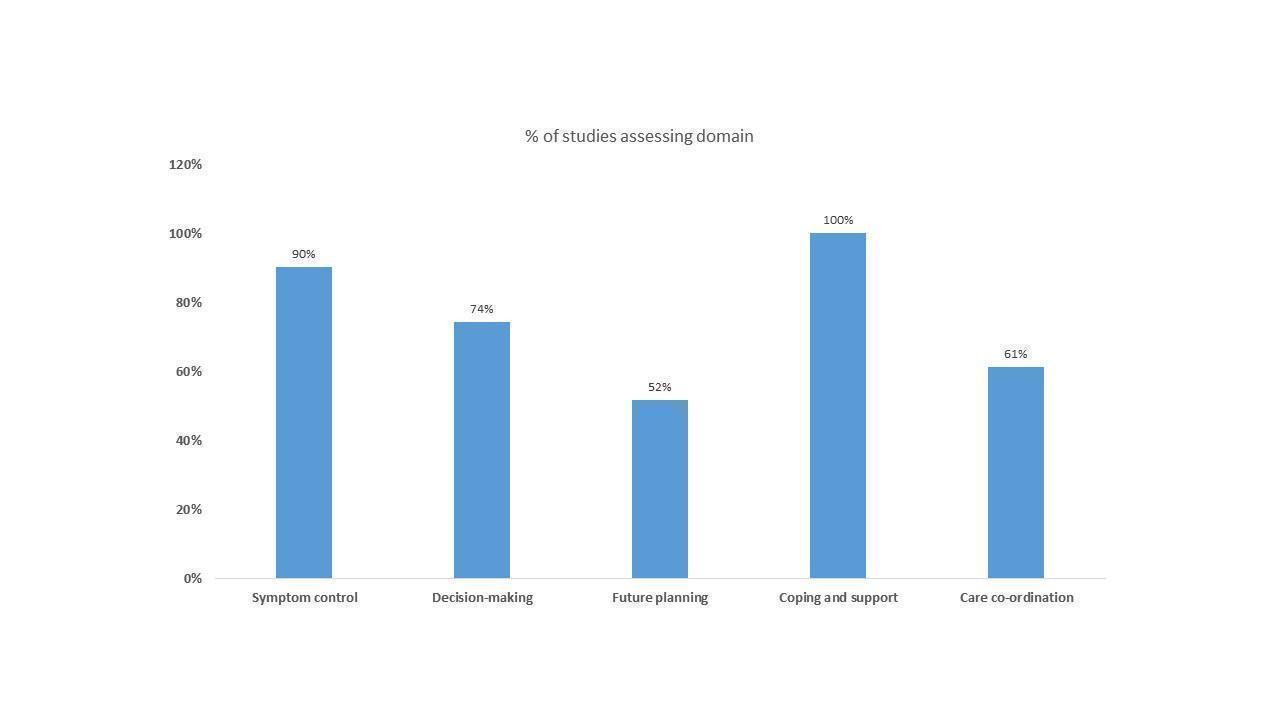
A figure showing the domains of HSPC in the studies that either included certified experts in palliative care or those described as palliative care clinicians

A figure showing the domains of HSPC in studies that were unclear about palliative care training

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot of comparison: 1 Patient health‐related quality of life, outcome: 1.1 HSPC versus usual care on patient HRQoL: adjusted endpoint values.

Comparison 1: Patient health‐related quality of life, Outcome 1: HSPC versus usual care on patient HRQoL: adjusted endpoint values

Comparison 1: Patient health‐related quality of life, Outcome 2: HSPC versus usual care on patient HRQoL: adjusted endpoint values (excluding McCorkle 2015)

Comparison 1: Patient health‐related quality of life, Outcome 3: HSPC versus usual care on patient HRQoL: unadjusted endpoint values

Comparison 1: Patient health‐related quality of life, Outcome 4: HSPC versus usual care on patient HRQoL: unadjusted endpoint values (excluding McCorkle 2015)

Comparison 1: Patient health‐related quality of life, Outcome 5: HSPC versus usual care on patient HRQoL: unadjusted change values

Comparison 2: Patient symptom burden, Outcome 1: HSPC versus usual care on patient symptom burden: adjusted endpoint values

Comparison 2: Patient symptom burden, Outcome 2: HSPC versus usual care on patient symptom burden: unadjusted endpoint values

Comparison 2: Patient symptom burden, Outcome 3: HSPC versus usual care on patient symptom burden: unadjusted endpoint values (excluding McCorkle 2015)

Comparison 2: Patient symptom burden, Outcome 4: HSPC versus usual care on patient symptom burden: adjusted change values

Comparison 2: Patient symptom burden, Outcome 5: HSPC versus usual care on patient symptom burden: adjusted change values (excluding McCorkle 2015)

Comparison 2: Patient symptom burden, Outcome 6: HSPC versus usual care on patient symptom burden: unadjusted change values

Comparison 3: Patient satisfaction with care, Outcome 1: HSPC versus usual care on patient satisfaction with care: adjusted endpoint values

Comparison 4: Achieving patient preferred place of death, Outcome 1: HSPC versus usual care on home deaths

Comparison 5: Pain, Outcome 1: HSPC versus usual care on pain: adjusted endpoint values

Comparison 5: Pain, Outcome 2: HSPC versus usual care on pain: adjusted change values

Comparison 5: Pain, Outcome 3: HSPC versus usual care on pain: unadjusted change values

Comparison 6: Patient anxiety, Outcome 1: HSPC versus usual care on patient anxiety: adjusted endpoint values

Comparison 6: Patient anxiety, Outcome 2: HSPC versus usual care on patient anxiety: adjusted endpoint values (excluding McCorkle 2015)

Comparison 6: Patient anxiety, Outcome 3: HSPC versus usual care on patient anxiety: unadjusted endpoint values

Comparison 6: Patient anxiety, Outcome 4: HSPC versus usual care on patient anxiety: unadjusted endpoint values (excluding McCorkle 2015)

Comparison 6: Patient anxiety, Outcome 5: HSPC versus usual care on patient anxiety: unadjusted change values

Comparison 6: Patient anxiety, Outcome 6: HSPC versus usual care on patient anxiety in different populations: adjusted endpoint values

Comparison 6: Patient anxiety, Outcome 7: HSPC versus usual care on patient anxiety in different populations: adjusted endpoint values (excluding McCorkle 2015)

Comparison 6: Patient anxiety, Outcome 8: EPC vs LPC on patient anxiety: adjusted endpoint values

Comparison 6: Patient anxiety, Outcome 9: Effect of MDT‐led services on patient anxiety: adjusted endpoint values

Comparison 6: Patient anxiety, Outcome 10: Effect of MDT‐led services on patient anxiety: adjusted endpoint values (excluding McCorkle 2015)

Comparison 6: Patient anxiety, Outcome 11: HSPC versus usual care on patient anxiety in different countries: adjusted endpoint values

Comparison 6: Patient anxiety, Outcome 12: HSPC versus usual care on patient anxiety in different countries: adjusted endpoint values (excluding McCorkle 2015)

Comparison 7: Unpaid caregiver anxiety, Outcome 1: HSPC versus usual care on unpaid caregiver anxiety: unadjusted endpoint values

Comparison 8: Patient depression, Outcome 1: HSPC versus usual care on patient depression: adjusted endpoint values

Comparison 8: Patient depression, Outcome 2: HSPC versus usual care on patient depression: unadjusted endpoint values

Comparison 8: Patient depression, Outcome 3: HSPC versus usual care on patient depression: unadjusted endpoint values (excluding McCorkle 2015)

Comparison 8: Patient depression, Outcome 4: HSPC versus usual care on patient depression: adjusted change values

Comparison 8: Patient depression, Outcome 5: HSPC versus usual care on patient depression: unadjusted change values

Comparison 8: Patient depression, Outcome 6: HSPC versus usual care on patient depression as a binary outcome

Comparison 9: Unpaid caregiver depression, Outcome 1: HSPC versus usual care on unpaid caregiver depression: adjusted endpoint values

Comparison 9: Unpaid caregiver depression, Outcome 2: HSPC versus usual care on unpaid caregiver depression: unadjusted endpoint values

Comparison 10: Unpaid caregiver quality of life, Outcome 1: HSPC versus usual care on unpaid caregiver quality of life: unadjusted endpoint values

Comparison 11: Unpaid caregiver burden, Outcome 1: HSPC versus usual care on unpaid caregiver burden: adjusted change values

Comparison 12: Patient breathlessness, Outcome 1: HSPC versus usual care on patient breathlessness: adjusted endpoint values

Comparison 12: Patient breathlessness, Outcome 2: HSPC versus usual care on patient breathlessness: unadjusted endpoint values

