Prebiotics for the prevention of hyperbilirubinaemia in neonates
Appendices
Appendix 1. Search strategy for CENTRAL (the Cochrane Library)
ID search
#1 MeSH descriptor: [Infant, Newborn] explode all trees
#2 newborn*:ti,ab,kw in Trials (Word variations have been searched)
#3 new‐born*:ti,ab,kw in Trials (Word variations have been searched)
#4 neonat*:ti,ab,kw in Trials (Word variations have been searched)
#5 baby*:ti,ab,kw in Trials (Word variations have been searched)
#6 babies*:ti,ab,kw in Trials (Word variations have been searched)
#7 child:ti,ab,kw in Trials (Word variations have been searched)
#8 boy*:ti,ab,kw in Trials (Word variations have been searched)
#9 girl*:ti,ab,kw in Trials (Word variations have been searched)
#10 {or #1‐#9}
#11 MeSH descriptor: [Hyperbilirubinemia] explode all trees
#12 MeSH descriptor: [Jaundice] explode all trees
#13 Hyperbilirubinaemia:ti,ab,kw in Trials (Word variations have been searched)
#14 Jaundice:ti,ab,kw in Trials (Word variations have been searched)
#15 Icter:ti,ab,kw in Trials (Word variations have been searched)
#16 {or #11‐#15}
#17 MeSH descriptor: [Prebiotics] explode all trees
#18 "prebiotic":ti,ab,kw in Trials (Word variations have been searched)
#19 oligosaccharides:ti,ab,kw in Trials (Word variations have been searched)
#20 {or #17‐#19}
#21 #10 and #16 and #20
Appendix 2. Search strategy for PubMed database
Search strategy for PubMed database
#1 Search “Infant, newborn”[Mesh]
#2 Search newborn* [TIAB]
#3 Search new‐born* [TIAB]
#4 Search neonat* [TIAB]
#5 Search baby* [TIAB]
#6 Search babies* [TIAB]
#7 Search child [TIAB]
#8 Search boy* [TIAB]
#9 Search girl* [TIAB]
#10 Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 Search Hyperbilirubinemia [Mesh]
#12 Search jaundice [Mesh]
#13 Search Hyperbilirubinaemia [TIAB]
#14 Search Jaundice [TIAB]
#15 Search Icter [TIAB]
#16 Search #11 OR #12 OR #13 OR #14 OR #15
#17 Search prebiotics [Mesh]
#18 Search Prebiotic [TIAB]
#19 Search oligosaccharides [TIAB]
#20 Search #17 OR #18 OR #19
#21 Search “clinical trials” [Mesh]
#22 Search “randomized controlled trial” [PT]
#23 Search “controlled clinical trial” [PT]
#24 Search “clinical trial” [PT]
#25 Search randomized [TIAB]
#26 Search placebo [TIAB]
#27 Search “drug therapy” [SH]
#28 Search randomly [TIAB]
#29 Search trial [TI]
#30 Search groups [TIAB]
#31 Search #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
#32 Search #10 AND #16 AND
Appendix 3. Embase session results
Embase session results
No.
Query
Results
3
#32
#11 AND #17 AND #21 AND #31
3,872,819
#31
#22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
2,078,392
#30
'groups':ab,ti
203,821
#29
'trial':ti
317,493
#28
'randomly':ab,ti
414,545
#27
'drug therapy'/de
234,682
#26
'placebo':ab,ti
511,199
#25
'randomized':ab,ti
364,274
#24
'controlled clinical trial'/de
398,755
#23
'randomized controlled trial'/de
1,318,524
#22
'clinical trial*'
26,456
#21
#18 OR #19 OR #20
21,036
#20
'oligosaccharides':ab,ti
3,864
#19
'prebiotic':ab,ti
4,152
#18
'prebiotics'/de
16,485
#17
#12 OR #13 OR #14 OR #15 OR #16
56
#16
'icter':ab,ti
18
#15
'juandice':ab,ti
1,565
#14
'hyperbilirubinaemia':ab,ti
18
#13
'juandice'
15,931
#12
'hyperbilirubinemia'/de
2,126,731
#11
#1 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
155,197
#10
'girl*':ab,ti
162,646
#9
'boy*':ab,ti
1,399,639
#8
'child*':ab,ti
41,052
#7
'babies*':ab,ti
43,681
#6
'baby*':ab,ti
274,173
#5
'neonat*':ab,ti
4,963
#4
'new‐born*':ab,ti
169,436
#3
'newborn*':ab,ti
509,521
#1
'infant newborn'/exp OR 'infant newborn'
Appendix 4. Sensitive key terms
1. For hyperbilirubinaemia, the following subject headings and text words will be used: Hyperbilirubinemia (explode) [MeSHTerms] OR jaundice (explode) [MeSH Terms] OR hyperbilirubinaemia OR hyperbilirubinaemia OR jaundice OR icter.
