Cirugía de derivación trabecular ab interno con iStent para el glaucoma de ángulo abierto
Resumen
Antecedentes
El glaucoma es la causa principal de ceguera irreversible. En los estadios iniciales, el glaucoma lleva a la pérdida progresiva de la visión periférica (lateral); en estadios posteriores, da lugar a la pérdida de la visión central que lleva a la ceguera. La presión intraocular (PIO) elevada es el principal factor de riesgo causal modificable de glaucoma. Las técnicas de cirugía para el glaucoma mínimamente invasivas (CGMI), como la cirugía de derivación trabecular ab interno con iStent (Glaukos Corporation, Laguna Hills, CA, EE.UU.), se han introducido como una nueva modalidad de tratamiento para la enfermedad. Sin embargo, no se conoce la efectividad de la CGMI para mantener a los pacientes "libres de gotas" (es decir, que no tengan que usar las gotas oculares para controlar la PIO) y otros resultados.
Objetivos
Evaluar la efectividad y la seguridad de la cirugía de derivación trabecular ab interno con iStent (o iStent inject) para el glaucoma de ángulo abierto en comparación con el tratamiento convencional médico, con láser o quirúrgico.
Métodos de búsqueda
El especialista en información del Grupo Cochrane de los Ojos y de la Visión (Cochrane Eyes and Vision's Information Specialist ) buscó en las siguientes bases de datos el 17 agosto 2018: Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), que contiene el el registro de ensayos del Grupo Cochrane de Trastornos de los Ojos y la Visión (Cochrane Eyes and Vision Trials Register, número 7, 2018), MEDLINE Ovid, Embase Ovid, en el ISRCTN registry, en ClinicalTrials.gov y en la World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). No se aplicaron restricciones de fecha ni de idioma. Se buscó en las listas de referencias de los informes de los estudios incluidos.
Criterios de selección
Se incluyeron ensayos controlados aleatorios (ECA) que habían comparado iStent o iStent inject con el tratamiento médico, el tratamiento con láser, la cirugía por glaucoma convencional (trabeculectomía) u otros procedimientos de CGMI. Se incluyeron ECA que habían comparado iStent o iStent inject en combinación con la facoemulsificación versus la facoemulsificación sola.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. Dos autores de la revisión examinaron de forma independiente los resultados de la búsqueda, evaluaron el riesgo de sesgo y extrajeron los datos de los informes de los ECA incluidos mediante un formulario electrónico de recopilación de datos.
Resultados principales
Se incluyeron siete ECA (765 ojos de 764 participantes; intervalo por estudio: 33 a 239 participantes) que evaluaron el iStent en pacientes con glaucoma de ángulo abierto. También se identificaron 13 estudios que están en curso o en espera de publicaciones de resultados. La mayoría de los participantes de los estudios incluidos fueron mujeres (417/764 (55%) participantes) y de mayor edad (intervalo de edad: 49 a 89 años). Se evaluó la mayoría de los ensayos como de riesgo de sesgo poco claro o alto: cuatro ensayos no informaron de manera clara sobre el método de generación de la secuencia aleatoria ni la ocultación de la asignación; cinco eran estudios no ocultados y no enmascarados, en que el riesgo de sesgo de realización y de detección se evaluó como alto. Los siete ensayos fueron financiados por la Glaukos Corporation. La certeza de la evidencia se calificó como muy baja.
Cuatro ECA compararon el iStent en combinación con la facoemulsificación versus la facoemulsificación sola. El cálculo resumido derivado de dos de los cuatro ECA indicó que en los participantes del grupo de iStent combinado con la facoemulsificación fue 1,38 veces más probable que no usaran gota a los seis a 18 meses que los del grupo de facoemulsificación sola (cociente de riesgos [CR] 1,38; intervalo de confianza [IC] del 95%: 1,18 a 1,63; I2 = 67%). Los datos de dos ECA también indicaron que el iStent en combinación con la facoemulsificación versus la facoemulsificación sola puede haber llevado a una reducción pequeña en el número de gotas para disminuir la PIO (diferencia de medias [DM] –0,42 gotas; IC del 95%: –0,60 a –0,23). No se sabe si hubo diferencias en cuanto a la reducción media de la PIO del valor inicial (ningún metanálisis).
Dos ECA compararon el tratamiento con iStent con el tratamiento médico; uno de los dos ensayos usó el iStent inject. Se determinó que los dos ensayos eran clínica y metodológicamente heterogéneos y no se realizó un metanálisis; sin embargo, los investigadores de ambos ensayos informaron que más de un 90% de los participantes en los grupos de tratamiento no utilizaban gotas en comparación con ningún participante de los grupos de tratamiento médico a los seis a 18 meses.
Un ECA comparó el tratamiento con uno versus dos versus tres iStents. No hubo diferencias en cuanto a los participantes que no utilizaban gotas a los 36 meses o menos; sin embargo, en el seguimiento más prolongado (es decir a 42 meses), fue más probable que los participantes del grupo de tratamiento con un iStent no usaran gotas en comparación con los grupos de dos iStent (CR 0,51; IC del 95%: 0,34 a 0,75) o tres iStent (CR 0,49; IC del 95%: 0,34 a 0,73). El estudio no informó del cambio medio en el número de gotas para disminuir la PIO.
El tipo y el momento de aparición de las complicaciones informadas variaron entre los ECA. Una proporción similar de participantes sometidos al tratamiento con iStent en combinación con la facoemulsificación y los sometidos a la facoemulsificación sola necesitaron una cirugía secundaria para el glaucoma. Ninguno de los ECA informó hallazgos relacionados con la calidad de vida.
Conclusiones de los autores
Hay evidencia de muy baja calidad de que el tratamiento con iStent puede llevar a una proporción mayor de participantes que no utilizan gotas o logran un mejor control de la PIO en el corto, medio o largo plazo. Los resultados de los 13 estudios con resultados aún no disponibles podrían aclarar los beneficios del tratamiento con iStent para los pacientes. Además, los estudios de CGMI futuros deben considerar la posibilidad de medir la calidad de vida y los resultados que representen la capacidad de los pacientes para realizar las actividades que dependen de la visión.
PICOs
Resumen en términos sencillos
iStent para el glaucoma de ángulo abierto
¿Cuál era el objetivo de esta revisión?
El objetivo de esta revisión Cochrane fue determinar si la implantación de uno o más iStent o dispositivos inject iStent ("iStents"), en comparación con los tratamientos convencionales médicos, con láser o quirúrgicos puede evitar que los pacientes con glaucoma primario de ángulo abierto utilicen gotas para el glaucoma (es decir, mantenerlos "libres de gotas"). Las gotas para el glaucoma se usan para controlar la presión del líquido en el interior de los ojos (presión intraocular [PIO]). También se consideró el cambio promedio del valor inicial en el número de gotas para el glaucoma necesarias para controlar la PIO, el cambio promedio del valor inicial (es decir, antes del tratamiento) en la PIO y la calidad de vida relacionada con la salud, según la definición de los investigadores de los estudios. Se examinaron todos los resultados a corto plazo (menos de seis meses), a medio plazo (seis a ≤ 18 meses), a largo plazo (> 18 meses y ≤ 36 meses) y en el plazo de más de 36 meses. Se recopilaron y analizaron todos los ensayos controlados aleatorios relevantes (ECA; estudios clínicos en que los pacientes se asignan al azar a uno de dos o más grupos de tratamiento) para responder esta pregunta y se hallaron siete ECA que evaluaron los iStents.
Mensajes clave
Hubo evidencia de calidad muy baja de que el tratamiento con iStents puede haber llevado a una proporción mayor de pacientes que no utilizaban gotas en el medio plazo o en quienes se logró un mejor control de la PIO. Ninguno de los siete ECA examinó cómo el iStent afectó la calidad de vida, y el informe sobre las complicaciones fue muy variable. Actualmente, las decisiones de práctica clínica deben basarse en el criterio del profesional y la preferencia del paciente, dada la incongruencia en los resultados y el riesgo de sesgo en los estudios relevantes publicados hasta la fecha.
¿Qué se estudió en esta revisión?
El glaucoma es un grupo de enfermedades oculares que causan daño al nervio óptico. Si no se trata, el glaucoma puede provocar ceguera. La PIO elevada es el único factor de riesgo conocido modificable de glaucoma de ángulo abierto, que es la forma más común de glaucoma. Los tratamientos convencionales de primera elección para el glaucoma de ángulo abierto incluyen intervenciones médicas (p.ej. gotas para el glaucoma) o con láser. La cirugía, que tiene un perfil de riesgos mayor, se ofrece cuando el glaucoma progresa a pesar del tratamiento con medicación o láser.
La cirugía para el glaucoma mínimamente invasiva incluye la implantación de dispositivos como el iStent. Se han propuesto como una opción más segura a las intervenciones quirúrgicas estándar por glaucoma en pacientes con formas leves a moderadas de glaucoma de ángulo abierto. El iStent crea una "derivación" entre las cámaras anteriores del ojo y la vía de drenaje natural. Esta derivación aumenta el flujo de líquido por fuera del ojo, que puede reducir la PIO y la necesidad de usar gotas para el glaucoma para controlarla.
¿Cuáles son los principales resultados de esta revisión?