Comparison 12: Patient breathlessness, Outcome 3: HSPC versus usual care on patient breathlessness: unadjusted change values
| Hospital‐based specialist palliative care compared to usual care for adults with advanced illness and their unpaid caregivers/families | |||||
| Patient or population: adults with advanced illness and their unpaid caregivers/families | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with usual care | Risk with hospital‐based specialist palliative care | ||||
| Patient health‐related quality of life (HRQoL)i, SD units | Mean (SD) ranging from ‐45.4 (26.83) to 131.14 (26.62) | SMD 0.26 SDs higher | ‐ | 1344 | ⊕⊕⊝⊝ |
| Patient symptom burden assessed with generalised measuresii, SD units (lower scores indicate lower symptom burden) | Mean (SD) ranging from ‐19.3 (4.2) to 268.59 (201.65) | SMD 0.26 SDs lower | ‐ | 761 | ⊕⊝⊝⊝ |
| Patient satisfaction with careiii, SD units | Mean (SD) ranging from 6.4 (1.1) to 68.37 (9.03) | SMD 0.36 SDs higher (0.41 higher to 0.57 higher) | ‐ | 337 (2 RCTs) | ⊕⊕⊝⊝ |
| Achieving patient preferred place of death (measured by number of patients with home death) Follow‐up: range 1 month to 13 months | 462 per 1000 | 583 per 1000 (513 to 649) | OR 1.63 higher (1.23 higher to 2.16 higher) | 861 | ⊕⊕⊝⊝ |
| Painiv, SD units | Mean (SD) ranging from 2.2 (3.7) to 28.19 (32.81) | SMD 0.16 SDs lower | ‐ | 525 | ⊕⊝⊝⊝ |
| Unpaid caregiver burdenv | Only two studies reported adjusted endpoint values but we could not pool them in a meta‐analysis. They both found no between‐group difference between HSPC and usual care | ‐ | 170 | ⊕⊝⊝⊝ | |
| Cost and cost‐effectiveness | Of 13 studies reporting costs of HSPC, nine studies found no difference between HSPC and usual care and two studies favoured HSPC over usual care. The difference in cost was unclear in one study, while another study reported mixed findings with lower cost of hospitalisation in favour of HSPC but no difference in the cost of emergency room visit. Four studies with full economic analysis were inconclusive on the cost‐effectiveness of HSPC. | ‐ | 2103 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). i. Assessed with the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ‐C30), Functional Assessment of Cancer Therapy ‐ Bone Marrow Transplant (FACT‐BMT), Functional Assessment of Cancer Therapy ‐ General Measure (FACT‐G), Functional Assessment of Cancer Therapy – Lung scale (FACT‐L), Functional Assessment of Chronic Illness therapy for Palliative Care (FACIT‐Pal), Functional Assessment of Chronic Illness Therapy ‐ Spiritual Well‐being Scale (FACIT‐Sp), McGill Quality of Life Questionnaire (McGill QoL questionnaire) and Minnesota Living with Heart Failure Questionnaire (MLHF questionnaire). ii. Assessed with the Edmonton Symptom Assessment Scale (ESAS) or a modified form of it, severity subscale of the Memorial Symptom Assessment Scale (MSAS), symptom impact subscale of the Quality of Life at End of life (QUAL‐E), Rotterdam Symptom Checklist (RSC ‐ Physical Symptoms Score) and lung cancer subscale of the FACT‐L. iii. Assessed with 16‐item Family Satisfaction with Care ‐ Patient Version (FAMCARE‐P16) and Modified City of Hope Patient Questionnaires ‐ Place of Care Environment Scale (MCOHPQ ‐ Place of Care Environment Scale). iv. Assessed with pain item of EORTC QLQ‐C30 and Brief Pain Inventory (BPI). v. Assessed with Montgomery‐Borgatta Caregiver Burden Scale and Zarit Burden Inventory | |||||
| GRADE Working Group grades of evidence | |||||
| a We downgraded by 2 levels for very serious study limitations due to a high risk of bias in studies. b We downgraded by 1 level due to inconsistency between our main meta‐analysis and sensitivity analyses. c We downgraded by 1 level for imprecision due to the small number of participants. d We downgraded by 1 level for inconsistency because the results were inconsistent across studies. | |||||
| Author | Symptom control (e.g. assess symptoms, prescribing of medications) | Decision‐making (e.g. enquire about goals of care) | Future planning (e.g. advance care planning) | Coping and support (e.g. emotional and practical support) | Care co‐ordination (e.g. helping with co‐ordinating care) |
|---|---|---|---|---|---|
| Yes | Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| No | Yes | No | Yes | No | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | No | No | Yes | No | |
| Yes | Yes | Yes | Yes | No | |
| Yes | Yes | Yes | Yes | No | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | Yes | Yes | No | |
| Yes | No | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | No | Yes | Yes | No | |
| Yes | Yes | Yes | Yes | No | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | No | Yes | Yes | |
| Unclear | Unclear | Unclear | Yes | Unclear | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | No | No | Yes | No | |
| Yes | Yes | Yes | Yes | Yes | |
| Yes | Yes | Yes | Yes | Yes | |
| Unclear | Unclear | Unclear | Yes | Unclear | |
| Yes | No | No | Yes | No | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | No | Yes | Yes | |
| Yes | Yes | No | Yes | Yes | |
| Yes | No | No | Yes | No |
| Author | Symptom control (e.g. assess symptoms, prescribing of medications) | Decision‐making (e.g. enquire about goals of care) | Future planning (e.g. advance care planning) | Coping and support (e.g. emotional and practical support) | Care co‐ordination (e.g. helping with co‐ordinating care) |
|---|---|---|---|---|---|
| Yes | No | Yes | Yes | No | |
| Unclear | Unclear | Unclear | Unclear | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | |
| Yes | Yes | Yes | Yes | No | |
| Yes | Yes | Yes | Yes | No | |
| Yes | Yes | No | Yes | No | |
| Unclear | Unclear | Unclear | Unclear | Yes | |
| Yes | No | No | Yes | No | |
| Yes | No | Yes | Yes | No | |
| Yes | No | Yes | Yes | No | |
| Yes | No | No | Yes | No |
| Studies, primary endpoint (PEP), disease group | Scales used | Dimensions covered in scales |
|---|---|---|
| PEP: 4 weeks Advanced fibrotic lung disease | KBILD (used in meta‐analysis) SGRQ | KBILD is a 15‐item questionnaire consisting of three domains (breathlessness and activities, chest symptoms and psychological) ‐ secondary outcome SGRQ is a 50‐item instrument designed to measure impact on overall health, daily life, and perceived well‐being in patients with obstructive airways disease. Part 1 has a symptoms component (frequency and severity) with a 1, 3 or 12 month recall (several scales); Part 2 has an activities component looking at activities that cause or are limited by breathlessness and an impact component looking at social functioning, psychological disturbances resulting from airways disease and referring to current state as the recall (dichotomous (true/false) except last question (4‐point Likert scale) – secondary outcome |
| PEP: 13 months Cancer | FACIT‐Pal | Measures physical, emotional, social, and functional well‐being in addition to concerns relevant to persons with life‐threatening illness (e.g. feeling peaceful, reconciling with others) – primary outcome |
| PEP: 3 months Cancer | FACIT‐Pal (used in meta‐analysis) Treatment Outcome Index | Measures physical, emotional, social, and functional well‐being and additional concern subscales – study did not specify whether primary or secondary outcome TOI, composed of FACIT‐Pal physical, functional, and additional concern subscales |
| PEP: 6 months Heart failure | KCCQ | KCCQ is a valid, reliable measure of heart failure–specific health status that is responsive to change. No further details provided in the study |
| PEP: 6 months Heart failure | EQ‐5D (used in meta‐analysis) KCCQ | A generic, single index that defines health in the five dimensions of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression ‐ did not specify primary or secondary outcomes Full data not shown in study |
| PEP: 12 weeks Multiple sclerosis | MSIS | Multiple Sclerosis Impact Scale (MSIS) is a 29‐item measure of disease impact. It has two subscales: physical and psychological subscales. |
| PEP: 2 weeks Cancer | FACT‐BMT | The 47‐item Functional Assessment of Cancer Therapy–Bone Marrow Transplant which includes subscales assessing physical, functional, emotional, social well‐being, and bone marrow transplant–specific concerns during the past week, was used to assess patients’ QoL – primary outcome |
| PEP: 12 weeks Cancer | FACT‐G | Functional Assessment of Cancer Therapy‐General (FACT‐G) scale. It is a 27‐item internationally validated questionnaire divided into four primary HRQoL domains: physical well‐being, social/family well‐being, emotional well‐being, and functional well‐being. The total FACT‐G score is the sum of the 4 subscale scores. |
| PEP: at hospital discharge Mixed diseases comprising cancer and non‐cancer | MCOHPQ | MCOHPQ Physical Area scale, emotional/relationship area and spiritual area scales and MCOHPQ place of care environment scale. Physical Area scale addresses pain, fatigue, sleep changes, nausea, constipation, diarrhoea, dry mouth, change in appetite, and shortness of breath. Emotional support items included: anxiety, burden to family, support they received, isolation, opportunity to discuss illness and possible death, and treatment wishes/goals. Spiritual support included: the importance of participation in spiritual or religious experiences from the Spiritual Area scale, and two items developed by the investigators: ability to find meaning in one’s life, and support given by religion or spiritual belief. MCOHPQ Place of Care Environment scale addressed experiences receiving pain management and symptom relief, psychological and social support, discharge planning, and end‐of‐life planning – primary outcome. |
| PEP: 12 weeks Cancer | FACT‐G | Functional Assessment of Cancer Therapy‐General Measure (not specified in study) – primary outcome |
| PEP: 6 weeks Mixed diseases comprising cancer and non‐cancer | CRQ HROL (presented in meta‐analysis) EQ‐5D | Measures breathlessness mastery, breathlessness, fatigue, and emotional function – secondary outcome A generic, single index that defines health in the five dimensions of mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression |
| PEP: 12 months COPD | SF‐36 | A generalised self‐assessment scale assessing different dimensions including vitality, mental health, general health, physical functioning, role physical, role emotional, bodily pain, social functioning and health transition |
| PEP: 3 months Cancer | EORTC QLQ‐C30‐Chinese version | Not specified as primary or secondary outcome |
| PEP: not stated but 3 months used in meta‐analysis Cancer | FACT‐G (presented in meta‐analysis) SF‐12 (not used in meta‐analysis because only its first item was used) | No information provided in study on dimensions covered by FACT‐G ‐ secondary outcome |
| PEP: 12 weeks Cancer | EORTC QLQ‐C30 | The EORTC QLQ‐C30 consists of 30 items in 15 scales. In the present study additional items measuring role functioning, cognitive functioning, social functioning, dyspnoea, pain, fatigue, insomnia, appetite loss, nausea/vomiting and constipation were added to the questionnaire to expand these scales to at least four items in each scale. |
| PEP: not stated but appeared to be 6 months. 6 months was used in meta‐analysis Heart failure | MLHF questionnaire | MLHF questionnaire measures heart failure–specific health–related quality of life. No further information provided |
| PEP: on discharge Cancer | EORTC QLQ‐C30 | The scale consists of the 2 subscales 'functional' and ‘symptom'. The functional section is divided into 6 subsections: physical, role, cognitive, emotional, social, and global quality of life. The symptom section includes the following symptoms: fatigue, nausea and vomiting, pain, dyspnoea, sleep disorders, loss of appetite, constipation, diarrhoea, and financial impact – primary outcome |
| PEP: 12 weeks Cancer | FACIT‐Sp | The scale covers physical, social/family, emotional, functional, and spiritual well‐being. |
| PEP: 6 months Heart failure | FACIT‐Pal (presented in meta‐analysis) KCCQ | Assesses quality of life in several domains, including physical well‐being, social/family well‐being, emotional well‐being, functional well‐being, and palliative care – primary outcome The overall summary score is derived from the physical function, symptom, social function, and quality‐of‐life domains. |
| PEP: not stated but data presented at 3 months used in meta‐analysis Heart failure | MLHF questionnaire | The MLHF Questionnaire was created to be representative of the ways HF and treatments can affect key physical, emotional, social, and mental dimensions of QoL. It assess how much a person’s HF has affected many aspects of their life during the prior month – primary outcome |