2. For neonates, the following subject headings and text words will be used: Infant, Newborn (explode) [MeSH heading] OR baby* OR babies OR newborn* OR new‐born* OR neonate* OR child OR boy* OR girl*
3. For prebiotics intervention, the following subject headings and text words will be used: prebiotics (explode) [MeSH Terms] OR prebiotic OR oligosaccharides.
4. Regarding proposed secondary outcomes (i.e. exchange transfusion, phototherapy, acute bilirubin encephalopathy, and frequency of stool), the following approach will be used:
a. For exchange transfusion, the following subject headings and text words will be used: exchange transfusion, whole blood (explode) [MeSH Terms] OR exchange transfusion OR exchange‐transfusion OR exsanguinotransfusion OR exsanguination transfusion.
b. For phototherapy, the following subject headings and text words will be used: phototherapy [MeSH Terms].
c. For acute bilirubin encephalopathy, the following subject headings and text words will be used: bilirubin encephalopathy [MeSH Terms] OR encephalopathy OR bilirubin‐encephalopathy OR acute bilirubin encephalopathy OR acute‐ bilirubin‐ encephalopathy OR kernicterus.
d. For frequency of stool, the following subject headings and text words will be used: Frequency stool [MeSH Terms] OR Stool OR Stool frequency OR stool number OR defecation frequency.
Appendix 5. Risk of bias tool
The following issues will be evaluated and entered into the 'Risk of bias' table.
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorise the method used to generate the allocation sequence as:
a. low risk (any truly random process, e.g. random number table; computer random number generator);
b. high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
c.unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorise the method used to conceal the allocation sequence as:
a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
c. unclear risk.
3. Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we will categorise the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding will be assessed separately for different outcomes or classes of outcomes. We will categorise the methods as:
a. low risk, high risk, or unclear risk for participants;
b. low risk, high risk, or unclear risk for personnel; and
c. low risk, high risk, or unclear risk for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion when reported, and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we will re‐include missing data in the analyses. We will categorise the methods as:
a. low risk (< 20% missing data);
b. high risk (≥ 20% missing data); or
c. unclear risk.
5. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. We will assess the methods as:
a. low risk (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
b. high risk (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes that were not prespecified outcomes of interest are reported incompletely and so cannot be used; or the study fails to include results of a key outcome that would have been expected to have been reported); or
c. unclear risk.
6. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns that we have about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We will assess whether each study was free of other problems that could put it at risk of bias as:
a. low risk;
b. high risk; or
c. unclear risk.
If needed, we will explore the impact of the level of bias by undertaking sensitivity analyses.

Guidelines for phototherapy in infants at ≥ 35 weeks' gestation.
(American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297.)
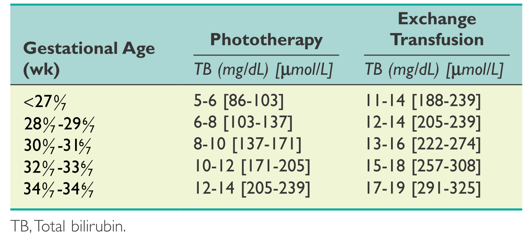
Suggested guidelines for initiating phototherapy or exchange transfusion in premature infants.
(Maisels MJ, Watchko JF, Bhutani VK, et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. Journal of Perinatology 2012;32:660‐664.)