Se identificaron cuatro ECA que asignaron al azar a los participantes al tratamiento con iStents en combinación con cirugía de cataratas (facoemulsificación) o con facoemulsificación sola. Además, se identificaron dos ECA que asignaron al azar a los participantes al tratamiento con iStents o a las intervenciones médicas. También se identificó un ECA que asignó al azar a los participantes al tratamiento con un iStent, con dos iStents, o con tres iStents. El fabricante del iStent aportó el financiamiento y patrocinio para todos los ECA de esta revisión.
Sobre la base de la evidencia de calidad baja, se halló que fue más probable que los participantes que recibieron iStent en combinación con cirugía de cataratas no utilicen gotas oculares y presenten una reducción moderada del número de gotas para el glaucoma usadas por día para el control de la PIO a medio plazo, en comparación con los participantes que fueron sometidos a la cirugía de cataratas sola; sin embargo, no hubo diferencias en el cambio promedio del valor inicial en la PIO entre los dos grupos.
Debido a la heterogeneidad significativa, no se realizó un análisis de los dos estudios que compararon el tratamiento con iStent con el tratamiento médico. Los investigadores de los dos estudios informaron que ningún participante del grupo de tratamiento médico utilizaba gotas a los 12 meses, en comparación con más de un 90% en los grupos de tratamiento con iStent. Los datos indicaron que el tratamiento con dos o tres iStents puede haber sido más efectivo que el tratamiento con un iStent en cuanto al control de la PIO.
Ninguno de los siete estudios incluidos en esta revisión aportó información sobre la calidad de vida, y las diferencias en las complicaciones o los efectos secundarios entre los grupos de tratamiento fueron inciertas, dado el escaso número de eventos y la efectividad variable.
¿Cuál es el grado de actualización de la revisión?
Se buscaron estudios publicados hasta 17 agosto 2018.
Authors' conclusions
Summary of findings
| iStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with phacoemulsification alone | Risk with iStent in combination with phacoemulsification | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | 583 per 1000 | 804 per 1000 | RR 1.38 | 239 | ⊕⊝⊝⊝ | Estimate based on data from 2 trials. |
| Mean change in number of IOP‐lowering drops from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in number of IOP‐lowering drops from baseline ranged from –1.0 to 0.9 drops | MD 0.42 drops fewer | — | 282 | ⊕⊝⊝⊝ | In addition, Fernandez‐Barrientos 2010 reported the change in number of IOP‐lowering drops was 0 (SD 0) in the iStent in combination of phacoemulsification treatment group and 0.7 (SD 1) in the phacoemulsification alone group. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline ranged from –8.5 to –1.6 mmHg | MD 1.24 mmHg lower | — | 284 | ⊕⊝⊝⊝ | — |
| Health‐related quality of life | — | — | — | — | Not reported in any of the 4 studies. | |
| Intraoperative complications | — | — | — | — | Samuelson 2011 reported that "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery." | |
| Postoperative complications | Based on available data, participants who were randomized to treatment with phacoemulsification in combination with iStent were less likely to experience elevated IOP (or IOP spikes) and loss of vision than those randomized to phacoemulsification alone. | — | 334 (4 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery Follow‐up: range 6 to ≤ 18 months | 1 participant randomized to treatment with phacoemulsification in combination with iStent and 1 participant randomized to phacoemulsification alone underwent selective laser trabeculoplasty at 12 months. | — | 290 (3 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high or unclear risk of bias for blinding of outcome assessor. | ||||||
| IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with medical therapy | Risk with iStent (or iStent inject) | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | At 12 months, 0/138 participants randomized to medical therapy were drop‐free at 12 months, while 141/148 (95%) participants randomized to treatment with iStent were drop‐free at 12 months. We did not derive an RR because no events occurred in the control groups of either trial. | — | 286 | ⊕⊝⊝⊝ | In addition, Vold 2016 noted that 48/54 (88%) participants in the iStent treatment group were drop‐free at 36 months. | |
| Mean change in number of IOP‐lowering drops from baseline | — | — | — | — | — | Not reported in either study. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline was –11.6 mmHg | MD 0.6 mmHg lower | — | 184 | ⊕⊝⊝⊝ | Vold 2016 did not report mean change in IOP but did provide mean IOP (without SD) at 6 months (14.2 mmHg), 18 months (13.5 mmHg), and 36 months (14.6 mmHg) in the iStent treatment groups; and at 6 months (13.8 mmHg), 18 months (14.6 mmHg), and 36 months (15.3 mmHg) in the medical therapy group. |
| Health‐related quality of life | — | — | — | — | Not reported in either study. | |
| Intraoperative complications | 1 participant in the iStent treatment group experienced hyphema which resolved by day 1 | — | 101 (1 RCT) | — | We did not conduct a meta‐analysis of complications. | |
| Postoperative complications | Vold 2016 noted that best‐corrected visual acuity was stable between both groups and did not report on any other postoperative complications. Fea 2014 reported that 1 participant in the iStent inject group experienced IOP decompensation with an elevated IOP of 48 mmHg. | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery | Fea 2014 reported that 1 participant needed laser treatment to remove an apparent obstruction | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision due to small sample size/wide confidence interval. | ||||||
Background
Description of the condition
Glaucoma is a group of diseases characterized by clinical and histopathological manifestations of optic nerve damage that leads to irreversible vision loss (Allingham 2010). Glaucoma is the second leading cause of blindness, affecting approximately 60 million people worldwide (Quigley 2006). One systematic review estimated that the global prevalence of glaucoma in people between 40 and 80 years of age may increase to 76 million by 2020 and to 111.8 million by 2040 (Tham 2014). Open‐angle glaucoma (OAG) is the most common type of glaucoma and accounts for approximately 74% of all cases (Quigley 2006). Women comprise 55% of OAG cases, and OAG disproportionately affects people of African ancestry and older adults (NEI 2015).
OAG is a progressive disease. In early mild‐to‐moderate stages, there are no symptoms (AAO 2015). Due to the 'silent' nature of OAG, people do not usually have any visual problems; there are optic nerve abnormalities consistent with glaucoma, but little to no aberrations in visual fields. In severe stages of glaucoma, people may notice vision loss or blind spots due to significant amounts of irreversible optic nerve damage (AAO 2015). Main signs of glaucoma include atrophied optic nerve and presence of an open angle, both of which can only be seen using specialized instruments.
Many people with OAG also experience elevated intraocular pressure (IOP); however, IOP is not a direct measure of structural or functional glaucomatous optic neuropathy and not all people with glaucoma present with elevated IOP (AAO 2015; Le 2016; Medeiros 2015). Nevertheless, because IOP is the only known modifiable risk factor, treatment for OAG has focused predominantly on lowering IOP (Li 2016; Quigley 2007).
Description of the intervention
Lowering IOP is achieved through medical, laser, and surgical interventions, typically implemented in a step‐wise manner (AAO 2015; Le 2018a; NICE 2009). Since the early 2000s, a series of new treatment modalities, which the US Food and Drug Administration (FDA) refers to as "minimally invasive glaucoma surgical" (MIGS) devices, has emerged. MIGS are ab interno procedures that require minimal to no conjunctival manipulation or scleral dissection, which are readily combined with another intraocular procedure such as cataract extraction by phacoemulsification (cataract surgery). MIGS typically lower IOP to a more modest degree than traditional filtering surgeries (e.g. trabeculectomy or tube shunt implantation); however, MIGS may pose fewer risks than those more invasive surgeries (Caprioli 2015; Francis 2011; Spaeth 2015). While MIGS generally are not used as first‐line therapy for glaucoma at this time, they may reduce the need for medication.
Examples of MIGS interventions include the iStent and iStent Inject, trabectome ab interno trabeculectomy, endoscopic cyclophotocoagulation (ECP), gonioscopy‐assisted transluminal trabeculotomy (GATT), the Hydrus Microstent intracanalicular scaffold, the XEN Gel Stent, and the Innfocus Microshunt. Of these, the first four are currently FDA approved for use in the US; the others are being evaluated in clinical trials.
This Cochrane Review examined the iStent and iStent inject (Glaukos Corporation, Laguna Hills, CA, USA), the former of which was the first MIGS device to have received FDA approval, for people with mild‐to‐moderate OAG.
-
The iStent is a heparin‐coated non‐ferromagnetic titanium 'L‐shaped' device, 1 mm in length with a head 0.3 mm in height facing the anterior chamber (Glaukos 2016). This MIGS device is preloaded into a single‐use injector and then inserted ab interno through the trabecular meshwork under direct gonioscopic view (Manasses 2016). The iStent creates a permanent opening that directly connects the anterior chamber to Schlemm's canal.
-
The iStent inject is a second‐generation 'mushroom‐shaped' MIGS device, 360 μm in length with a conical head with maximum width of 230 μm. Like the iStent, the iStent inject is made of heparin‐coated titanium but the conical head contains four evenly spaced outlets that allow fluid to pass from the anterior chamber into Schlemm's canal (Bahler 2012). The injector is preloaded with two iStent inject MIGS devices and is designed to deliver both stents, ab interno, into Schlemm's canal while entering the eye only once (Bahler 2012; Klamann 2015).