| PEP: 6 months | SEIQoL‐DW questionnaire | Schedule for the Evaluation of Individual Quality of Life‐Direct Weighting (SEIQoL‐DW). The SEIQoL‐DW is administered in an interview in which respondents nominate the five areas of life that are most important in determining their QoL, and rate the satisfaction/functioning and weight/importance in each of these areas. The SEIQoL‐DW index can range from 0 to 100 (best). |
| PEP: one year Cancer | McGill QoL Questionnaire | Physical symptoms, psychological symptoms, outlook on life, and meaningful existence – primary outcome |
| PEP: 12 weeks Cancer | FACT‐L (presented in meta‐analysis) LCS TOI | Assesses multiple dimensions of the quality of life (physical, functional, emotional, and social well‐being) during the previous week. In addition, the lung cancer subscale (LCS) of the FACT‐L scale evaluates seven symptoms specific to lung cancer – primary outcome |
| PEP: 12 weeks Cancer | FACT‐G | Assesses four dimensions of QoL (physical, functional, emotional, and social well‐being) – primary outcome |
| PEP: 12 weeks Cancer | EORTC QLQ‐C30 (presented in meta‐analysis) McGill QoL questionnaire | Global health status/quality of life scale of the European Organisation for Research and Treatment of Cancer Quality‐of‐Life Questionnaire Core 30 items (EORTC QLQ‐C30; version 3) Single item scale and overall summary score of the McGill Quality of Life questionnaire (MQoL). The MQoL incorporates a single item scale of global quality of life and four subscales, measuring four relevant domains of quality of life (i.e. physical, psychological, existential/spiritual, and social). |
| PEP: 4 weeks Cancer | EORTC QLQ‐C30 (Korean version) | EORTC QLQ‐C30 (Korean version) assesses multiple dimensions of QoL (physical, functional, emotional and social well‐being) during the previous week. |
| COPD: | ||
| Author | Results for Mortality/Survival | P value |
|---|---|---|
| Number of deaths in the sample Intervention: 12 (25%) Control: 12 (25%) | 0.96 | |
| Number of deaths in the sample Intervention: 8 (32%) Control: 13 (54%) | Not stated | |
| Number of deaths in the sample Intervention: 112 (69.6%) Control: 119 (73.9%) Survival time (median, 95% CI) Intervention: 14 months (10.6 to 18.4) Control: 8.5 months (7 to 11.1) | Cox proportional hazards model estimate demonstrated a reduced relative risk of death (HR, 0.67 (95% CI: 0.496 to 0.906) P = .009) in the HSPC group during the first year of the study and a greater relative risk after one year, (HR, 1.56 (95% CI: 0.908 to 2.655)). P for survival time = 0.14 | |
| Number of deaths (authors stated that there were 109 deaths (52.7%) Intervention: numbers not provided Control: numbers not provided Survival time (median) Intervention: 18.3 months Control: 11.8 months | Kaplan‐Meier curves illustrated a 15% difference in survival at 1 year (HSPC, 63% vs control, 48%; P = 0.038). However, for the overall log‐rank test, P = 0.18), suggesting a convergence in overall survival after 12 months. | |
| Number of deaths in the sample Intervention: 10 (6.4%) Control: 13 (8.3%) | 0.52 | |
| Number of deaths in the sample Intervention: 8 (22%) Control: 4 (11.1%) | 0.34 | |
| Number of deaths (authors highlighted 75% deaths among participants) Intervention: numbers not provided Control: numbers not provided Survival time (mean (SD)) Intervention: 196 days (SD:164) Control: 242 days (SD:200) | P = 0.03 However, results of the Kaplan‐Meier survival analysis did not show differences in survival time between study groups (P = 0.08). | |
| Survival time (median, IQR) Intervention: 19 days (12 to 37) Control: 23 days (12 to 39) | P for survival time = 0.51 90‐day survival (HR, 0.95 (95% CI: 0.65 to 1.38), P = 0.96). Post hoc adjustment for baseline activities of daily living and study site did not alter the outcome (HR,1.01 (95% CI; 0.69 to 1.47), P = 0.96) | |
| Number of deaths in the sample Intervention: 7 (70%) Control: 9 (90%) | P = 0.58 | |
| Number of deaths in the sample Intervention: 1 (70%) Control: 3 (11.5%) | P value not stated | |
| Number of deaths in the sample Intervention: 3 (3.7%) Control: 0 | P value not stated | |
| Number of deaths in the sample Intervention: 2 (5.7%) Control: 0 | P value not stated | |
| Number of deaths in the sample Intervention: 1 (2.3%) Control: 1 (2.3%) | P value not stated | |
| Number of deaths in the sample Intervention: 52 (37.4%) Control: 30 (36.6%) | P value not stated | |
| Number of deaths in the sample Intervention: 173 (63%) Control: 132 (56%) Survival time (median, IQR) Intervention: 30 days (6 to 104) Control: 36 days (13 to 106) | P (for difference in number of deaths) = 0.08 P (for difference in survival time) = 0.08 | |
| Number of deaths in the sample Intervention: 25 (27%) Control: 22 (23%) Survival time (median) Intervention: 323 days Control: 364 days | P (for difference in survival time) = 0.16, but in the adjusted analysis P = 0.39 | |
| Number of deaths in the sample Intervention: 41 (59.4%) Control: 44 (65.7%) Survival time (median, 95% CI) Intervention: 289 days (128 to 453) Control: 132 days (80 to 302) | The P value for difference in median survival was 0.20 (log‐rank test) | |
| Number of deaths in the sample Intervention: 1 (3.8%) Control: 3 (11.5%) | P value not stated | |
| Number of deaths in the sample Intervention: 3 (5.7%) Control: 13 (25%) Survival time (median, range) Intervention: 745 (338 to1075) Control: 711 (345 to1045) | P (for survival rate) was 0.048. In subgroup analysis, this pattern was not recorded for patients with cancer (P = 0·97); but it became more marked for patients with diseases other than cancer (P = 0·01). | |
| Number of deaths in the sample (denominator unclear) Intervention: 11 Control: 8 | P = 0.47 | |
| Number of deaths in the sample Intervention: 4 (15.4%) Control: 4 (17.4%) Survival time (unclear if mean or median reported) Intervention: 454 days (95% CI: 382 to 525) Control: 425 days (95% CI: 339 to 509) | Survival did not differ between groups (log‐rank test, P = 0.913). | |
| One‐third of the sample died within 45 days after enrollment, the second third within 120 days but numbers were not provided for the intervention and control groups | Authors reported no difference in the survival patterns of HSPC and control patients | |
| Number of deaths in the sample Intervention: 3 (5%) Control: 0 | P value not stated | |
| Number of deaths in the sample Intervention: 34 (35.1%) Control: 37 (36.3%) | P = 0.87 | |
| Number of deaths in the sample Intervention: 16 (69.6%) Control: 5 (62.5%) | Increment (95% CI) reported as 7 (‐45.1 to 30.4) | |
| Number of deaths in the sample Intervention: 7 (10.6%) Control: 3 (3.8%) | P value not stated | |
| Authors reported that 36 (24.7%) patients died before one month but did not provide numbers in the intervention and control group. | ||
| Number of deaths in the sample Intervention: 1 (4.5%) Control: 1 (5.6%) | P value not stated | |
| Number of deaths in the sample Intervention: 23 (30.7%) Control: 20 (26.7%) | P value not stated | |
| Number of deaths in the sample Intervention: 14 (12.1%) Control: 5 (4.3%) | Results of the survival analysis found no association between study group assignment and death within 6 months after adjustment for age, gender, and marital status. | |
| Number of deaths in the sample Intervention: 3 (3%) Control: 0 | P value not stated | |
| Number of deaths in the sample Intervention: 39 (65%) Control: 31 (51.7%) Survival time (median, 95% CI) Intervention: 7 months (5.2 to 9.8) Control: 11.7 months (9.8 to 18.8) | P (log rank) = 0.014. The estimated HR was 1.6 (95% CI: 1.1 to 2.3; P = 0.015). This estimate changed to 1.5 (95% CI 0.99 to 2.2; P = 0.06) when adjusted for the oncologist’s baseline estimate of likely survival, diagnosis, months since diagnosis, and gender. | |
| Number of deaths (authors stated 105 participants (70%) had died by the time of analysis) Intervention: numbers not provided Control: numbers not provided Survival time (median, 95% CI) Intervention: 11.6 (6.4 to 16.9) months Control: 8.9 (6.3 to 11.4) months | Log‐rank P = 0.02 After adjustment for age, sex, and baseline Eastern Cooperative Oncology Group performance status, the group assignment remained a predictor of survival (HR for death in the standard care group, 1.70; 95% CI, 1.14 to 2.54; P = 0.01). | |
| Number of deaths in the sample Intervention: 33 (18.9%) Control: 41 (23.4%) | P value not stated | |
| Number of deaths (authors stated that 121 (65%) of participants had died by the end of the study) Intervention: numbers not provided Control: numbers not provided Survival time (median, 95% CI) Intervention: 312 days (190 to 434) Control: 343 days (253 to 433) | P = 0.97 | |
| Authors reported that there was no difference in survival between HSPC and usual care but did not present any data | ||
| CI: | ||
| Studies | Participants | Adverse effects in patients/caregivers |
|---|---|---|
| Patients and caregivers | Authors reported no worsening of any outcome after receiving the intervention. | |
| Patients | There were no harmful adverse events attributed to the intervention. | |
| Patients | Authors did not observe any harmful effect of the intervention. | |
| Patients (and caregivers if present) | Authors did not observe any harmful effect of the intervention. | |
| Patients | Authors did not observe any harmful effect of the intervention. | |
| Patients | Authors reported no adverse events during the study. | |
| Patients and caregivers | Authors reported 15 serious adverse events in 13 patients in the HSPC group and 7 in 7 patients in the control group. Serious adverse events reported included aspiration pneumonia, generalised anxiety, breathing difficulty, urine retention/infection, anarthria, contact dermatitis, dysphagia, vomiting, bladder catheter malfunctioning, fever, arrhythmia, necrotising fasciitis, traumatic wound, macrohaematuria, constipation, abdominalgia and bronchitis. Three patients in the HSPC group died but this was considered to be unrelated to the intervention. | |
| Patients | Authors reported that more patients in the HSPC group had poorer appetite compared to the control group (P = 0.04). | |
| HSPC: | ||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | Wilcoxon rank sum test P = 0.53 | Intervention: 0.86 visits Control: 0.63 visits Note: not clear if the figures were means or medians | |
| Total use covering period before and after enrollment | Poisson generalised linear model P = 0.32 for baseline (total sample of 207) P = 0.21 for total use in 109 decedents | Intervention for baseline sample (days, 95% CI): 0.16 (0.1 to 0.25) Control for baseline sample: 0.21 (0.15 to 0.31 Intervention (total use in 50 decedents): 0.14 (0.09 to 0.2) Control (total use in 59 decedents): 0.19 (0.14 to 0.26) | |
| During study period | Reduced ED use in intervention group Cramer’s V 0.15; P = 0.01 linear regression adjusted for survival, age and severity of illness showed intervention reduced ED visits by 0.35 (P = 0.02) | Intervention: 20% had ED visits Control: 33% had ED visits | |
| Admissions to the emergency ward in the year before study enrollment | There was no difference in admissions to the emergency ward in the intervention group compared to the control group (Incidence rate ratio 1.27, 95% CI: 0.72 to 2.26, P = 0.384). | Number of admissions to emergency ward Intervention: 33 Control: 23 | |
| During study period | Admission to the emergency ward was twice as often in the intervention group compared to the control group (incidence rate ratio 2.05, 95% CI: 1.11 to 3.94, P = 0.014). However, after the Benjamini and Hochberg correction for multiple testing, this difference was not significant. | Number of admissions to emergency ward Intervention: 37 Control: 16 | |
| During study period and post‐discharge | Patients in the intervention group had fewer ED visits compared to usual care (P = 0.0067) | % of ED visits: Intervention: 1.3% Control: 12.5% P: 0.0067 | |
| Unclear | P = 0.074 | Intervention: 39 Control: 50 | |
| During study period | P value not stated | Frequency of interactions occurring between patients and providers Emergency department/urgent care: Intervention, mean (SD): 0.4 (0.12) Control, mean (SD): 0.5 (0.11) | |
| During study period | P value not stated | Any emergency department visit from enrollment to death: Intervention: 53.1% Control: 57.1% | |
| P value not stated | Any emergency department visit within 30 days of death: Intervention: 22.4% Control: 30.4% | ||
| CI: confidence intervals | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | Wilcoxon rank sum test P > 0.99 | Intervention: 0.06 days Control: 0.06 days Note: not clear if the figures were means or medians | |
| Total use covering period before and after enrollment | Poisson generalised linear model P = 0.10 for baseline (total sample of 207) P = 0.49 for total use in 109 decedents | Intervention for baseline sample (days, 95% CI): 0.52 (0.28 to 0.95) Control for baseline sample: 0.22 (0.1 to 0.5) Intervention (total use in 50 decedents): 0.1 (0.04 to 0.24) Control (total use in 59 decedents): 0.15 (0.07 to 0.3) | |
| Interviewed surrogate decision‐makers immediately after the second support and information team meeting for the intervention group and 10 days after randomisation for the control group, unless the patient had died. All surrogate decision‐makers were interviewed again by telephone for follow‐up beginning 90 days after randomisation. | Differences between groups for other patient outcomes were analysed based on t tests, nonparametric tests, χ2 tests (including the Fisher exact test), or log‐rank tests as appropriate. For total ICU days, P = 0.51 P value for after randomisation, P = 0.72 | ICU days Total: Intervention, median (IQR): 19 (15 to 26) Control, median (IQR): 20 (15 to 30) After randomisation: Intervention, median (IQR): 9 (6 to 15) Control, median (IQR): 10 (5 to 17) | |
| Enrollment to ICU discharge | Fisher’s exact test and the Mann‐Whitney test P = 0.97 | Intervention: median (IQR) ICU length of stay: 3 (7) days Control: median (IQR) ICU length of stay: 5 (8) days | |
| During study period | Index‐admission Fisher exact test P > 0.99 Up to 180 days Fisher exact test P > 0.99 | Hospital days at 180 days Index‐admission: Since only 1 participant had more than 1 ICU admission, the authors treated the ICU admission as a binary outcome. During the index‐admission, there was no difference between the 2 groups. (Fisher exact test P > 0.99) Up to 180 days: There was no difference between the 2 groups (Fisher exact test, P > 0.99). | |
| 6 months post‐index hospitalisation | P = 0.04 Continuous measures for intervention and usual care patients were compared using t tests for normally distributed measures and Wilcoxon two‐sample tests for measures with skewed distributions. | ICU admissions, median n: Intervention: 12 Control: 21 | |
| Admissions to ICU for respiratory failure in the year before study enrollment | There was no difference in ICU admissions for respiratory failure in the intervention group compared to the control group (Incidence rate ratio 0.88, 95% CI: 0.26 to 2.96, P = 0.82). | Number of ICU admissions for respiratory failure in the year before inclusion: Intervention: 7 Control: 7 | |
| During study period | There was no difference in ICU admissions for respiratory failure in the intervention group compared to the control group (Incidence rate ratio 4.42, 95% CI: 0.49 to 20.92, P = 0.16). | Number of ICU admissions for respiratory failure during the study period: Intervention: 5 Control: 1 | |
| During study period | P value not stated | Mean number of ICU days per patient: Intervention, mean per patient: 0.2 Control, mean per patient: 0.3 | |
| During study period | No difference in ICU duration between intervention and control group (P = 0.38) | ICU duration in days, median (IQR): Intervention: 5 (3 ‐ 8) Control: 5.5 (3 ‐ 10) P: 0.38 | |
| CI: confidence intervals | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| Interviewed surrogate decision‐makers immediately after the second support and information team meeting for the intervention group and 10 days after randomisation for the control group, unless the patient had died. All surrogate decision‐makers were interviewed again by telephone for follow‐up beginning 90 days after randomisation. | Differences between groups for other patient outcomes were analysed based on t tests, nonparametric tests, χ2 tests (including the Fisher exact test), or log‐rank tests as appropriate. Mechanical ventilation, P = 0.41 Dialysis, P = 0.64 Nutrition, P = 0.60 Vasopressors, P = 0.86 | Limitations of ICU treatment Mechanical ventilation: Intervention, median (IQR): 40 (31) Control, median (IQR): 33 (26) Dialysis: Intervention, median (IQR): 13 (10) Control, median (IQR): 15 (12) Nutrition: Intervention, median (IQR): 18 (14) Control, median (IQR): 21 (17) Vasopressors: Intervention, median (IQR): 18 (14) Control, median (IQR): 19 (15) | |
| During study period | The following were lower in the intervention group compared to the control group: tracheostomy (P = 0.035) and days on mechanical ventilation (P = 0.042). | % of patients using mechanical ventilation: Intervention: 53.6% Control: 56.9% P: 0.64 Haemodialysis: Intervention: 15.5% Control: 23.5% P: 0.15 Vasopressors: Intervention: 48.5% Control: 50% P: 0.83 Tracheostomy: Intervention: 1% Control: 7.8% P: 0.035 Cardiopulmonary resuscitation: Intervention: 5.2% Control: 6.9% P: 0.61 Number of days on mechanical ventilation, median (IQR): Intervention: 4 (3 ‐ 7) Control: 6 (3 ‐ 13) P: 0.042 Number of days on vasopressors, median (IQR): Intervention: 3 (1 ‐ 6) Control: 3 (2 ‐ 6) P: 0.91 | |
| ICU: | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | P = 0.92 | Mean number of total admissions Intervention: 1.94 Control: 1.90 | |
| During study period | P = 0.61 | Number of hospitalisations Intervention: 18 patients had 1 hospitalisation 9 patients had 2 or more hospitalisations Control: 30 patients had 1 hospitalisation 6 patients had 2 or more hospitalisations | |
| During study period | P = 0.009 | Number of hospitalisations, mean (SD) Intervention: 0.42 ± 0.60 Control: 1.47 ± 1.81 Total number of hospitalisations: Intervention: 15 Control: 53 | |
| During study period | Reduced hospitalisation in intervention group Cramer’s V 0.23; P < 0.001 | Intervention: 36% were admitted Control: 59% were admitted | |
| During study period | P value not stated | Inpatient: Intervention, n (%), mean (SD) contacts: 2 (7%), 3.0 (2.8) Control, n (%), mean (SD) contacts: 3 (12%), 6.3 (6.8) | |
| During study period | P value not stated | Inpatient: Intervention, n (%), mean (SD) contacts: 6 (15%), 11.5 (8.3) Control, n (%), mean (SD) contacts: 4 (11%), 6.0 (3.4) | |
| Hospital admissions for respiratory failure in the year before study enrollment | There was no difference in hospital admissions for respiratory failure in the intervention group compared to the control group (incidence rate ratio 1.18, 95% CI: 0.61 to 2.31, P = 0.60). | Number of hospital admissions for respiratory failure in the year before inclusion: Intervention: 24 Control: 18 | |
| During study period | Hospital admission for respiratory failure was almost twice as often in the intervention group compared to the control group (incidence rate ratio 1.87, 95% CI: 1.04 to 3.48, P = 0.026). However, after the Benjamini and Hochberg correction for multiple testing, this difference was not significant. | Number of hospital admissions for respiratory failure during study period: Intervention: 38 Control: 18 | |
| Hospital admissions for respiratory failure in the year before study enrollment | There was no difference in hospital admissions for respiratory failure in the intervention group compared to the control group (incidence rate ratio 1.18, 95% CI: 0.36 to 4.12, P = 0.77). | Other hospitalisations in the year before inclusion: Intervention: 8 Control: 6 | |
| During study period | There was no difference in hospital admissions for respiratory failure in the intervention group compared to the control group (incidence rate ratio 1.01, 95% CI: 0.32 to 3.28, P = 0.99). | Other hospitalisations during study period: Intervention: 8 Control: 7 | |
| During study period and post‐discharge | Patients in the intervention group had fewer hospital readmissions compared to usual care (P = 0.024) | % of hospital readmissions: Intervention: 17.3% Control: 33.3% P: 0.024 | |
| Unclear | There was no difference in number of hospitalisations. P value not given | Intervention: 48% Control: 51% | |
| During study period | During the 6‐month follow‐up, 30% of patients were hospitalised for HF. No differences were seen between the 2 treatment groups in this clinical endpoints through the 6‐month follow‐up point. For hospitalisation for non‐heart failure/cardiovascular and hospitalisation for non‐cardiovascular, P value was not stated | Hospitalisation for HF: Intervention: 30.7% Control: 29.3% Hospitalisation for non‐heart failure/cardiovascular: Intervention: 16% Control: 13% Hospitalisation for non‐cardiovascular: Intervention: 10.7% Control: 24% | |
| Inpatient readmission for any cause within 30 days | Survival analysis using proportional hazards regression P = 0.50 | There was no association between study group assignment and 30‐day inpatient readmission (adjusting for age, gender, and marital status). | |
| During study period | P value not stated | Any admission from enrollment to death: Intervention: 73.5% Control: 76.8% | |
| P value not stated | Any admission within 30 days of death: Intervention: 36.7% Control: 53.6% | ||
| CI: | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | Student’s t‐test was used P = 0.46 | Intervention (mean (range)): 8.8 (1 ‐ 93) Control (mean (range)): 9.7 (1 ‐ 63) | |
| During the study | Wilcoxon rank sum test P = 0.14 | Number of hospital days (unclear if mean or median reported) Intervention: 6.6 days Control: 6.5 days | |
| Total use covering period before and after enrollment | Poisson generalised linear model P = 0.03 for baseline (total sample of 207) P = 0.26 for total use in 109 decedents | Intervention for baseline sample (days, 95% CI): 0.69 (0.4 to 1.18) Control for baseline sample: 1.39 (0.97 to 1.97) Intervention (total use in 50 decedents): 0.95 (0.61 to 1.46) Control (total use in 59 decedents): 1.3 (0.91 to 1.86) | |
| During the study period | P value for total hospital days = 0.011. The number of days spent in hospital was also significantly lower in the intervention group at the Departments of Medicine‐Geriatrics (100, range 1–45 vs. 242, range 2–46 days) and Surgery (0 vs. 56, range 2–21 days). Days in other departments did not differ significantly. | Total hospital days, mean (SD) Intervention: 2.9 (8.3) Control: 8.5 (12.4) Days in Department of Medicine‐Geriatrics: Intervention: 100 (range 1 ‐ 45) Control: 242 (range 2 ‐ 46) Days in Department of Surgery: Intervention: 0 Control: 56 Days in other departments: Intervention: 3 (range 1 ‐ 2) Control: 7 (1 ‐ 6) | |
| During the study | Fewer hospital days in intervention group. Linear regression adjusted for survival, age and severity of illness showed intervention reduced hospital days by 4.36 (P < 0.001) | No descriptive data provided | |
| Interviewed surrogate decision‐makers immediately after the second support and information team meeting for the intervention group and 10 days after randomisation for the control group, unless the patient had died. All surrogate decision‐makers were interviewed again by telephone for follow‐up beginning 90 days after randomisation. | Differences in the number of hospital days were analysed using nonparametric methods. P value for total hospital days, P = 0.78 P value for deceased patients, P = 0.60 P value for after randomisation, P = 0.51 | Hospital days Total hospital days: Intervention, median (IQR): 35 (23 to 52) Control, median (IQR): 36 (23 to 54) For deceased patients: Intervention (49 deaths), median (IQR): 25 (18 to 36) Control (51 deaths), median (IQR): 24 (14 to 39) After randomisation: Intervention, median (IQR): 19 (12 to 37) Control, median (IQR): 23 (12 to 39) | |
| During study period | Fisher’s exact test and the Mann‐Whitney test P = 0.44 | Intervention: median (IQR) hospital length of stay: 5 (8) days Control: median (IQR) hospital length of stay: 11 (27) days | |
| During study period | P value not stated | Duration of HCT hospitalisation, median (range): Intervention: 20 (12 – 102) days Control: 21 (13 – 40) days | |
| 6 months post‐index hospitalisation | P value for admission to study enrollment (days), P = 0.36 P value for study enrollment to discharge or death in the hospital (days), P = 0.10 P‐value for index hospital length of stay (days), P = 0.57 Continuous measures for intervention and usual care patients were compared using t tests for normally distributed measures and Wilcoxon two‐sample tests for measures with skewed distributions. | Admission to study enrollment (days), median (IQR): Intervention: 3 (2, 7) Control: 4 (2, 7) Study enrollment to discharge or death in the hospital (days), median (IQR): Intervention: 3 (1, 6) Control: 2 (1, 5) Index hospital length of stay (days), median (IQR): Intervention: 7 (4, 12) Control: 7 (4, 12) | |