How the intervention might work
IOP increases when there is an imbalance between production and outflow of aqueous humor (a clear fluid that provides avascular ocular structures with nutrition). Aqueous humor drains through a complex network of cells and tissue (trabecular meshwork, Schlemm's canal, and collector channels) in an area known as the drainage angle (AAO 2015).
Given that the trabecular meshwork is the primary site of aqueous outflow and that resistance to aqueous humor outflow in this region largely determines IOP (Manasses 2016), bypassing the trabecular meshwork is a viable method to decrease IOP. Ab interno implantation of MIGS devices such as the iStent and iStent inject may increase outflow facility by providing direct access to Schlemm's canal and downstream collector channels via a permanent opening through trabecular meshwork (Francis 2011).
Why it is important to do this review
Most treatments for OAG rely primarily on lowering IOP (AAO 2015; AGIS 2000; EGS 2014), but they all have limitations. Many people with mild‐to‐moderate OAG elect to start with medical treatment (e.g. topical eye drops) as first‐line therapy (AAO 2015; Li 2016; NICE 2009); commercially available eye drops have short durations of effect and adherence is poor (Friedman 2009; Okeke 2009). Conventional surgical procedures to bypass the trabecular meshwork and drainage angle, such as trabeculectomy and tube shunts or valves, are associated with variable frequencies of success and complications (Gedde 2012a; Gedde 2012b; Spaeth 2015). Trabeculectomies fail after about five years in approximately 50% of cases (Gedde 2012a; Gedde 2012b; Kirwan 2013; Lichter 2001). Laser trabeculoplasty (LTP) represents an intermediate intervention between drops and surgery, or can be used as an alternative first line to drops, but its efficacy has been noted to decrease over time and most people ultimately require repeat LTP or surgery (Leahy 2015; Patel 2015; Rolim de Moura 2007; Woo 2015).
MIGS procedures are becoming increasingly common, with their proponents claiming better safety profiles than other glaucoma surgical techniques (Brandao 2013; Larsen 2017). In this review, we specifically examined the evidence for the effectiveness and safety of one type of MIGS device – the iStent and iStent inject – in people with mild‐to‐moderate OAG of any type. This review was undertaken as part of the Cochrane Eyes and Vision MIGS Consortium. The Consortium also reviewed other types of MIGS techniques and devices including the Trabectome (NeoMedix, Tustin, CA, USA), Hydrus Microstent (Ivantis, Irvine, CA, USA) (Otarola 2017), ECP (Endo Optiks, Waltham, MA, USA) (Tóth 2019), and XEN Glaucoma Implant (AqueSys Implant, Aliso Viejo, CA, USA) (King 2018).
Objectives
To assess the effectiveness and safety of ab interno trabecular bypass surgery with iStent (or iStent inject) for open‐angle glaucoma in comparison to conventional medical, laser, or surgical treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs), prepared in any language, irrespective of their publication status.
Types of participants
We included studies of people with mild‐to‐moderate OAG of any type, including primary and secondary OAG. Primary OAG refers to glaucoma that develops due to an unknown cause; secondary OAG develops from a known cause, such as trauma to the eye or ocular inflammatory diseases. In the absence of a universally accepted definition for glaucoma, we permitted studies to use their own criteria to define OAG; however, we excluded studies of participants with angle‐closure glaucoma (where increased IOP occurs because abnormal iris anatomy obstructs aqueous flow to the drainage angle). In addition, we allowed studies that included participants with ocular hypertension (OHT), normal tension glaucoma, or possible OAG (i.e. suspected).
Types of interventions
We included studies that compared iStent or iStent inject (Glaukos Corporation, Laguna Hills, CA, USA) to any of the following:
-
laser treatment (selective LTP or argon LTP);
-
other MIGS procedures/techniques;
-
conventional glaucoma surgery (trabeculectomy);
-
medical therapy; or
-
in combination with phacoemulsification compared with phacoemulsification alone. iStent devices are approved in people undergoing phacoemulsification.
Additionally, we conducted stratified analyses based on iStent procedures (e.g. iStent versus iStent inject).
Types of outcome measures
We did not use reporting of particular outcomes as a criterion for including a trial into our systematic review. We, as with other review teams in the Consortium, adapted primary and secondary outcomes from a Cochrane systematic review prepared by Hu and colleagues (Hu 2016).
We evaluated each outcome at a time point in the six to 18 months (medium‐term) time window, in addition to less than six months (short‐term), over 18 but less than or equal to 36 months (long‐term), and over 36 months time windows. We recognized that our primary outcome may not be relevant in RCTs that randomized participants to medical therapy in lieu of an iStent procedure.
Primary outcomes
-
Proportion of participants who were drop‐free (i.e. not using eye drops) at a time point in each of the time windows.
Secondary outcomes
-
Mean change in number of IOP‐lowering drops taken per day from baseline to a time point in each of the time windows.
-
Mean change in IOP, measured using Goldmann applanation tonometry, from baseline to a time point in each of the time windows.
-
Any health‐related quality of life measures, measured as mean change from baseline or proportion meeting a threshold at a time point in each of the time windows, as defined by the investigators of the included trials.
Adverse outcomes
-
Proportions of participants experiencing intra‐ and postoperative complications at a time point in each of the time windows, including but not restricted to the following:
-
loss of visual acuity of more than 2 Snellen lines, or more than 0.3 logMAR, according to the method of recording visual acuity; or loss of light perception;
-
bleeding, as recorded by the investigators;
-
endophthalmitis, as recorded by the investigators;
-
IOP spikes, defined as postoperative rise in IOP, measured using Goldmann applanation tonometry, of more than 10 mmHg compared to the previous assessment, including during the first postoperative month;
-
secondary glaucoma surgery, including laser, as recorded by the investigators of the included trials.
-
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. We used no restrictions on language or year of publication.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 7; which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 17 August 2018; Appendix 1).
-
MEDLINE Ovid (1946 to 17 August 2018; Appendix 2).
-
Embase Ovid (1980 to 17 August 2018; Appendix 3).
-
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 17 August 2018; Appendix 4).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 17 August 2018; Appendix 5).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 17 August 2018; Appendix 6).
-
US FDA website (www.fda.gov; searched 17 August 2018; Appendix 7).
Searching other resources
We searched the reference lists of included studies for possible studies and the website of the manufacturer for information regarding forthcoming trials (Glaukos 2016).
Data collection and analysis
Selection of studies
Two review authors (JL and TL) worked independently to screen titles and abstracts of all records identified by the search using web‐based review management software (Covidence 2015). We removed duplicates from the search results. The review authors classified each record as either relevant (a 'Yes' vote) or not relevant (a 'No' vote) for full‐text review. The two review authors independently assessed the full‐text copies of all titles and abstracts that they identified as relevant to determine if the reports met the inclusion criteria (an 'Include' vote) or not (an 'Exclude' vote). We did not need to contact the trial authors of any record to clarify details necessary to make a complete assessment of the eligibility. We documented reasons for exclusion for each study assessed as not eligible after review of the full‐text articles. We resolved all discrepancies between review authors by discussion at each stage of the screening process. We then linked multiple reports originating from the same trial.
Data extraction and management
Two review authors (JL and LW) independently extracted data using a web‐based electronic data collection form in SRDR (srdr.ahrq.gov/). We extracted the information as described in Appendix 8, including: study setting, countries where recruitment took place, sample size, study duration and follow‐up time, study design, analysis choice, sources of funding, and potential conflicts of interests; characteristics of the participants (e.g. inclusion/exclusion criteria), underlying disease conditions, and medical history (including IOP at baseline, number of glaucoma medications at baseline, visual acuity, and other vision‐related characteristics); interventions (e.g. iStent or iStent inject) and comparators (e.g. type of laser, drugs, surgery, duration, and timing); outcomes (e.g. domain, specific measurement, specific metric, method of aggregation, and time frame); and quantitative results.
The review authors compared the extracted data and resolved discrepancies by discussion. One review author (JL) completed data entry into Review Manager 5 (Review Manager 2014), and a second review author (LW) verified the data entered.
Assessment of risk of bias in included studies
Two review authors (JL and LW) independently assessed the risk of bias in included studies, following guidance described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Specific items for consideration included random sequence generation and allocation concealment (selection bias), masking of study personnel (performance bias), masking of outcome assessors (detection bias) for number of IOP‐lowering drops used and for IOP measurement, missing data and intention‐to‐treat analysis (attrition bias), and selective outcome reporting (reporting bias).
We assigned each item as having low risk, high risk, or, if the information provided was insufficient to make an assessment, unclear risk. We documented reasons for those assessments and resolved discrepancies through discussion. We presented the overall assessments as the 'Risk of bias' summary figure (Higgins 2017).
Measures of treatment effect
We used mean difference (MD) as the measure of effect for all continuous outcomes, with 95% confidence intervals (CI). We used risk ratio (RR) with 95% CIs as the measure of effect for all binary and categorical outcomes.
Unit of analysis issues
We assessed whether the included trials included one or both eyes from each participant and whether the trial investigators randomized (and analyzed) at the participant‐level or at the eye‐level. When both eyes were randomized to different treatments, we planned to extract the results that had accounted for the correlation.