| During study period | Index‐admission Wilcoxon test P = 0.67 Up to 180 days Wilcoxon test P = 0.14 | Hospital days at 180 days Index‐admission: The authors found no difference in hospital days between the intervention and usual care groups during the index‐admission (Wilcoxon test P = 0.67). Up to 180 days: The intervention group had slightly more hospital days at 180 days than the usual care group (Wilcoxon test P = 0.14). | |
| 12 weeks following enrollment | Authors stated increased institutional days in control group but P value was not stated. “The control care patients were more likely to be (...) admitted to or seen in hospital”. | Intervention: 4/26 (17%) were institutionalised for mean 19.0 days (SD 21.6) Control: 6/28 (29%) were institutionalised for mean 30.7 days (SD 32.1) | |
| Three months before baseline interview | P value not stated | Hospital inpatient days Intervention, mean (SD): 4.5 (6.8) Control, mean (SD): 4.6 (7.6) | |
| During study period | P value for general medical inpatient days, P < 0.05 P value for intermediate care inpatient days P < 0.05 | Total inpatient days: Intervention, mean per patient: 51 Control, mean per patient: 47.5 General medical: Intervention, mean per patient: 13.2 Control, mean per patient: 20.7 Intermediate care: Intervention, mean per patient: 8.3 Control, mean per patient: 26.5 | |
| During study period | No difference in hospital duration between intervention and control group (P = 0.43) | Hospital duration in days, median (IQR) Intervention: 10 (6 ‐ 15) Control: 11 (6 ‐ 19) P: 0.43 | |
| Unclear | P = 0.808 | Intervention: 78 days Control: 90 days | |
| During study period | P = 0.07 | Intervention, mean (SD): 9.4 (6.27) days Control, mean (SD): 13.9 (11.5) days | |
| During study period | P value not stated | Median inpatient days (range) from enrollment to death: Intervention: 5 (0 – 50) Control: 7 (0 – 45) | |
| IQR: interquartile range | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | P value not stated | Palliative care visits, median (range): All intervention patients had at least 2 palliative care visits during the first 2 weeks of their hospitalisation (median number of visits, 4; range, 2‐7). Intervention participants had at least 4 palliative care visits during their entire hospitalisation (median number of visits, 8; range, 4‐40). Two control patients received a palliative care consultation. A total of 41.8% (146/349) of palliative care visits occurred while a family member was present. | |
| During study period | P = 0.37 | Palliative care contact during the last acute hospital admission: Intervention: 42 patients (86%) Control: 29 patients (78%) |
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | P value for physician visit, P = 0.000 P value for physician, phone calls and prescriptions, P = 0.012 P value for nurse visits, P = 0.003 P value for nurse visits, phone calls and prescriptions P = 0.003 | Hospital outpatient clinic Physician visit, n, median (range): Intervention: 27, 1 (4 – 30) Control: 133, 3 (2 ‐11) Physician, phone calls and prescriptions, n, median (range): Intervention: 42, 3 (0 – 8) Control: 86, 3 (0 ‐10) Nurse visits, n, median (range): Intervention: 4, 1 (0 – 4) Control: 60, 2 (0 ‐27) Nurse, phone calls and prescriptions, n, median (range): Intervention: 8, 1 (0 – 4) Control: 44, 2 (0 ‐ 8) | |
| During study period | P values not stated | Contact with the HSPC team, (numbers): Intervention: 138 patients had at least one face‐to‐face contact Control: 13 patients had at least one face‐to‐face contact | |
| 12 weeks following enrollment | Hospital specialist visits differences and P value not stated | Hospital specialist visits: Intervention: 8 patients (35%) received; mean 1.0 contacts (SD 0.0) Control: 16 patients (76%) received; mean 1.3 contacts (SD 0.7) | |
| During study period | P value not stated | Frequency of interactions occurring between patients and providers Total number of clinic encounter records: Intervention, mean (SD): 21.9 (1.99) Control, mean (SD): 20.8 (1.92) Cardiology: Intervention, mean (SD): 2.3 (0.55) Control, mean (SD): 3.2 (1.0) Rehabilitation clinic: Intervention, mean (SD): 1.4 (0.68) Control, mean (SD): 0.9 (0.48) | |
| During study period | P values not stated | Contact with palliative care physician consultant: Intervention: 51 patients (85%) Control: 8 patients (13.3%) Contact with palliative care physician in the last month of life: Intervention: 16 patients (26.7%) Control: 6 patients (10%) | |
| During study period | P values not stated | PC visits: All the patients assigned to early palliative care, except for one patient who died within 2 weeks after enrollment, had at least one visit with the palliative care service by the 12th week. The average number of visits in the palliative care group was 4 (range, 0 to 8). Ten patients who received standard care (14%) had a palliative care consultation in the first 12 weeks of the study, primarily to address the management of symptoms, with seven patients having one visit and three having two visits. | |
| During study period | P value not stated | Mean number of palliative care visits: Intervention, mean (range): 6.54 (0 to 14) Control, mean (range): 0.89 (0 to 7) Number of palliative care visits split on lung and GI cancer: The authors stated that “we explored characteristics between patients with lung and GI cancer and found no differences in baseline measures or in the number of PC visits among those patients who received intervention. However, the GI cancer cohort had a higher proportion of male patients and a greater number of hospitalisations (P = 0.038) from baseline to week 24 compared with the lung cancer cohort". | |
| During study period | P value not stated for some of the comparisons. However, the authors reported a difference between intervention and control groups for number of consultations with a psychologist (P = 0.02) | Number of consultations from the palliative care team nurse at 18 weeks: Intervention, median (IQR): 3 (1 – 4). 82 patients (89%) had at least one consultation Control, median (IQR): 17 patients (18%) had at least one consultation PC physician at 18 weeks: Intervention: 25 patients (27%) Control: 1 patient (1%) Nurses at 24 weeks: Intervention, median (IQR): 3 (2 – 5). 55 patients (60%) had at least 3 consultations Control, median (IQR): 12 patients (13%) had at least 3 consultations PC physician at 24 weeks: Intervention: 32 patients (35%) had at least one consultation Control: 1 (1%) had one consultation Number of consultations with a psychologist: 18 weeks: Intervention: 34 patients (37%) had at least one consultation Control: 21 patients (22%) had at least one consultation 24 weeks: No difference was found between intervention and control groups. Number of consultations with other professionals: There were no differences between study groups in the number of consultations with a social care nurse (P = 0·87), dietician (P = 0·32), or specialist nurse (P = 0·28) between 18 weeks and baseline; or between 24 weeks and baseline with social care nurse (P = 0·07), dietician (P = 0·95), or specialist nurse (P = 0·99). | |
| During study period | Forwards from enrollment | Consultation with a psychiatrist: The proportions that consulted a psychiatrist (12% vs 12%) were similar in the intervention and control groups. | |
| HSPC: hospital‐based specialist palliative care | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| Total use covering period before and after enrollment | Poisson generalised linear model P = 0.62 | Hospice use: Intervention, rate 95% CI: 0.68 (0.55 to 0.84) Control, rate 95% CI: 0.63 (0.51 to 0.78) | |
| During study period | Primary Healthcare Centre: P‐value for physician, primary healthcare centre (PHC), P = 0.027 P value for physician, phone calls and prescriptions, P = 0.000 P‐value for nurse visits, PHC, P = 0.25 P value for nurse visits, phone calls and prescriptions P = 0.010 Home: P‐value for physician visits, home, P not stated P value for nurse visits, home, P = 0.032 Within the PREFER team there were 158 additional physician visits and 1031 nurse visits at the patient’s home, and 36 phone call and/or drug prescriptions by the physician and 225 phone calls and/or prescriptions by the nurses. Summarising all this, the most striking difference was found between nurse visits in the PREFER group and the usual care group (1075 vs. 230; P =0.000). On the other hand, phone calls and prescriptions by doctors were more common in the usual care group (108 vs. 231), while physician’s visits were somewhat similar (194 vs. 201). | Primary Healthcare Centre Physician, primary healthcare centre (PHC), n, median (range): Intervention: 9, 1 (0 – 3) Control: 54, 2 (0 ‐ 8) Physician, phone calls and prescriptions, n, median (range): Intervention: 30, 1 (0 – 5) Control: 145, 1 (1 ‐ 14) Nurse visits, PHC, n, median (range): Intervention: 29, 1 (0 – 12) Control: 61, 2 (0 ‐ 14) Nurse, phone calls and prescriptions, n, median (range): Intervention: 59, 3 (0 – 9) Control: 153, 4 (1 ‐ 21) Home: Physician visits, home, n, median (range): Intervention: 0, 0 (0 – 0) Control: 14, 2 (1 ‐ 5) Nurse visits, home, n, median (range): Intervention: 11, 2 (1 – 3) Control: 109, 5 (1 ‐ 23) | |
| During study period | Days in hospice care (1of 2 sites only) t 0.52 P = 0.60 | Days in hospice care (1 of 2 sites only): descriptive data not provided | |
| During study period | P values not stated | Breathlessness intervention service: Intervention, n (%), mean (SD) contacts: 27 (96%), 1.9 (2.0) Control, n (%), mean (SD) contacts: 2 (8%), 1.5 (0.7) | |
| P values not stated | GP: Intervention, n (%), mean (SD) contacts: 10 (36%), 1.2 (0.6) Control, n (%), mean (SD) contacts: 13 (50%), 1.3 (0.5) | ||
| During study period | P values not stated | Breathlessness intervention service: Intervention, n (%), mean (SD) contacts: 39 (95%), 2.1 (1.0) Control, n (%), mean (SD) contacts: 2 (5%), 1.5 (0.7) | |
| P values not stated | GP: Intervention, n (%), mean (SD) contacts: 25 (61%), 1.8 (1.2) Control, n (%), mean (SD) contacts: 24 (63%), 1.6 (0.7) | ||
| 6 months post‐index hospitalisation | P = 0.09 Continuous measures for intervention and control patients were compared using t tests for normally distributed measures and Wilcoxon two‐sample tests for measures with skewed distributions | Study enrollment to hospice admission (days), median (IQR): Intervention: 2 (0, 23) Control: 3 (0, 37) | |
| P = 0.04 Continuous measures for intervention and control patients were compared using t tests for normally distributed measures and Wilcoxon two‐sample tests for measures with skewed distributions. | Hospice length of stay (days), median (IQR) Intervention: 24 (7, 94) Control: 12 (4, 48) | ||
| P = 0.5 Categorical measures were tested using 2 tests or Fisher’s exact test. | Patients admitted to hospice, n (%): Intervention: 103 (37.1%) Control: 96 (40.7%) | ||
| During study period | Fisher’s exact test P = 0.85 Chi2 test P = 0.93 | Hospice use at 180 days: Intervention: 28% Control: 25% | |
| 12 weeks following enrollment | General practice: Authors stated less GP contact in intervention group but P values not stated District/practice nurse: P values not stated MS nurse: Authors stated there were no differences (P values not stated) Social services: P values not stated Specialist home visit: P values not stated | General practice: Intervention: 8 (35%) received; M 3.8 contacts (SD 0.5) Control: 11 (52%) received; M 3.4 contacts (SD 1.2) “Control care patients were more likely to be in contact with general practitioners” District/practice nurse: Intervention: 20 (87%) received; M 12.3 contacts (SD 19.7) Control: 13 (62%) received; M 31.9 contacts (SD 50.7) MS nurse: Intervention: 11 (48%) received; M 1.8 contacts (SD 1.8) Control: 7 (33%) received; M 1.1 contacts (SD 0.2) “Receipt of MS nurses was similar in the two groups”. Social services: Intervention: 10 (43%) received; M 6.4 contacts (SD 7.7) Control: 8 (38%) received; M 4.1 contacts (SD 2.4) Specialist home visit: Intervention: 5 (22%) received; M 5.2 contacts (SD 4.5) Control: 0 received Note: authors stated that specialist home visits were most likely to be from the intervention home palliative care team. | |
| During study period | P value not stated | Days at home: Intervention, mean per patient: 44.8 Control, mean per patient: 37.9 | |
| During study period | No difference as increment, mean (95% CI) = 1 (‐6.8, 8.6) | Days at home: Intervention, mean (95% CI): 13.1 (8.5, 17.7) Control, mean (95% CI): 12.1 (5.9, 18.4) | |
| During study period | P values not stated | Frequency of interactions occurring between patients and providers Primary care: Intervention, mean (SD): 4.4 (0.93) Control, mean (SD): 5.2 (0.82) | |
| Hospice use within 6 months of study hospitalisation | Survival analysis using proportional hazards regression P = 0.36 | There was no significant association between study group assignment and hospice use within 6 months (adjusting for age, gender, and marital status). | |
| During study period | P = 0.09 | Median duration of hospice care: Intervention: 11 days Control: 4 days | |
| CI: | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | P value not stated | Breathlessness intervention service: Intervention, n (%), mean (SD) contacts: 22 (79%), 20.3 (20.8) Control, n (%), mean (SD) contacts: 25 (96%), 23.4 (25.2) | |
| 12 weeks following enrollment | P value not stated | Care by informal caregiver: Intervention: 15/23 (65%) received; Mean 152.5 contacts (SD 53.7) Control: 16/21 (76%) received; Mean 151.1 contacts (SD 57.7) | |
| n: number | |||
| Study | Time horizon | Significance and direction | Details |
|---|---|---|---|
| During study period | Pearson chi2 test P = 0.79 | New feeding tube Intervention: 22 (45.8%) Control: 22 (43.1%) | |
| Pearson chi2 test P = 0.66 | Total feeding tube Intervention: 34 (70.8%) Control: 34 (66.7%) | ||
| Pearson chi2 test P = 0.44 | Mechanical ventilation Intervention: 2 (4.2%) Control: 4 (7.8%) | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | Tracheostomy Intervention: 0 Control: 1 | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | CPR Intervention: 0 Control: 3 (5.9%) | ||
| Pearson chi2 test P = 0.16 | Systemic antibiotics (unclear if mean or median presented) Intervention: 73 (79.3) Control: 69 (70.4) | ||
| Interventions during 190 admissions | |||
| Pearson chi2 test P = 0.025 | IV for entire admission (unclear if mean or median presented) Intervention: 61 (66) Control: 79 (81) | ||
| Pearson chi2 test P = 0.30 | Indwelling urinary catheter (unclear if mean or median presented) Intervention: 41 (44.6) Control: 51 (52) | ||
| Pearson chi2 test P = 0.33 | Mechanical restraints (unclear if mean or median presented) Intervention: 13 (54.2) Control: 11 (45.8) | ||
| Student’s t‐test P = 0.14 | Days with restraints (mean) Intervention: 5.18 Control: 6.56 | ||
| Pearson chi2 test P = 0.089 | Daily phlebotomy for at least 50% of admission (unclear if mean or median presented) Intervention: 32 (34.8) Control: 46 (46.9) | ||
| Pearson chi2 test P = 0.461 | Daily sc/im injection for at least 50% of admission (unclear if mean or median presented) Intervention: 16 (17.4) Control: 21 (21.6) | ||
| ns Pearson chi2 test P = 0.12 | >1 complex non‐invasive test (unclear if mean or median presented) Intervention: 10 (11) Control: 4 (4) | ||
| ns Pearson chi2 test P = 0.215 | >1 invasive test (unclear if mean or median presented) Intervention: 5 (4.3) Control: 2 (2) | ||
| Pearson chi2 test P = 0.15 | Number of fingersticks per day in patients receiving insulin (unclear if mean or median presented) Intervention: 1.56 Control: 2.01 | ||
| Decisions to forgo treatments | |||
| Not calculated because expected frequencies < 5 in at least 2 cells | Enteral feeds Intervention: 3 (6.3%) Control: 4 (7.8%) | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | Mechanical ventilation Intervention: 3 (6.3%) Control: 0 | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | Intravenous lines Intervention: 5 (10.4%) Control: 1 (2%) | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | Blood draws Intervention: 4 (8.3%) Control: 0 | ||
| Not calculated because expected frequencies < 5 in at least 2 cells | Antibiotics Intervention: 3 (6.3%) Control: 0 | ||
| Pearson chi2 test P = 0.65 | CPR in‐hospital (unclear if mean or median presented) Intervention: 62 (67.4) Control: 63 (64.3) | ||
| Pearson chi2 test P = 0.10 | CPR nonhospital (unclear if mean or median presented) Intervention: 47 (51.1) Control: 38 (38.8) | ||
| During study period | P = 0.34 Referral to hospice care Fisher exact test P = 0.75 | Referral to palliative care Intervention: 34/145 (23.4%) Control: 39/134 (29.1%) Referral to hospice care Intervention: 6/161 (3.7%) Control: 4/161 (2.5%) | |
| Total use covering period before and after enrollment | Poisson generalised linear model P = 0.54 | Chemotherapy in last 2 weeks of life Intervention, rate (95% CI): 0.08 (0.03 to 0.2) Control, rate (95% CI): 0.05 (0.02 to 0.15) | |
| During study period | Referral to hospice care (1of 2 sites only) Chi2 P = 0.15 Days in hospice care (1of 2 sites only) t 0.52 P = 0.60 | Referral to hospice care (1 of 2 sites only) Intervention: 25% Control: 36% Days in hospice care (1 of 2 sites only) descriptive data not provided | |
| Interviewed surrogate decision‐makers immediately after the second support and information team meeting for the intervention group and 10 days after randomisation for the control group, unless the patient had died. All surrogate decision‐makers were interviewed again by telephone for follow‐up beginning 90 days after randomisation. | Total ventilator days, P = 0.59 After randomisation, P = 0.42 | Ventilator days Total Intervention, median (IQR): 19 (15 to 31) Control, median (IQR): 21 (14 to 35) After randomisation Intervention, median (IQR): 10 (5 to 20) Control, median (IQR): 12 (5 to 27) | |
| Interviewed surrogate decision‐makers immediately after the second support and information team meeting for the intervention group and 10 days after randomisation for the control group, unless the patient had died. All surrogate decision‐makers were interviewed again by telephone for follow‐up beginning 90 days after randomisation. | P = 0.62 | Hospital discharge disposition (81 patients discharged from the hospital in intervention group and 75 in control group). Home Intervention, median (IQR): 15 (19) Control, median (IQR): 18 (24) Home with paid assistance: Intervention, median (IQR): 10 (12) Control, median (IQR): 7 (9) Hospice Intervention, median (IQR): 3 (4) Control, median (IQR): 4 (5) Acute rehabilitation facility Intervention, median (IQR): 22 (27) Control, median (IQR): 15 (20) Long‐term acute care hospital Intervention, median (IQR): 12 (15) Control, median (IQR): 12 (16) Other acute care facility Intervention, median (IQR): 0 Control, median (IQR): 1 (1) Skilled nursing facility Intervention, median (IQR): 19 (23) Control, median (IQR): 16 (21) Other Intervention, median (IQR): 0 Control, median (IQR): 2 (3) | |
| During study period | P value not stated | Other hospital care Intervention, n (%), mean (SD) contacts: 15 (54%), 1.5 (0.8) Control, n (%), mean (SD) contacts: 14 (54%), 1.4 (0.6) | |
| P value not stated | Nurse Intervention, n (%), mean (SD) contacts: 11 (39%), 3.0 (3.8) Control, n (%), mean (SD) contacts: 12 (46%), 1.8 (1.6) | ||
| P value not stated | Other health professionals Intervention, n (%), mean (SD) contacts: 5 (18%), 1.2 (0.4) Control, n (%), mean (SD) contacts: 3 (12%), 1.0 (0.0) | ||
| Social care Intervention, n (%), mean (SD) contacts: 4 (14%), 4.3 (6.5) Control, n (%), mean (SD) contacts: 3 (12%), 15.7 (22.9) | |||
| During study period | P value not stated | Other hospital services Intervention, n (%), mean (SD) contacts: 20 (49%), 1.7 (1.0) Control, n (%), mean (SD) contacts: 19 (50%), 2.5 (3.5) | |
| P value not stated | Nurse Intervention, n (%), mean (SD) contacts: 21 (51%), 2.7 (3.3) Control, n (%), mean (SD) contacts: 16 (42%), 2.5 (2.5) | ||
| P value not stated | Other health services Intervention, n (%), mean (SD) contacts: 14 (34%), 1.5 (1.1) Control, n (%), mean (SD) contacts: 4 (11%), 1.0 (0.0) | ||
| P value not stated | Social and other care Intervention, n (%), mean (SD) contacts: 8 (20%), 5.4 (4.6) Control, n (%), mean (SD) contacts: 9 (24%), 11.3 (22.8) | ||
| During study period | P value not stated | Telephone contact with the HSPC team, n Intervention: 116 patients had at least one telephone contact Control: 9 patients had at least one telephone contact | |
| 12 weeks after enrollment | P value not stated | Palliative care nurse Intervention: 9 (39%) received; M 3.0 (SD 1.5) Control: 0 received Other nurse Intervention: 7 (30%) received; M 40.0 (SD 63.8) Control: 7 (33%) received; M 95.0 (SD 79.6) Specialist (ward) Intervention: 5 (22%) received; M 1.0 (SD 0.0) Control: 7 (33%) received; M 9.6 (SD 12.1) Specialist (other) Intervention: 4 (17%) received; M 1.1 (SD 0.3) Control: 5 (24%) received; M 1.0 (SD 0.0) Occupational therapist/physiotherapist Intervention: 16 (70%) received; M 10.6 (SD 9.9) Control: 14 (67%) received; M 22.5 (SD 47.7) Dietitian/chiropodist Intervention: 12 (52%) received; M 3.5 (SD 2.5) Control: 13 (62%) received; M 2.6 (SD 1.3) Day centre Intervention: 5 (22%) received;M 20.2(SD 21.0) Control: 5 (24%) received; M 20.4 (SD 15.9) Respite care Intervention: 2 (9%) received; M 9.5 (SD 0.7) Control: 5 (24%) received; M 10.0 (SD 5.9) | |
| During study period | P = 0.819 | Use of antibiotics The use of antibiotics (for exacerbations not leading to hospital admission) did not differ between groups during the observation period. | |
| During study period | Major surgical procedures P < 0.05 | Major surgical procedures Intervention, mean per patient: 0.09 Control, mean per patient: 0.01 Minor surgical procedures Intervention, mean per patient: 0.42 Control, mean per patient: 0.30 | |
| Over 80% of both hospice and control patients had no radiation treatments. However, those few who did had as many as 48 treatments, hence the large number. | Radiation treatments Intervention, mean per patient: 7.4 Control, mean per patient: 7.7 | ||
| P = 0.03 | Chemotherapy treatments Intervention, mean per patient: 1.3 Control, mean per patient: 0.49 | ||
| Markgren 2016 (linked to Brannstrom 2014) | During study period | Only the change in patients receiving full target doses of the ACEIs/angiotensin receptor blockers, BBs and MRAs were higher (P = 0.0009) in the intervention arm than in the control arm. | Prescribed medication use In the intervention arm, the percentages of angiotensin converting enzyme inhibitors (ACEIs) and mineralocorticoid receptor antagonists (MRAs) increased at the end of the study from baseline, while loop diuretics decreased. Beta‐receptor blockers (BBs) decreased somewhat in both groups. The number of patients treated with MRAs differed the most between groups, and increased from 10 (28%) to 15 (48%) in the PREFER arm compared with 13 (35%) vs 13 (39%) in the control group. The change in patients receiving full target doses (+8 vs. +1) of the ACEIs/angiotensin receptor blockers, BBs and MRAs were higher (P = 0.0009) in the intervention arm than in the control arm. |
| During study period | CRT device, P = 0.3 ACE1/ARB device, P = 0.2 Diuretics, P = 0.2 Spironolactone/eplerenone, P = 0.9 Beta‐blockers, P = 0.4 | Medications (prescription and over‐the‐counter) in the medication list of patients Guideline‐driven HF therapies CRT device Intervention: 20% Control: 35.7% ACE1/ARB Intervention: 60% Control: 35.7% Diuretics Intervention: 86.7% Control: 64.3% Spironolactone/eplerenone Intervention: 26.7% Control: 28.6% Beta‐blockers Intervention: 66.7% Control: 50% Medications for other conditions Cholesterol‐lowering medication Intervention: 73.3% Control: 50% Anti‐anginal Intervention: 20% Control: 14.3% Diabetes medication Intervention: 13.3% Control: 14.3% Antidepressants Intervention: 20% Control: 28.6% Pain medication (NSAIDS and opioids) Intervention: 53.3% Control: 21.4% Anxiety medication Intervention: 0 Control: 7.1% Constipation Intervention: 26.7% Control: 28.6% | |
| During study period | P value not stated | Referral to palliative care Intervention: 22 (100%) Control: 1 (5%) Referral to social work Intervention: 22 (100%) Control: 20 (100%) Referral to psychiatry Intervention: 1 (4.5%) Control: 1 (5%) | |
| During study period | P value not stated | Frequency of interactions occurring between patients and providers Total number of hospital encounter records Intervention, mean (SD): 2.5 (0.45) Control, mean (SD): 2.4 (0.35) Telephone contact Intervention, mean (SD): 12.6 (1.2) Control, mean (SD): 10.6 (0.88) | |
| During study period | P = 0.05 | Aggressive end‐of‐life care among 105 decedents (chemotherapy within 14 days before death, no hospice care, or admission to hospice 3 days or less before death) Intervention: 54% Control: 33% | |
| Chemotherapy within 30 days of death Intervention: 32.5% Control: 42% | |||
| ACEI: | |||
| Studies | Participants interviewed | Qualitative approach | Findings of the qualitative study | Findings of the quantitative component |
|---|---|---|---|---|