Dealing with missing data
Where data on included studies were unclear or missing, we planned to write to the authors and analyze the data using the best information available if we received no response within two weeks. We planned to consider multiple imputation or other imputation approaches for handling missing data if needed. If the quality of the available data prevented any meaningful analysis, we planned to omit the study from quantitative analyses and note this decision in the discussion.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining participant characteristics, MIGS techniques and devices, and outcomes, taking into consideration potential risk of bias. We assessed forest plots and examined the I2 statistic and its CI for statistical heterogeneity. Similar to other protocols on MIGS procedures, we considered an I2 statistic greater than 50% as indicative of substantial heterogeneity, suggesting that a meta‐analysis may not be appropriate. However, if all estimates were in the same direction, we pursued a meta‐analysis despite substantial statistical heterogeneity, and we interpreted the findings taking into consideration the heterogeneity.
Assessment of reporting biases
We planned to assess small‐study effects using funnel plots if there were more than 10 trials for each meta‐analysis. We assessed selective reporting as part of the 'Risk of bias' assessment, for example, examining differences between trial registration, protocol, and publication.
Data synthesis
We followed Chapter 9 of theCochrane Handbook for Systematic Reviews of Interventions for data synthesis and analysis (Deeks 2017). We first provided a descriptive, qualitative synthesis of studies and their results. We used fixed‐effect models for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to conduct a subgroup analysis by type of iStent (iStent or iStent inject).
Sensitivity analysis
We planned to conduct additional sensitivity analyses to determine the impact of any post hoc decisions made during the review process.
'Summary of findings' tables
We prepared 'Summary of findings' tables using the GRADE approach to assess the certainty of the evidence (GRADEpro GDT 2015). We planned to include following outcomes in the summary.
-
Proportion of participants who were drop‐free (not using eye drops) at six to 18 months.
-
Mean change in number of IOP‐lowering drops taken per day from baseline to six to 18 months
-
Mean change in IOP, measured using Goldmann applanation tonometry, from baseline to six to 18 months.
-
Health‐related quality of life at six to 18 months.
-
Intraoperative complications at six to 18 months.
-
Postoperative complications up to six to 18 months.
-
Secondary glaucoma surgery, including laser, as recorded by the investigators of the included trials between baseline and six to 18 months.
We summarized findings from two comparison groups: in combination with phacoemulsification compared with phacoemulsification alone and iStent compared to medical therapy. We downgraded the level of certainty of the evidence if the contributing studies were at high or unclear risk of bias for masking of outcome assessors (one level), provided inconsistent estimates (one level) or imprecise estimates (one level) due to small sample size or wide CIs, or may have been subject to publication bias (one level). Guyatt 2011 noted that inclination to downgrade for publication bias should increase when evidence comes from small studies which are "industry sponsored or likely to be industry sponsored (or if the investigators share another conflict of interest)." We also presented results of one trial which compared one iStent with two iStents with three iStents, but did not assess the certainty of the evidence or present a 'Summary of findings' table for this single trial.
Results
Description of studies
Results of the search
The electronic search yielded 362 records (Figure 1). After removal of 90 duplicates, we screened the remaining 272 records and excluded a further 237 records based on information in the title and abstract. We obtained full‐text reports of 35 records for further investigation. We included 19 reports of seven studies (see Characteristics of included studies table) and excluded three reports of three studies (see Characteristics of excluded studies table). We identified 13 ongoing studies that potentially met the inclusion criteria, these studies will be assessed when data become available (see Characteristics of ongoing studies table for further details).
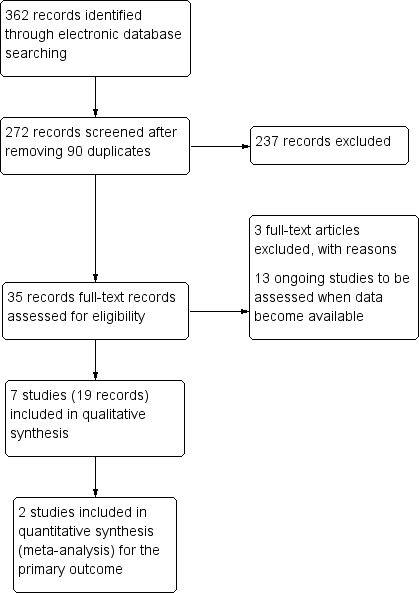
Study flow diagram.
Included studies
Type of studies
We included seven RCTs (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011; Vold 2016). Most RCTs were multi‐center trials in which participants were recruited from Armenia (two RCTs); Italy (one RCT); Spain (one RCT); the US (two RCTs); or the previous four countries in addition to Germany and the UK (one RCT) (see Characteristics of included studies table). Six trials began enrollment of participants prior to 2010, and the maximal planned length of follow‐up ranged from one to five years (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011). All seven RCTs reported having received support – including financial, non‐study financial, or non‐financial support (e.g. study devices, editorial assistance, or payment of article processing charges) – from the Glaukos Corporation (Laguna Hills, CA, USA), manufacturer of the iStent and iStent inject.
Type of participants
The seven RCTs enrolled 764 participants (765 eyes; range per study: 33 to 239 participants). Most participants were white, female sex (417/764 (55%)), and older age (range: 49 to 89 years). The diagnosis of participants varied between studies: some included people with OHT, pseudoexfoliative glaucoma (PXG), or pigmentary glaucoma (PG) in addition to OAG. Most excluded participants with prior incisional glaucoma surgery, and four trials included only participants with OAG in need of cataract surgery (Fea 2010; Fernandez‐Barrientos 2010; NCT00721968; Samuelson 2011). Four trials reported that participants were washed out of current glaucoma medication (Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011); one trial recruited only treatment‐naïve participants (Vold 2016). Table 1 provides a synopsis of the trial‐level eligibility criteria.
| Study | Diagnosis | Intraocular pressure | Number of glaucoma medication currently taking | Visual acuity (Snellen; BCVA) | Prior incisional glaucoma surgery | Prior laser surgery | Washout period |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | |||||||
| OAG in need of cataract surgery | > 18 mmHg (medicated) | ≥ 1 | 20/80 or worse | Excluded | NR | NR | |
| OHT or OAG in need of cataract surgery | > 17 mmHg and < 31 mmHg (medicated); > 21 mmHg and < 36 mmHg (unmedicated) | ≤ 2 | 20/40 or worse | Excluded | Excluded | Yes | |
| OAG in need of cataract surgery | NR | NR | NR | NR | NR | NR | |
| OAG, PEXG, or PG, in need of cataract surgery | ≤ 24 mmHg (medicated); ≥ 22 mmHg and ≤ 36 mmHg (unmedicated) | ≥ 1 and ≤ 3 | 20/40 or worse | Excluded (except for iridectomy) | Excluded | Yes | |
| Comparison 2: iStent or iStent inject vs medical therapy | |||||||
| OAG, PEXG, or PG | ≥ 22 mmHg and < 38 mmHg (unmedicated) | 1 | 20/200 or better | Excluded | Includeda | Yes | |
| OHT, OAG, or PEXG | ≥ 21 mmHg and ≤ 40 mmHg (unmedicated) | 0 | NR | Excluded | Excluded | NA | |
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | |||||||
| OAG, PEXG, or PG; and phakic | ≥ 18 mmHg and ≤ 30 mmHg (medicated); > 22 mmHg and < 38 mmHg (unmedicated) | 2 | 20/200 or better | Excluded | Includeda | Yes | |
aAs long as the procedure was not performed within 30 days prior to screening.
BCVA: best‐corrected visual acuity; OAG: open‐angle glaucoma; OHT: ocular hypertension; PEXG: pseudoexfoliative glaucoma; PG: pigmentary glaucoma; NA: not available; NR: not reported.
Type of interventions
Four RCTs compared treatment with iStent in combination with phacoemulsification to phacoemulsification alone; specifically: Fea 2010, NCT00721968, and Samuelson 2011 compared the iStent combined with phacoemulsification to phacoemulsification alone, and Fernandez‐Barrientos 2010 compared two iStents combined with phacoemulsification to phacoemulsification alone.
The remaining three RCTs did not use phacoemulsification as a concomitant intervention: Vold 2016 compared two iStents with topical travoprost (Travatan; Alcon, Fort Worth, TX, USA); Fea 2014 compared the iStent inject with fixed combination of latanoprost/timolol (Xalacom; Pfizer, New York, NY, USA); and Katz 2015 compared one iStent with two iStents and with three iStents.
Type of outcomes
Five RCTs reported our primary outcome (proportion of participants who were drop‐free at two years) (Fea 2010; Fea 2014; Katz 2015; Samuelson 2011; Vold 2016). The two RCTs that did not report on our primary outcome (Fernandez‐Barrientos 2010; NCT00721968), along with Samuelson 2011, provided data on the mean change in number of IOP‐lowering drops. Four RCTs reported mean change in IOP postwashout of any glaucoma medications (Fernandez‐Barrientos 2010; Katz 2015; NCT00721968; Samuelson 2011). Proportions of participants experiencing complications were reported variably among the seven RCTs. No RCTs reported quality of life.
Three RCTS described calculating sample sizes, based on the ability to detect a 19.5% difference in proportion of participants with IOP 21 mmHg or less at one year (Samuelson 2011); a difference in IOP of approximately 3 mmHg at 15 months (Fea 2010); or a 0.3 μL/minute/mmHg difference in the outflow facility at one year (Fernandez‐Barrientos 2010).
We summarized clinically important and surgery‐related adverse events of interest in Table 2.