| Bajwah 2015 (patients with interstitial lung disease (ILD)) | 5 patients 5 carers 1 ILD consultant 1 ILD CNS 1 community matron 1 community palliative care nurse 1 GP | Semi‐structured interviews analysed using a constant comparison approach within framework analysis | Findings: Patients and carers interviewed valued the case conference as they felt that it "laid everything on the table" and importantly addressed concerns and anxieties that had been playing on patients’ and carers’ minds. The qualitative work also identified lack of early referral to palliative care by community health professionals, despite requests from patients and carers, and some gatekeeping by hospital health professionals. Themes from patients: Support in the community Crisis management Palliative care, psychological support Advance care planning Themes from health professionals: GPs ‐ collaboration of care and efficiency Community palliative care clinical nurse specialist – individual care plans and practical problems addressed ILD consultant – symptom control ILD CNS – empowering health professionals | Primary outcome: Symptom burden Mean (SD) POS scores at 4 weeks were ‐5.7 (7.5) fast‐track vs ‐0.4 (8.0) control, (mean change difference between the two arms was ‐5.3 (95% CI ‐9.8 to ‐0.7) independent t test P = 0.02); effect size (95% CI) ‐0.7 (‐1.2 to ‐0.1). Secondary outcomes: The secondary outcomes of quality of life, anxiety and depression were superior in the fast‐track arm, and none were worse. |
| Bakitas 2013 (linked to Bakitas 2009) (ENABLE II) (cancer patients) | 35 oncology clinicians comprising 21 physicians and 14 nurse practitioner | Semi‐structured interviews analysed using thematic analysis | Findings: Oncologists believed that integrating palliative care at the time of an advanced cancer diagnosis enhanced patient care and complemented their practice. Five themes comprised oncologists' views on the complementary role of palliative care: (1) “refer early and often,” (2) referral challenges: “palliative” equals “hospice”; “Heme patients are different,” (3) palliative care as consultants or co‐managers, (4) palliative care “shares the load,” and (5) ENABLE II facilitated palliative care integration. Self‐assessment of their practice with advanced cancer patients comprised four themes: (1) treating the whole patient, (2) focussing on quality versus quantity of life, (3) “some patients just want to fight,” and (4) helping with transitions; timing is everything. | Primary outcomes: Quality of life: The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of 4.6 (2) for quality of life (P = 0.02) Symptom intensity The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of ‐27.8 (15) for symptom intensity (P = 0.06) Resource use: Intensity of service did not differ between the 2 groups. Secondary outcomes: The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of ‐1.8 (0.81) for depressed mood (P = 0.02). |
| Maloney 2013 (linked to Bakitas 2009 ) (ENABLE II) (cancer patients) | 53 patients (28 females included) | Semi‐structured interviews analysed using thematic analysis | Findings: Participants' perceptions of intervention benefits were represented by four themes: enhanced problem‐solving skills, better coping, feeling empowered, and feeling supported or reassured. Three themes related to trial participation: helping future patients and contributing to science, gaining insight through completion of questionnaires, and trial/intervention aspects to improve. Participants did not describe participation as burdensome but rather described some inconveniences or disappointments such as non‐attendance of meetings by other participants and disappointment at not being randomised to the intervention group. | Primary outcomes: Quality of life: The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of 4.6 (2) for quality of life (P = 0.02) Symptom intensity The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of ‐27.8 (15) for symptom intensity (P = 0.06) Intensity of service did not differ between the 2 groups. Secondary outcomes: The estimated treatment effects (intervention minus usual care) for all participants were a mean (SE) of ‐1.8 (0.81) for depressed mood (P = 0.02). |
| Talabani 2017 (linked to Brannstrom 2014) (heart failure (HF) patients) | 12 patients from the intervention group (8 men included) | Semi‐structured interviews analysed using content analysis | Findings: Two themes and a total of five categories were identified. The first theme was feeling secure and safe through receiving care at home with the categories: having access to readily available care at home, being followed up continuously and having trust in the team members' ability to help. The second theme was being acknowledged as both a person and a patient, with the following two categories: being met as a person, participating in decisions about one's care and receiving help for symptoms of both HF and comorbidities. The team also offered relatives support, which patients appreciated. | Outcomes: Quality of life: Between‐group analysis revealed that patients receiving HSPC had improved HRQoL compared with controls (57.6 ± 19.2 vs. 48.5 ± 24.4, age‐adjusted P = 0.05). Within‐group analysis revealed a 26% improvement in the HSPC group for HRQoL (P = 0.046) compared with 3% (P = 0.82) in the control group. Quality of life improved by 24% (P = 0.047). Symptom burden: Total symptom burden improved by 18% (P = 0.035) Resource use: Fifteen rehospitalisations (103 days) occurred in the HSPC group, compared with 53 (305 days) in the control group. |
| Farquhar 2014 (cancer patients) | 20 patients (and associated carers) | Semi‐structured interviews analysed using framework analysis | Findings: Breathlessness intervention service (BIS) reduced fear and worry, and increased confidence in managing breathlessness. Patients and carers consistently identified specific and repeatable aspects of the BIS model and interventions that helped. The multidisciplinary staff expertise was repeatedly noted. How interventions were delivered was important with a suggestion that the intervention was delivered through the provision of knowledge, with specialist expertise, which increased patients’ and carers’ confidence. BIS legitimised breathlessness and increased knowledge whilst making patients and carers feel ‘not alone’. | Primary outcome: BIS reduced patient distress due to breathlessness (primary outcome: −1.29; 95% CI −2.57 to −0.005; P = 0.049) significantly more than the control group; 94% of respondents reported a positive impact (51/53) Secondary outcomes: Mean CRQ mastery scores improved only negligibly in the intervention arm and remained stable for controls. No differences were found between trial arms on other CRQ domains (dyspnoea, fatigue or emotional function). Mean anxiety scores (HADS) remained fairly stable (both arms). Mean depression scores decreased slightly in the intervention arm, increasing slightly for controls. There was little change in other patient or carer outcomes. BIS had a 66% likelihood of better outcomes in terms of reduced distress due to breathlessness at lower health/social care costs than standard care (81% with informal care costs included). |
| Farquhar 2016 (Non‐cancer (mostly COPD) | 20 patients (and associated carers) | Semi‐structured interviews analysed using framework analysis | Findings: Patients with non‐malignant conditions and their carers described a range of impacts including reduced fear, anxiety, worry, and feelings of panic, as well as feeling more confident about breathlessness. They valued the multidisciplinary staff expertise (their knowledge and understanding of life with breathlessness), the characteristics of the BIS staff (their approachability and attentiveness) and their reassuring and positive approach, and the time BIS gave them to talk about breathlessness with an expert. They reported that being seen at home was especially helpful. The findings suggest that it was not only the provision of these interventions that was important, but also that how they were delivered was key to their impact: delivery of interventions through the provision of knowledge (why and how interventions work or specific guidance on how and when to use a particular intervention) increased patients’ and carers’ confidence. | Primary outcome: There was no difference between groups in the primary outcome ("distress due to breathlessness"), when compared to standard care, of –0.24 (95 % CI: –1.30, 0.82). Secondary outcomes: Mean CRQ mastery scores improved slightly on both arms with greater improvement in the intervention arm. No differences were found between trial arms on other CRQ domains (dyspnoea, fatigue or emotional function). Mean patient anxiety scores decreased slightly for the intervention arm and increased slightly for the control arm and mean depression scores decreased slightly in the intervention arm and remained stable for controls; no between‐group difference was found. Mean anxiety scores for carers achieved a greater, 1.65‐point, reduction in the intervention arm compared with a 0.15‐point reduction for controls, adjusted difference of –1.22 (95 % CI: –2.84 to 0.40), P = 0.14. There was little change in other patient or carer secondary outcomes. Carers of patients randomised to the intervention arm achieved a greater, 1.03‐point, reduction in their distress due to their patient’s breathlessness compared with a 0.2‐point increase for controls, adjusted difference of –0.42 (95 % CI: –1.86 to 1.02), P = 0.56. BIS resulted in extra mean costs of GBP799, reducing to GBP100 when outliers were excluded. |
| Hopp 2016 (patients with heart failure) | 85 patients | Unclear although the authors stated that clinical records were qualitatively reviewed | Findings: Patients expressed concerns about hospital palliative care as it might prevent them from receiving more aggressive treatment. Most patients did not engage with advanced care options. | Primary outcome: There was no difference between groups in the primary outcome (election vs non‐election of measure of comfort‐oriented care) (difference 9.3%, 95% CI ‐11.8% to 30%; P = 0.12). |
| Veron 2018 (linked to Janssens 2019) (COPD patients) | 18 patients (44.4% females) | Semi‐structured interviews analysed using thematic content analysis | Findings: Patients described poor recollection of the RCT and difficulties understanding the palliative care intervention. No major differences were observed between patients who received the specialised intervention and those who did not. Content analysis emphasised that although they experienced disabling symptoms, participants tended to attribute their limitations to problems other than COPD and some declared that they were not sick. Patients reported restrictions due to oxygen therapy, and the burden of becoming dependent on it. This dependence resulted in intense anxiety, leading participants to focus on the present only. A strong feeling of perceived helplessness emerged from the patients' interviews. | Primary outcomes: Patients in the HSPC group were hospitalised for respiratory failure (incidence rate ratio (IRR) 1.87, 95% CI 1.04 to 3.48, P = 0.026) and admitted to the emergency ward (IRR 2.05, 95% CI 1.11 to 3.94, P = 0.014) twice as often during follow‐up than the control group. However, after the Benjamini and Hochberg correction for multiple testing, none of these differences was significant. Furthermore, median values were identical in both groups (hospitalisation: median (IQR): 0.0 (1 to 2) vs. 1.5 (1 to 4), P = 0.219; admissions to emergency wards: 1.0 (0; 3) vs. 1.0 (0; 4), P = 0.484). Secondary outcomes: There was no difference in HRQoL assessed using the SF‐36 between the HSPC and control group. There was no difference in anxiety and depression measured by the HADS‐anxiety and HADS‐depression between the intervention and control group. At inclusion, 3 patients in each group had completed their advanced care planning (ACP) directives (P = 1.00). At the end of the study, 9 patients (35%) of the intervention group versus 3 (13%) of the control group had completed ACP directives (P = 0.194). There was therefore a difference in the number of patients who wrote their ACP directives in favour of the intervention group (P = 0.023). Survival did not differ between the groups (P = 0.913). 8 deaths occurred, 4 in each group. In the intervention group, survival was 454 days (1.24 years; 95% CI: 382 to 525 vs. 425 days (1.16 years; 95% CI: 339 to 509) in the control group; P = 0.592. |