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| — | — | |
| Adverse events | "No postoperative stent‐related adverse events were observed in these eyes [N = 24] through 48 months. IOP was well controlled in both groups throughout the entire follow‐up period; no secondary surgical intervention was required to control IOP." | |
| 2 iStents in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Stent malposition | Authors noted that "six of the 34 (18%) implanted stents appeared to be malpositioned" | NA |
| Need for selective trabeculoplasty | 0 | 1/16 |
| iStent in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Posterior capsule opacification | 4/27 | 1/17 |
| IOP increase ≥ 10 mmHg vs baseline IOP at any visit | 3/27 | 9/17 |
| Conjunctivitis | 3/27 | 2/17 |
| Corneal abrasion | 2/27 | 1/17 |
| Iritis | 2/27 | 0 |
| Punctate corneal staining | 1/27 | 1/17 |
| Superficial punctate keratitis | 1/27 | 1/17 |
| Blurry vision | 1/27 | 1/17 |
| BCVA loss ≥ 1 line after 3 months postoperative | 0 | 2/17 |
| Eye pain | 0 | 2/17 |
| Retinal detachment | 0 | 1/17 |
| iStent in combination with phacoemulsification at 2 years | Phacoemulsification alone at 2 years | |
| Anticipated early postoperative event (as defined by investigators) | 20/116 | 22/117 |
| Posterior capsule opacification | 7/116 | 12/117 |
| Elevated IOP | 5/116 | 8/117 |
| Stent obstruction | 5/116 | NA |
| Blurry vision or visual disturbance | 4/116 | 8/117 |
| Stent malposition | 3/116 | NA |
| Iritis | 1/116 | 6/117 |
| Conjunctival irritation due to hypotensive medication | 1/116 | 3/117 |
| Disk hemorrhage | 1/116 | 3/117 |
| Comparison 2: iStent (or iStent inject) vs medical therapy | ||
| iStent inject at 1 year (94 eyes of 94 participants) | Medical therapy at 1 year (98 eyes of 98 participants) | |
| IOP decompensation | 1/94 | 0 |
| Soreness/discomfort | 1/94 | 0 |
| Eye burning | 0 | 1/98 |
| Medical allergy | 0 | 1/98 |
| Secondary glaucoma surgery | 1/94 | NA |
| "Safety was favorable in both groups [Two iStents, N = 54; Medical therapy, N = 47; at 36 months). Two complications were reported during stent insertion in the surgery group, both of which were attributed to subject movement during surgery: one of these subjects had hyphema which resolved by day 1 and one subject had a small iridodialysis which resulted in no postoperative ocular sequelae...In the remaining non‐operated subjects, three‐year BCVA was 20/40 or better in 6 eyes (2 in stent group and 4 in med group), 20/100 in 1 eye (stent group), and 20/200 in 6 eyes (3 per group). No other post‐treatment adverse events were reported in either group." | ||
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | ||
| "No complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hyphema, nor iridodialysis [One iStent, N=38; two iStents, N= 41; three iStents, N=40]. During 42 months of postoperative follow‐up, no device‐related or sight‐threatening adverse events occurred; furthermore, no eyes required additional glaucoma surgery. In this cohort of almost entirely phakic subjects (117 of 119) with mean baseline age between 62 and 69 years, the most common (and expected) adverse event over 3.5 years of follow‐up was progression of preexisting cataract. By Month 42 postoperatively, a total of eight one‐stent eyes, five two‐stent eyes, and seven three‐stent eyes had BCVA loss 1 line due to cataract progression. Of these cases, five one‐stent eyes, two two‐stent eyes, and three three‐stent eyes underwent cataract surgery by Month 42, and their IOP and medication data thereafter were excluded from efficacy analyses; two additional eyes (three‐stent group) had cataract surgery shortly after the Month 42 visit." | ||
BCVA: best‐corrected visual acuity; IOP: intraocular pressure; N: number; NA: not available.
Excluded studies
We excluded two studies that were not RCTs. Bacharach 2014 was a subsequent observational extension of Samuelson 2011, where a non‐randomized population of 46 participants were added to the treatment arm. Vlasov 2017 was a retrospective case series review of one versus two iStent implantations in combination with phacoemulsification. We also excluded one study which was withdrawn before enrolling the first participant (NCT03274323).
Ongoing studies
We identified 13 ongoing studies awaiting classification. All 13 are described as RCTs and are recruiting participants from Armenia, Australia, Germany, Japan, Spain, Turkey, and the US. Participants are randomized to treatment with iStent compared to treatment with no iStent (e.g. phacoemulsification alone), to medical therapy (e.g. latanoprost and timolol), to different number of iStents implanted (e.g. one versus two stents), to SLT laser treatment, to the Hydrus Microstent (see Characteristics of ongoing studies table). The studies are funded mostly by manufacturers of the devices.
Risk of bias in included studies
We summarized the risk of bias in the included trials in Figure 2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Random sequence generation
Three RCTs reported how investigators generated the random allocation sequence: Fea 2010, Fernandez‐Barrientos 2010, and Samuelson 2011 used a computer‐based random generator, a method that we considered to be at low risk of bias. We assessed the remaining four RCTs, which did not report the method of generating the allocation sequence, at unclear risk of bias.
Allocation concealment
No studies described the method used to conceal the allocation sequence. We assessed all seven trials at unclear risk of bias.
Blinding
One RCT reported that the same examiner, "who was masked to the type of surgery performed" performed all postoperative evaluations (Fernandez‐Barrientos 2010). Accordingly, we assessed the risk of bias in blinding of outcome assessments as low for both 'number of IOP‐lowering drops' and 'IOP measurement' outcome domains. Although Fea 2010 noted that "staff members who measured IOP throughout the study" were masked (low risk of bias), it was unclear whether this same staff member also assessed the number of IOP‐lowering drops that participants were taking per day (unclear risk of bias). We assessed the remaining five trials, which investigators described as "not masked" (Fea 2014) or "open‐label" (Katz 2015; NCT00721968; Samuelson 2011; Vold 2016), at high risk of detection bias.
Incomplete outcome data
We considered three RCTs at low risk of bias for incomplete outcome data because there were no missing data on outcomes of our review (Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011). We assessed two RCTs at high risk of bias for incomplete outcome data: Fea 2010 excluded 3/36 participants (all randomized to the phacoemulsification alone group) from the final analysis and Vold 2016 conducted an available‐case analysis of 73/101 (72%) participants at 36 months. We assessed the remaining two RCTs at unclear risk of bias because the full publication is not yet available (NCT00721968), or the completeness of outcome data varied by time points reported (Fea 2014).
Selective reporting
We considered the risk of selective reporting to be low for five RCTs because outcomes described in the results matched those specified in methods section and in the trial registrations (Fea 2010; Fea 2014; Fernandez‐Barrientos 2010; Katz 2015; Samuelson 2011). We considered the risk of selective reporting was unclear for NCT00721968, because the full publication of trial results is not yet available; and for Vold 2016, because of differences between the primary outcomes specified on ClinicalTrials.gov ("change from screening in mean diurnal IOP (mm Hg) at the Month 12 visit") and in the published trial report (mean IOP up to 36 months; "diurnal measurements of IOP were not performed").
Effects of interventions
See: Summary of findings for the main comparison IStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma; Summary of findings 2 IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma
Based on the data available, the comparisons that we could make at time of writing this review were: 1. iStent in combination with phacoemulsification versus phacoemulsification alone and 2. iStent (or iStent inject) versus medical therapy. We also summarized findings from one RCT that compared one iStent with two iStents with three iStents. We presented our analyses by comparison, outcome, and duration of follow‐up in the order described.
Comparison 1: iStent in combination with phacoemulsification versus phacoemulsification alone
Four RCTs, which randomized 353 participants with OAG in need of cataract surgery, compared iStent in combination with phacoemulsification versus phacoemulsification alone. Three RCTs implanted one iStent (Fea 2010; NCT00721968; Samuelson 2011); one RCT implanted two iStents (Fernandez‐Barrientos 2010).
Proportion of participants who were drop‐free
Two RCTs reported proportion of participants who were drop‐free in the medium‐term (Figure 3): Fea 2010 at 15 months (RR 2.80, 95% CI 1.18 to 6.64) and Samuelson 2011 at 12 months (RR 1.31, 95% CI 1.11 to 1.54). Although estimates from both studies were consistent in favoring treatment with one iStent in combination with phacoemulsification over phacoemulsification alone, we observed substantial statistical heterogeneity in the meta‐analytical estimate (RR 1.38, 95% CI 1.18 to 1.63; I2 = 67%). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.1 Proportion of participants who were drop‐free.
Mean change in number of intraocular pressure‐lowering drops
Samuelson 2011 observed a greater reduction from baseline in the number of IOP‐lowering drops in the iStent in combination with phacoemulsification group than the phacoemulsification alone group at 12 months (MD –0.40 drops, 95% CI –0.60 to –0.20). NCT00721968 and Fernandez‐Barrientos 2010 reported the mean number (rather than mean change from baseline) of IOP‐lowering drops in each group at medium‐term (Figure 4). Overall, iStent in combination with phacoemulsification reduced the number of IOP‐lowering drops compared with phacoemulsification alone at medium term (MD –0.42, 95% CI –0.60 to –0.23; I2 = 0%). Additionally, Fernandez‐Barrientos 2010 found no statistically significant difference between treatment iStent in combination with phacoemulsification and phacoemulsification alone in the short‐term (MD –0.40, 95% CI –0.82 to 0.02 at 6 months). We graded the certainty of evidence as very low for this outcome, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
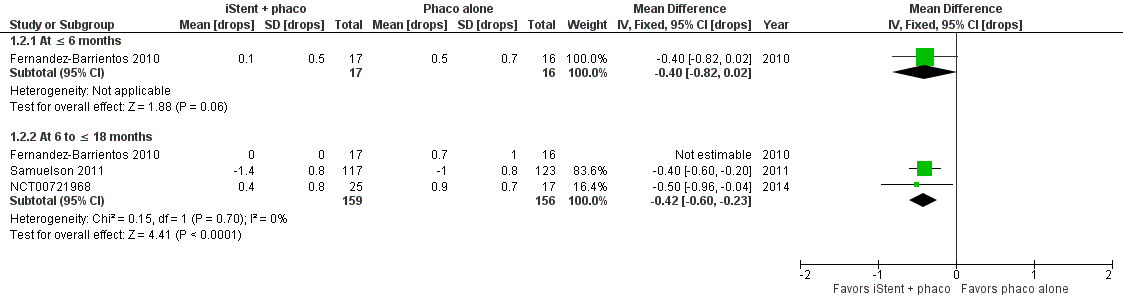
Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.
Mean change in intraocular pressure
Three RCTs reported mean change in IOP at medium‐term (Figure 5): Fea 2010 provided data at 15 months (MD –1.60, 95% CI –3.78 to 0.58); Fernandez‐Barrientos 2010 at 12 months (MD –2.70, 95% CI –4.65 to –0.75); and Samuelson 2011 at 12 months (MD 0.10, 95% CI –0.95 to 1.15). We observed substantial statistical heterogeneity (I2 = 71%) and did not conduct a meta‐analysis; instead, we present the point estimates in a forest plot (Figure 5). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), inconsistency (one level) and potential publication bias (one level).

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.3 Mean change in IOP.
Of note, Samuelson 2011, in which one iStent was implanted at the time of phacoemulsification in each study eye, reported that although mean reduction in IOP appeared similar in both groups at 12 months, "a substantially higher level of medications was used in the control [phacoemulsification only] group to maintain this similar IOP level." Additionally, at 24 months, the investigators of this RCT observed that mean IOP in the one iStent treatment group was 8.4 mmHg lower than baseline IOP, compared to 7.5 mmHg in the phacoemulsification alone group (Samuelson 2011); no standard deviations (SD) were provided and therefore we could not derive any between‐group estimates.
Health‐related quality of life
No trials reported health‐related quality of life.
Intra‐ and postoperative complications from baseline
Samuelson 2011 reported one intraoperative complication where "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery."
The reporting of postoperative complications varied by RCT (Table 3).
| Study | Pharmaceutical industry involvement | Other financial support |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| Glaukos Corporation provided funding/support (including study devices) | Ricerca Finalizaata della Regione Pimonte 2007 | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support (Investigators were consultants to Glaukos for the conduct of this study) | — | |
| Comparison 2: iStents vs medical therapy | ||
| Glaukos Corporation provided funding/support (including study devices, editorial assistance, payment of article processing charges, financial support, and non‐study financial support) | — | |
| Glaukos Corporation provided funding/support (including non‐financial, financial, and non‐study financial support to some/all authors) | — | |
| Additional comparison: iStent vs 2 iStents vs 3 iStents | ||
| Glaukos Corporation provided funding/support (including study devices and non‐financial, financial, and non‐study financial support to some/all authors) | — | |
-
In Samuelson 2011, one participant in each treatment group experienced best‐corrected visual acuity (BCVA) loss and two participants in each treatment group experienced "subconjunctival hemorrhage" at 12 months. Two participants in the iStent treatment group experienced and elevation of IOP requiring treatment at 12 months compared to one participant in the phacoemulsification alone group. Data comparing number of participants who needed secondary surgical interventions at 24 months in the iStent in combination with phacoemulsification group versus the phacoemulsification groups available for a subset "safety population" that excluded six participants who were terminated from the study before receiving surgery of any type. In this population, one participant treated with phacoemulsification in combination with iStent underwent a secondary glaucoma surgical intervention (trabeculoplasty).
-
In NCT00721968, participants in the iStent treatment group were less likely to experience IOP spikes at 12 months (RR 0.21, 95% CI 0.07 to 0.67).
-
In Fernandez‐Barrientos 2010, one participant randomized to phacoemulsification underwent selective LTP at 12 months to manage their glaucoma.
-
In Fea 2010, none of the 24 participants (10 randomized to iStent in combination with phacoemulsification and 14 randomized to phacoemulsification alone) still under follow‐up at 48 months needed secondary glaucoma surgery.
Comparison 2: iStent (or iStent inject) versus medical therapy
Two RCTs, which randomized 293 participants with OAG, compared treatment with either two iStents (Vold 2016) or the iStent inject (Fea 2014) with medical therapy. Medical therapy consisted of either a fixed combination of latanoprost/timolol (Fea 2014) or topical travoprost (Vold 2016). All participants in Fea 2014 were using one IOP‐lowering medication at recruitment and, "in the opinion of the investigator, required additional IOP lowering." All participants enrolled in Vold 2016 were newly diagnosed and had not "undergone prior treatment of any kind" for their glaucoma. Because the patient population, intervention, and comparison were clinically heterogenous between these two RCTs, we did not conduct a meta‐analysis for any outcomes.
Proportion of participants who were drop‐free
Both RCTs reported proportion of participants who were drop‐free in the medium‐term. As one would expect based on study design, no participants who were randomized to medical therapy in either RCTs were drop‐free at 12 months, compared with 96% (90/94; Fea 2014) and 94% (51/54; Vold 2016) of participants in the iStent treatment groups. Additionally, Vold 2016 observed that at 36 months, no participants in the medical therapy group were drop‐free compared to 88% (48/54) of participants in the iStent treatment group. We did not derive an RR because no events occurred in the control group. We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
Mean change in number of intraocular pressure‐lowering drops
Vold 2016 specifically reported medications required over and above the travoprost used as a study intervention, while Fea 2014 reported total number of medications required, regardless of response to medical therapy. Thus, neither Fea 2014 nor Vold 2016 provided mean change in number of IOP‐lowering drops that could be analyzed for this review.
Mean change in intraocular pressure
Fea 2014 reported the mean change in IOP in the short‐term (MD 0.10, 95% CI –0.72 to 0.92, at 6 months) and medium‐term (MD –0.60, 95% CI –1.28 to 0.08, at 12 months), comparing iStent inject to medical therapy. Vold 2016 did not report mean change in IOP but provided mean IOP (without SD) at six months (14.2 mmHg in the iStent group versus 13.8 mmHg in the medical therapy group), 18 months (13.5 mmHg in the iStent group versus 14.6 mmHg in the medical therapy group), and 36 months (14.6 mmHg in the iStent group versus 15.3 mmHg in the medical therapy group). We graded the certainty of evidence as very low, downgrading for risk of bias (one level), imprecision (one level), and potential publication bias (one level).
Health‐related quality of life
No studies reported health‐related quality of life.
Intra‐ and postoperative complications from baseline
The reporting of intraoperative and postoperative complications varied by RCT.
-
Vold 2016 observed one participant with hyphema that resolved by day one and another participant with a small iridodialysis. The investigators also noted that six participants (five in the iStent treatment group and one in the medical therapy group) underwent cataract surgery at 36 months, but they did not report on need for secondary glaucoma surgery.
-
Fea 2014 reported one participant in the iStent inject treatment group who experienced "IOP decompensation with an elevated IOP (48 mmHg)" that resolved after treatment with medication; and one participant, also in the iStent inject treatment group, who required laser treatment to remove an apparent obstruction.
Additional comparison: one iStent versus two iStents versus three iStents
One three‐arm RCT randomized 119 participants with OAG to treatment with one iStent, two iStents, or three iStents (Katz 2015). The investigators recruited participants from a single center in Armenia and followed them for five years. Results up to 42 months were available.
Proportion of participants who were drop‐free
Comparing treatment with one iStent to treatment with two iStents, there was no difference in terms of participants who were drop‐free at time points in the short‐term (RR 0.94, 95% CI 0.85 to 1.05), medium‐term (RR 0.99, 95% CI 0.85 to 1.15), and long‐term (RR 0.98, 95% CI 0.85 to 1.15) (Figure 6). However, at more than 36 months, participants who were randomized to treatment with one iStent were less likely to be drop‐free than those to treatment with two iStents (RR 0.51, 95% CI 0.34 to 0.75, at 42 months).
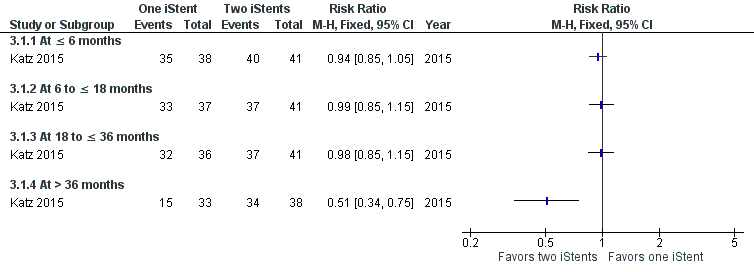
Forest plot of comparison: 3 One iStent (without phacoemulsification) versus two iStents (without phacoemulsification), outcome: 3.1 Proportion of participants who were drop‐free.