| Lowther 2018 (linked to Lowther 2015) (HIV patients) | 20 patients (predominantly females (85%)) from the intervention group | Semi‐structured interviews analysed using thematic content analysis | Findings: Patients reported that having time to talk, appropriate pain medication and effective health education was of therapeutic value for their psychological well‐being. Integration of mixed method findings suggested that positive effect in quantitative measures of mental health and well‐being were attributable to the active ingredients of: appropriate medication, effective health education and counselling, and having time to talk in clinical encounters. Mechanisms of action included symptom relief, improved understanding of illness and treatment, and support focussed on articulated concerns. Participants whose quality of life remained static or deteriorated reported concurrent intractable physical or social problems which prevented them from fulfilling their social roles and led to financial difficulties. This in turn led to stress, which was a barrier to positive psychological well‐being. | Primary outcome: In the control group, median pain score on the pain item of the APOS (range: 0 to 5; 0 indicates worst pain) improved from 1.0 (IQR 0.0 to 2.0) at baseline to 5.0 (3.0 to 5.0) at 4 months; in the HSPC group, it improved from 1.0 (0.0 to 2.0) at baseline to 4.5 (3.0 to 5.0) at 4 months. There was no between‐group difference (coefficient ‐0.01, 95% CI ‐0.36 to 0.34, P = 0.95). Secondary outcomes: Person‐centred assessment and care delivered by staff who had received additional training had positive effects on self‐reported mental health‐related quality of life and psychosocial well‐being. |
| Giovannetti 2018 (linked to Solari 2018) (multiple sclerosis) | 12 patients, 15 caregivers, 8 physicians and nine members of HSPC team | Semi‐structured interviews analysed using framework method | Findings: Three themes emerged from the interviews: 'expectations,' 'met and unmet needs', and 'barriers'. Participants described benefits from the intervention such as improved control of symptoms and reduced sense of isolation of the patient‐caregiver dyads. Patient‐caregiver dyads valued the expertise of the HSPC team. Limitations identified that included factors related to experimental design (difficulty of dyads in identifying examiner and team roles, additional burden for caregivers); team issues (insufficient team building/supervision, competing priorities); limitations of the intervention itself (insufficient length, lack of rehabilitation input); and external factors (resource limitations, under‐responsive services/professionals). The referring physician focus groups provided little experiential data. | Primary outcomes: There was greater reduction in symptom burden (POS‐S‐MS) in the HSPC group compared to usual care (P = 0.047). Effect size was 0.20 at 3 months and 0.32 at 6 months. Changes in quality of life (SEIQoL‐DW index) did not differ between the two groups. Secondary outcomes: There were no differences between the secondary patient (POS, HADS, FIM total score) and carer outcomes (ZBI) at three and six months. There were 22 serious adverse events in 20 patients, 15 events in 13 patients in the HSPC group (30%) and 7 events in 7 patients in the control group (27%; P = 0.78). |
| Slota 2014 (linked to Wallen 2012) (cancer patients) | In Wallen 2012, n was unclear while Slota 2014 had 34 participants | Open‐ended, qualitative questions on a questionnaire. Method of analysis stated in Wallen 2012 was transcript‐based analysis while thematic analysis was stated in Slota 2014 | Findings: Patients identified consistent communication, emotional support, and pain and symptom management as positive contributions delivered by the intervention. Consistent communication was described in terms of the team as a whole and their focus on individualising patients’ pain and comfort needs. When describing emotional support or 'being there' participants emphasised the support and reassurance they felt knowing the Pain and Palliative Care Team was available across time. They saw team members as their advocates. | Primary outcomes and secondary outcomes: There was no difference between HSPC and control group. However, for those who remained on study for 12 months, the HSPC group performed better than their standard of care counterparts. |
| ACP: | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 HSPC versus usual care on patient HRQoL: adjusted endpoint values Show forest plot | 10 | 1344 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.15, 0.37] |
| 1.2 HSPC versus usual care on patient HRQoL: adjusted endpoint values (excluding McCorkle 2015) Show forest plot | 9 | 1280 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.18, 0.40] |
| 1.3 HSPC versus usual care on patient HRQoL: unadjusted endpoint values Show forest plot | 9 | 1201 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.11, 0.70] |
| 1.4 HSPC versus usual care on patient HRQoL: unadjusted endpoint values (excluding McCorkle 2015) Show forest plot | 8 | 1137 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.13, 0.78] |
| 1.5 HSPC versus usual care on patient HRQoL: unadjusted change values Show forest plot | 9 | 1278 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.16, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 HSPC versus usual care on patient symptom burden: adjusted endpoint values Show forest plot | 6 | 761 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.41, ‐0.12] |
| 2.2 HSPC versus usual care on patient symptom burden: unadjusted endpoint values Show forest plot | 6 | 833 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.54, 0.20] |
| 2.3 HSPC versus usual care on patient symptom burden: unadjusted endpoint values (excluding McCorkle 2015) Show forest plot | 5 | 769 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.62, 0.24] |
| 2.4 HSPC versus usual care on patient symptom burden: adjusted change values Show forest plot | 4 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐3.27, 0.64] |
| 2.5 HSPC versus usual care on patient symptom burden: adjusted change values (excluding McCorkle 2015) Show forest plot | 3 | 289 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.79 [‐4.29, 0.70] |
| 2.6 HSPC versus usual care on patient symptom burden: unadjusted change values Show forest plot | 6 | 641 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.94, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 HSPC versus usual care on patient satisfaction with care: adjusted endpoint values Show forest plot | 2 | 337 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.14, 0.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 HSPC versus usual care on home deaths Show forest plot | 7 | 861 | Odds Ratio (M‐H, Random, 95% CI) | 1.63 [1.23, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 HSPC versus usual care on pain: adjusted endpoint values Show forest plot | 4 | 525 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.33, 0.01] |
| 5.2 HSPC versus usual care on pain: adjusted change values Show forest plot | 2 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.74, ‐0.20] |
| 5.3 HSPC versus usual care on pain: unadjusted change values Show forest plot | 2 | 291 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐3.05, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 HSPC versus usual care on patient anxiety: adjusted endpoint values Show forest plot | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.2 HSPC versus usual care on patient anxiety: adjusted endpoint values (excluding McCorkle 2015) Show forest plot | 4 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.56, ‐0.65] |
| 6.3 HSPC versus usual care on patient anxiety: unadjusted endpoint values Show forest plot | 4 | 273 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.52, 0.71] |
| 6.4 HSPC versus usual care on patient anxiety: unadjusted endpoint values (excluding McCorkle 2015) Show forest plot | 3 | 209 | Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐3.52, 0.56] |
| 6.5 HSPC versus usual care on patient anxiety: unadjusted change values Show forest plot | 4 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.02, ‐0.21] |
| 6.6 HSPC versus usual care on patient anxiety in different populations: adjusted endpoint values Show forest plot | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.6.1 Cancer populations | 3 | 275 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐3.03, 1.74] |
| 6.6.2 Non‐cancer populations | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐2.45, 0.80] |
| 6.7 HSPC versus usual care on patient anxiety in different populations: adjusted endpoint values (excluding McCorkle 2015) Show forest plot | 4 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.56, ‐0.65] |
| 6.7.1 Cancer populations | 2 | 211 | Mean Difference (IV, Random, 95% CI) | ‐1.91 [‐3.12, ‐0.70] |
| 6.7.2 Non‐cancer populations | 2 | 109 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐2.45, 0.80] |
| 6.8 EPC vs LPC on patient anxiety: adjusted endpoint values Show forest plot | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.8.1 Early palliative care (EPC) | 2 | 221 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐3.94, 2.79] |
| 6.8.2 Late palliative care (LPC) | 3 | 163 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐2.14, 0.52] |
| 6.9 Effect of MDT‐led services on patient anxiety: adjusted endpoint values Show forest plot | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.9.1 MDT‐led services | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.10 Effect of MDT‐led services on patient anxiety: adjusted endpoint values (excluding McCorkle 2015) Show forest plot | 4 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.56, ‐0.65] |
| 6.10.1 MDT‐led services | 4 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.56, ‐0.65] |
| 6.11 HSPC versus usual care on patient anxiety in different countries: adjusted endpoint values Show forest plot | 5 | 384 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.22, 0.96] |
| 6.11.1 Studies from USA | 3 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐3.04, 2.39] |
| 6.11.2 Studies from UK | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.45, 0.42] |
| 6.12 HSPC versus usual care on patient anxiety in different countries: adjusted endpoint values (excluding McCorkle 2015) Show forest plot | 4 | 320 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.56, ‐0.65] |
| 6.12.1 Studies from USA | 2 | 187 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐3.90, 1.00] |
| 6.12.2 Studies from UK | 2 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐2.45, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 HSPC versus usual care on unpaid caregiver anxiety: unadjusted endpoint values Show forest plot | 2 | 351 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐4.27, 2.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 HSPC versus usual care on patient depression: adjusted endpoint values Show forest plot | 8 | 1096 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.34, ‐0.10] |
| 8.2 HSPC versus usual care on patient depression: unadjusted endpoint values Show forest plot | 5 | 350 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.55, 0.04] |
| 8.3 HSPC versus usual care on patient depression: unadjusted endpoint values (excluding McCorkle 2015) Show forest plot | 4 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.65, ‐0.03] |
| 8.4 HSPC versus usual care on patient depression: adjusted change values Show forest plot | 2 | 231 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.10, 0.45] |
| 8.5 HSPC versus usual care on patient depression: unadjusted change values Show forest plot | 4 | 488 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.58, ‐0.18] |
| 8.6 HSPC versus usual care on patient depression as a binary outcome Show forest plot | 3 | 338 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 HSPC versus usual care on unpaid caregiver depression: adjusted endpoint values Show forest plot | 2 | 413 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.18] |
| 9.2 HSPC versus usual care on unpaid caregiver depression: unadjusted endpoint values Show forest plot | 3 | 420 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.70, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 HSPC versus usual care on unpaid caregiver quality of life: unadjusted endpoint values Show forest plot | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 6.11 [0.42, 11.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 HSPC versus usual care on unpaid caregiver burden: adjusted change values Show forest plot | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐5.95, ‐1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 HSPC versus usual care on patient breathlessness: adjusted endpoint values Show forest plot | 5 | 616 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.12] |
| 12.2 HSPC versus usual care on patient breathlessness: unadjusted endpoint values Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.70, ‐0.00] |
| 12.3 HSPC versus usual care on patient breathlessness: unadjusted change values Show forest plot | 2 | 292 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.55, 0.61] |