Comparing treatment with one iStent to treatment with three iStents, there was also no difference in terms of participants who were drop‐free at time points in the short‐term (RR 0.94, 95% CI 0.85 to 1.05), medium‐term (RR 0.97, 95% CI 0.84 to 1.12), and long‐term (RR 0.97, 95% CI 0.83 to 1.12) (Figure 7). At more than 36 months, participants who were randomized to treatment with one iStent were less likely to be drop‐free than those to treatment with three iStents (RR 0.49, 95% CI 0.34 to 0.73, at 42 months).

Forest plot of comparison: 4 One iStent (without phacoemulsification) versus three iStents (without phacoemulsification), outcome: 4.1 Proportion of participants who were drop‐free.
Mean change in number of intraocular pressure‐lowering drops
Katz 2015 did not report the mean change in number of IOP‐lowering drops; however, the investigators noted that one participant in the two‐iStent treatment group was prescribed two medications at 18 months. The remaining 13 participants who were using drops at 18 months were on one medication to control their IOP.
Mean change in intraocular pressures
Katz 2015 reported the mean change in IOP at 18 months as –3.94 mmHg in the one‐iStent, –5.99 mmHg in the two‐iStent, and –8.19 mmHg in the three‐iStent treatment groups. No SD were reported; however, investigators provided mean IOP at six, 18, and 42 months with SDs. The investigators reported a statistically significant difference in mean IOP at 18 months, comparing treatment with one iStent to treatment with two iStents (MD 1.80 mmHg, 95% CI 1.17 to 2.43) and to treatment with three iStents (MD 3.50 mmHg, 95% CI 2.88 to 4.12), and no difference in mean IOP comparing treatment with one iStent to treatment with two iStents or to treatment with three iStents at six and 42 months
Health‐related quality of life
Katz 2015 did not report health‐related quality of life.
Intra‐ and postoperative complications from baseline
Katz 2015 noted that "no complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hypema, nor iridodialysis." By 42 months, eight (21%) participants in the one‐iStent, five (12%) participants in the two‐iStent, and seven (18%) participants in three‐iStent treatment groups had BCVA loss of 1 line or more due to cataract progression. Of these, five participants in the one‐iStent, two participants in the two‐iStent, and three participants in the three‐iStent treatment groups underwent cataract surgery by 42 months.
Discussion
Summary of main results
We identified seven RCTs that compared treatment of people with the iStent (or iStent inject) to treatment with phacoemulsification alone, medical therapy, or different numbers of iStents. We summarized our findings after reviewing the available evidence in the 'Summary of findings' tables for the main comparison section (summary of findings Table for the main comparison; summary of findings Table 2).
There was considerable variability among the trials with respect to interventions evaluated, outcomes reported, and length of participant follow‐up. The certainty of the evidence was very low.
-
In comparison 1, we examined four RCTs comparing treatment with iStent in combination with phacoemulsification to phacoemulsification alone. Data from two trials suggest that participants randomized to iStent were 1.38 times more likely to be drop‐free between six and 18 months postsurgery. However, we observed uncertainty in this estimate (95% CI 1.18 to 1.63) and substantial statistical heterogeneity (I2 = 67%).
-
In comparison 2, we examined RCTs comparing treatment with iStent (without phacoemulsification) to medical therapy. We determined the two studies in this comparison to be clinically and methodologically heterogeneous and did not conduct a meta‐analysis. In both studies, at 12 months, no participants in the medical therapy groups were drop‐free, while over 90% of participants in the iStent groups were drop‐free.
-
Additionally, we found one RCT that compared participants randomized to treatment with one iStent to treatment with two iStents, and to treatment with three iStents. About 53% of participants in the one iStent treatment group were drop‐free at 42 months compared to over 90% of participants in the two‐iStent treatment group (RR 0.51, 95% CI 0.34 to 0.75) and three‐iStent treatment group (RR 0.49, 95% CI 0.34 to 0.73).
All trials that reported mean change in IOP noted modest to no difference in IOP reduction. No trials reported on health‐related quality of life, and the seven trials reported proportions of participants experiencing complications variably. Therefore, the association between treatment with iStent and quality of life or adverse events could not be estimated reliably from the data provided.
Overall completeness and applicability of evidence
Despite limiting this review to only RCTs, the included studies differed from one another in several regards. Recognizing these differences, we must consider several factors when interpreting the evidence.
-
Type and number of iStents may matter. Included studies suggested that implantation of two iStents, compared to one iStent, may be associated with greater proportions of participants who are drop‐free and greater mean reduction in IOP in the long‐term (Fernandez‐Barrientos 2010; Katz 2015). However, a dearth of information precludes formal assessments of quality of life and adverse events associated with procedures involving implantation of multiple devices.
-
Specific racial or ethnic groups may be under‐represented. Most participants randomized were white.
-
Prior treatments that participants received for glaucoma differed. All trials excluded people with laser glaucoma surgery performed within 30 days of screening and those with any prior incisional glaucoma surgery. Most trials required participants to be on one or more glaucoma medication at time of enrollment, except one trial that recruited treatment‐naïve participants.
-
All seven trials received support from the Glaukos Corporation. Support included financial support to some/all authors, study devices, payment of article processing charges, and editorial assistance.
Quality of the evidence
The certainty of the evidence was low across comparisons included in this review. Most trials did not report how the random sequence was generated or the method of concealing allocation. There was high or unclear risk of detection bias in most trials because the outcome assessors were not masked. Attrition bias was either at high or unclear risk for four of the seven included trials. Additionally, few meta‐analyses were possible due to considerable clinical, methodological, and statistical heterogeneity in interventions evaluated and length of participant follow‐up. We also downgraded the evidence because only data from small studies sponsored by industry are available (Guyatt 2011). All seven studies were sponsored by the same industry sponsor (Table 3).
Potential biases in the review process
We worked with an information specialist to conduct a highly sensitive search of the literature and searched multiple databases including trial registries. Two review authors independently completed all steps outlined in the Methods section of this review to reduce bias during study selection, 'Risk of bias' assessment, and data extraction.
Agreements and disagreements with other studies or reviews
One Cochrane systematic review of combined surgery versus phacoemulsification alone for eyes with cataract and glaucoma examined interventions including the iStent (Zhang 2015). The authors included three trials of four that were also included in our review (Fea 2010; Fernandez‐Barrientos 2010; Samuelson 2011). The authors reported a summary estimate for mean reduction in IOP at one year (MD –1.37 mmHg, 95% CI –2.76 to 0.03; I2 = 56%), which we did not report due to substantial statistical heterogeneity and qualitative differences in effect estimates. Additionally, the authors extracted values for mean change from the medicated screening IOP rather than change from unmedicated (postwashout) IOP at baseline for one trial (Samuelson 2011).
We also identified two additional systematic reviews involving the iStent (Lavia 2017; Malvankar‐Mehta 2015). Both reviews used inappropriate statistical methods in analyzing their data.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.1 Proportion of participants who were drop‐free.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.3 Mean change in IOP.

Forest plot of comparison: 3 One iStent (without phacoemulsification) versus two iStents (without phacoemulsification), outcome: 3.1 Proportion of participants who were drop‐free.

Forest plot of comparison: 4 One iStent (without phacoemulsification) versus three iStents (without phacoemulsification), outcome: 4.1 Proportion of participants who were drop‐free.

Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 1 Proportion of participants who were drop‐free.

Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.

Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 3 Mean change in IOP.

Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 1 Proportion of participants who were drop‐free.

Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 2 Mean change in intraocular pressure.

Comparison 3 One iStent versus two iStents, Outcome 1 Proportion of participants who were drop‐free.

Comparison 3 One iStent versus two iStents, Outcome 2 Mean change in intraoperative pressure.

Comparison 4 One iStent versus three iStents, Outcome 1 Proportion of participants who were drop‐free.

Comparison 4 One iStent versus three iStents, Outcome 2 Mean change in intraocular pressure.
| iStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with phacoemulsification alone | Risk with iStent in combination with phacoemulsification | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | 583 per 1000 | 804 per 1000 | RR 1.38 | 239 | ⊕⊝⊝⊝ | Estimate based on data from 2 trials. |
| Mean change in number of IOP‐lowering drops from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in number of IOP‐lowering drops from baseline ranged from –1.0 to 0.9 drops | MD 0.42 drops fewer | — | 282 | ⊕⊝⊝⊝ | In addition, Fernandez‐Barrientos 2010 reported the change in number of IOP‐lowering drops was 0 (SD 0) in the iStent in combination of phacoemulsification treatment group and 0.7 (SD 1) in the phacoemulsification alone group. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline ranged from –8.5 to –1.6 mmHg | MD 1.24 mmHg lower | — | 284 | ⊕⊝⊝⊝ | — |
| Health‐related quality of life | — | — | — | — | Not reported in any of the 4 studies. | |
| Intraoperative complications | — | — | — | — | Samuelson 2011 reported that "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery." | |
| Postoperative complications | Based on available data, participants who were randomized to treatment with phacoemulsification in combination with iStent were less likely to experience elevated IOP (or IOP spikes) and loss of vision than those randomized to phacoemulsification alone. | — | 334 (4 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery Follow‐up: range 6 to ≤ 18 months | 1 participant randomized to treatment with phacoemulsification in combination with iStent and 1 participant randomized to phacoemulsification alone underwent selective laser trabeculoplasty at 12 months. | — | 290 (3 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high or unclear risk of bias for blinding of outcome assessor. | ||||||
| IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with medical therapy | Risk with iStent (or iStent inject) | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | At 12 months, 0/138 participants randomized to medical therapy were drop‐free at 12 months, while 141/148 (95%) participants randomized to treatment with iStent were drop‐free at 12 months. We did not derive an RR because no events occurred in the control groups of either trial. | — | 286 | ⊕⊝⊝⊝ | In addition, Vold 2016 noted that 48/54 (88%) participants in the iStent treatment group were drop‐free at 36 months. | |
| Mean change in number of IOP‐lowering drops from baseline | — | — | — | — | — | Not reported in either study. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline was –11.6 mmHg | MD 0.6 mmHg lower | — | 184 | ⊕⊝⊝⊝ | Vold 2016 did not report mean change in IOP but did provide mean IOP (without SD) at 6 months (14.2 mmHg), 18 months (13.5 mmHg), and 36 months (14.6 mmHg) in the iStent treatment groups; and at 6 months (13.8 mmHg), 18 months (14.6 mmHg), and 36 months (15.3 mmHg) in the medical therapy group. |
| Health‐related quality of life | — | — | — | — | Not reported in either study. | |
| Intraoperative complications | 1 participant in the iStent treatment group experienced hyphema which resolved by day 1 | — | 101 (1 RCT) | — | We did not conduct a meta‐analysis of complications. | |
| Postoperative complications | Vold 2016 noted that best‐corrected visual acuity was stable between both groups and did not report on any other postoperative complications. Fea 2014 reported that 1 participant in the iStent inject group experienced IOP decompensation with an elevated IOP of 48 mmHg. | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery | Fea 2014 reported that 1 participant needed laser treatment to remove an apparent obstruction | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision due to small sample size/wide confidence interval. | ||||||
| Study | Diagnosis | Intraocular pressure | Number of glaucoma medication currently taking | Visual acuity (Snellen; BCVA) | Prior incisional glaucoma surgery | Prior laser surgery | Washout period |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | |||||||
| OAG in need of cataract surgery | > 18 mmHg (medicated) | ≥ 1 | 20/80 or worse | Excluded | NR | NR | |
| OHT or OAG in need of cataract surgery | > 17 mmHg and < 31 mmHg (medicated); > 21 mmHg and < 36 mmHg (unmedicated) | ≤ 2 | 20/40 or worse | Excluded | Excluded | Yes | |
| OAG in need of cataract surgery | NR | NR | NR | NR | NR | NR | |
| OAG, PEXG, or PG, in need of cataract surgery | ≤ 24 mmHg (medicated); ≥ 22 mmHg and ≤ 36 mmHg (unmedicated) | ≥ 1 and ≤ 3 | 20/40 or worse | Excluded (except for iridectomy) | Excluded | Yes | |
| Comparison 2: iStent or iStent inject vs medical therapy | |||||||
| OAG, PEXG, or PG | ≥ 22 mmHg and < 38 mmHg (unmedicated) | 1 | 20/200 or better | Excluded | Includeda | Yes | |
| OHT, OAG, or PEXG | ≥ 21 mmHg and ≤ 40 mmHg (unmedicated) | 0 | NR | Excluded | Excluded | NA | |
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | |||||||
| OAG, PEXG, or PG; and phakic | ≥ 18 mmHg and ≤ 30 mmHg (medicated); > 22 mmHg and < 38 mmHg (unmedicated) | 2 | 20/200 or better | Excluded | Includeda | Yes | |
| aAs long as the procedure was not performed within 30 days prior to screening. | |||||||
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| — | — | |
| Adverse events | "No postoperative stent‐related adverse events were observed in these eyes [N = 24] through 48 months. IOP was well controlled in both groups throughout the entire follow‐up period; no secondary surgical intervention was required to control IOP." | |
| 2 iStents in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Stent malposition | Authors noted that "six of the 34 (18%) implanted stents appeared to be malpositioned" | NA |
| Need for selective trabeculoplasty | 0 | 1/16 |
| iStent in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Posterior capsule opacification | 4/27 | 1/17 |
| IOP increase ≥ 10 mmHg vs baseline IOP at any visit | 3/27 | 9/17 |
| Conjunctivitis | 3/27 | 2/17 |
| Corneal abrasion | 2/27 | 1/17 |
| Iritis | 2/27 | 0 |
| Punctate corneal staining | 1/27 | 1/17 |
| Superficial punctate keratitis | 1/27 | 1/17 |
| Blurry vision | 1/27 | 1/17 |
| BCVA loss ≥ 1 line after 3 months postoperative | 0 | 2/17 |
| Eye pain | 0 | 2/17 |
| Retinal detachment | 0 | 1/17 |
| iStent in combination with phacoemulsification at 2 years | Phacoemulsification alone at 2 years | |
| Anticipated early postoperative event (as defined by investigators) | 20/116 | 22/117 |
| Posterior capsule opacification | 7/116 | 12/117 |
| Elevated IOP | 5/116 | 8/117 |
| Stent obstruction | 5/116 | NA |
| Blurry vision or visual disturbance | 4/116 | 8/117 |
| Stent malposition | 3/116 | NA |
| Iritis | 1/116 | 6/117 |
| Conjunctival irritation due to hypotensive medication | 1/116 | 3/117 |
| Disk hemorrhage | 1/116 | 3/117 |
| Comparison 2: iStent (or iStent inject) vs medical therapy | ||
| iStent inject at 1 year (94 eyes of 94 participants) | Medical therapy at 1 year (98 eyes of 98 participants) | |
| IOP decompensation | 1/94 | 0 |
| Soreness/discomfort | 1/94 | 0 |
| Eye burning | 0 | 1/98 |
| Medical allergy | 0 | 1/98 |
| Secondary glaucoma surgery | 1/94 | NA |
| "Safety was favorable in both groups [Two iStents, N = 54; Medical therapy, N = 47; at 36 months). Two complications were reported during stent insertion in the surgery group, both of which were attributed to subject movement during surgery: one of these subjects had hyphema which resolved by day 1 and one subject had a small iridodialysis which resulted in no postoperative ocular sequelae...In the remaining non‐operated subjects, three‐year BCVA was 20/40 or better in 6 eyes (2 in stent group and 4 in med group), 20/100 in 1 eye (stent group), and 20/200 in 6 eyes (3 per group). No other post‐treatment adverse events were reported in either group." | ||
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | ||
| "No complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hyphema, nor iridodialysis [One iStent, N=38; two iStents, N= 41; three iStents, N=40]. During 42 months of postoperative follow‐up, no device‐related or sight‐threatening adverse events occurred; furthermore, no eyes required additional glaucoma surgery. In this cohort of almost entirely phakic subjects (117 of 119) with mean baseline age between 62 and 69 years, the most common (and expected) adverse event over 3.5 years of follow‐up was progression of preexisting cataract. By Month 42 postoperatively, a total of eight one‐stent eyes, five two‐stent eyes, and seven three‐stent eyes had BCVA loss 1 line due to cataract progression. Of these cases, five one‐stent eyes, two two‐stent eyes, and three three‐stent eyes underwent cataract surgery by Month 42, and their IOP and medication data thereafter were excluded from efficacy analyses; two additional eyes (three‐stent group) had cataract surgery shortly after the Month 42 visit." | ||
| BCVA: best‐corrected visual acuity; IOP: intraocular pressure; N: number; NA: not available. | ||
| Study | Pharmaceutical industry involvement | Other financial support |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| Glaukos Corporation provided funding/support (including study devices) | Ricerca Finalizaata della Regione Pimonte 2007 | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support (Investigators were consultants to Glaukos for the conduct of this study) | — | |
| Comparison 2: iStents vs medical therapy | ||
| Glaukos Corporation provided funding/support (including study devices, editorial assistance, payment of article processing charges, financial support, and non‐study financial support) | — | |
| Glaukos Corporation provided funding/support (including non‐financial, financial, and non‐study financial support to some/all authors) | — | |
| Additional comparison: iStent vs 2 iStents vs 3 iStents | ||
| Glaukos Corporation provided funding/support (including study devices and non‐financial, financial, and non‐study financial support to some/all authors) | — | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤ 18 months | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.18, 1.63] |
| 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.82, 0.02] |
| 2.2 At 6 to ≤ 18 months | 3 | 315 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.60, ‐0.23] |
| 3 Mean change in IOP Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 At ≤ 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 6 to ≤ 18 months (unmedicated IOP) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤18 months | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 125.43 [17.80, 883.89] |
| 1.2 At 18 to ≤ 36 months | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 84.65 [5.36, 1336.23] |
| 2 Mean change in intraocular pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.72, 0.92] |
| 2.2 At 6 to ≤ 18 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraoperative pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraocular pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

